1 Department of Obstetrics and Gynecology, Ankara Bilkent City Hospital, 06800 Ankara, Turkey
2 Department of Obstetrics and Gynecology, Ondokuz Mayıs University, Faculty of Medicine, 55280 Samsun, Turkey
3 Department of Obsteterics and Gynecology, Sağlık Bilimleri University, 06290 Ankara, Turkey
4 Department of Obsteterics and Gynecology, Mediliv Medical Center, IVF Specialist, 55100 Samsun, Turkey
5 Department of Obstetrics and Gynecology, McGill University Health Centre Reproductive Centre, Montreal, QC H4A 3J1, Canada
Abstract
The purpose of this research is to compare the efficacy of 8% micronized vaginal progesterone gel (VAG) at 180 mg daily versus intramuscular progesterone (IMP) in oil at 100 mg daily for luteal phase support (LPS) after fresh single embryo transfer (SET) in Patient-Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) Group 1b patients, and to ascertain whether the type of LPS predicts live birth in these patients.
A total of 2420 assisted reproductive technology (ART) cycles performed in Ondokuz Mayıs University IVF Unit were analysed retrospectively. The data of POSEIDON Group 1b (unexpected suboptimal responders) who underwent antagonist in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles were included. All patients were categorized into groups according to the form of LPS, specifically VAG and IMP. Pregnancy, clinical pregnancy rate (CPR), live birth rate (LBR), spontaneous abortion rate and predictors of live birth (age, infertility duration, body mass index (BMI), type of progesterone used for luteal support, serum day 3 estradiol, serum progesterone on the day of human coryonic gonadotrophin (HCG), total duration of induction, serum estradiol on the day of HCG, the total number of oocytes retrieved, number of mature oocytes obtained, average gonadotropin dose per day, total gonadotropin dose administered and total number of embryos retrieved) were analyzed.
There was no statistically significant difference between the micronized VAG and IMP groups in terms of age (31 (23–35) vs. 27 (23–35), p = 0.319). There were no statistically significant differences in pregnancy outcomes between the two groups concerning CPR per transfer (70.6% vs. 75.6%; p = 0.364), ongoing pregnancy per cycle (36.2% vs. 39.5%; p = 0.577), and LBR per cycle (34.4% vs. 36.1%; p = 0.785). The spontaneous abortion rates (36.2% vs. 36.8%; p = 0.921) were similar between VAG and IMP groups. The type of LPS did not emerge as a parameter predicting pregnancy (odds ratio (OR): 0.718, 95% confidence interval (95% CI): 0.652–1.313, p = 0.451), clinical pregnancy (OR: 0.598, 95% CI: 0.592–1.289, p = 0.562) and live birth (OR: 0.802, 95% CI: 0.661–1.202, p = 0.580). The logistic regression analysis aimed at assessing the influence of confounding factors, namely age, BMI, and duration of infertility on pregnancy rate, CPR and LBR, did not reveal statistically significant results (p > 0.05).
VAG 180 mg daily provide similar pregnancy outcomes compared to 100 mg daily IMP in POSEIDON Group 1b patients undergoing antagonist fresh IVF/ICSI cycles.
Keywords
- intramuscular
- luteal support
- progesterone
- suboptimal responders
- vaginal
Progesterone (P4) is crucial for both endometrial development and embryo implantation [1]. The transition of the endometrium to its receptive phase relies on adequate serum P4 levels [2]. In natural menstrual cycles, P4 is synthesized by the corpus luteum (CL) and is indispensable for maintaining pregnancy. P4 protects the embryo by stimulating the production of anti-inflammatory cytokines, and decreased serum P4 levels can increase the risk of miscarriage [1, 3]. Generally, there is a belief that endogenous production of progesterone is sufficient to support implantation in a natural cycle [1].
Luteal phase deficiency (LPD) can occur following in vitro fertilization (IVF) due to follicular damage during oocyte retrieval, and suppression of endogenous P4 production resulting from the use of supraphysiological dose of follicle-stimulating hormone (FSH) during ovarian stimulation [4]. Additionally, in ovarian stimulation protocols utilizing gonadotropin-releasing hormone (GnRH) analogs, serum P4 levels remain low and LPD occurs [4]. The necessity of luteal phase support (LPS) is unquestionable for both managing luteal insufficiency and ensuring the continuation of pregnancy during the early gestational period in IVF cycles [4, 5]. Various routes of LPS are utilized in IVF cycles, including vaginal, intramuscular, and oral administration of progesterone [5]. Both vaginal and intramuscular routes have demonstrated efficacy in fresh stimulated IVF cycles [6, 7].
Insufficient response to standard treatment protocols, characterized by inadequate
follicle recruitment, is referred to as ‘poor ovarian response’. [8]. Even women with
normal ovarian reserve parameters may fail to achieve the optimal oocyte yield.
The Patient-Oriented Strategies Encompassing Individualized Oocyte Number
(POSEIDON) criteria encompass women demonstrating poor or suboptimal responses to
standard ovarian stimulation. This includes unexpected poor responder women
(retrieving
Although research exists on LPS in normal responders and poor responders [9, 12, 13], there is currently a scarcity of studies on LPS administration routes within POSEIDON Group 1b, which includes unexpected suboptimal responders undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) fresh embryo transfer cycles.
The purpose of this study is to compare the efficacy of 8% micronized vaginal progesterone gel (VAG) at a daily dosage of 180 mg compared to intramuscular progesterone (IMP) in oil at a daily dosage of 100 mg for LPS following fresh single embryo transfer (SET) in POSEIDON Group 1b patients and to assess whether the type of LPS influences live birth rates in these patients.
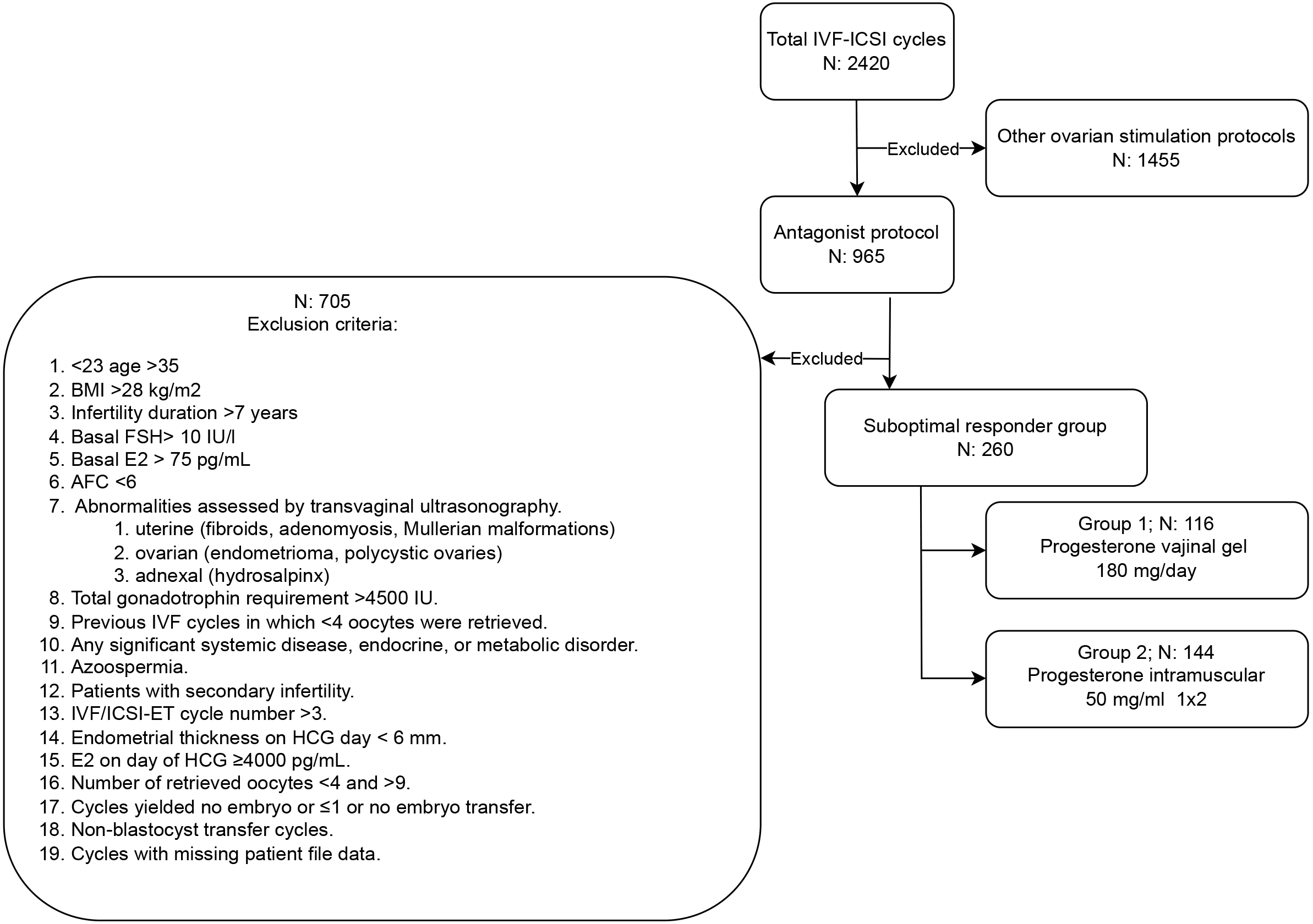
A retrospective analysis was conducted on a total of 2420 assisted reproductive technology (ART) cycles performed at Ondokuz Mayıs University IVF Unit from January 2010 to December 2015. An approval was recieved from the ethics committee at the Ondokuz Mayıs University Medical Faculty in Samsun (Approval No: 2017/121). This study encompassed unexpected suboptimal responders (POSEIDON Group 1b) [10] undergoing fresh SET, who yielded 4–9 oocytes postantagonist stimulation. These individuals displayed normal ovarian reserve parameters, which included a serum anti-mullerian hormone (AMH) level of 1 ng/mL or higher, a basal follicle-stimulating hormone (FSH) level below 10 IU/L, and antral follicle counts (AFC) exceeding 10. The study involved a total of 260 patients. Fig. 1 illustrates all the inclusion and exclusion criteria. Information regarding demographic characteristics, ovarian stimulation parameters, and pregnancy outcomes was extracted from the patient files.
 Fig. 1.
Fig. 1.
The study flowchart depicted the included and excluded cycles. BMI, body mass index; E2, estradiol; AFC, antral follicle count; IVF/ICSI-ET, in vitro fertilization/intracytoplasmic sperm injection-embryo transfer; FSH, follicle-stimulating hormone; HCG, human chorionic gonadotropin.
FSH injections were initiated on cycle day 3 (D3) if functional ovarian cysts were not present. Ovarian response was observed by serial transvaginal ultrasonography with or without hormonal monitoring. Dosage adjustments were based on the ovarian response. When encountering a follicle measuring 14 mm in diameter, the administration of cetrorelix acetate as GnRH antagonist (Cetrotide® 0.25 mg, Merck KGaA, Darmstadt, Germany) at a daily subcutaneous dosage of 0.25 mg persisted until the day of human chorionic gonadotropin (HCG) administration [14]. A dosage of 10,000 IU of HCG (Pregnyl® 5000 IU, Organon, Oss, Netherlands) was administered intramuscularly to stimulate the final maturation of oocytes. This procedure was executed when the dominant follicle reached a size of 18 mm, and at least half of the remaining follicles exhibited a mean diameter of no less than 14 mm. Oocyte retrieval, guided by transvaginal ultrasound, was carried out 36 hours following the HCG injection.
All oocyte retrieval and embryo transfer procedures were performed by the same experienced IVF specialist (D.G., Director, IVF Unit, Ondokuz Mayıs University Hospital, Samsun, Turkey). Fluid was collected from the follicles during oocyte retrieval using a 17-gauge needle and 100 mmHg aspiration pressure. Under ultrasound guidance (GE Logiq Alpha 200®, General Electric Medical Systems, Milwaukee, Brookfield, WI, USA), a single embryo with the highest morphological grade was transferred into the uterine cavity. Transferred embryos were day 5 (D5) and were good quality blastocysts, which was characterized by early cavitation, resulting in the formation of an eccentric and expanded cavity lined by a distinct inner-cell mass and a trophectoderm layer with a thin zona pellucida. The transfer procedure utilized an ultra-soft catheter (Frydman® Ultrasoft Catheter 4.5, CCD Laboratoire, Paris, France).
LPS began on the evening of oocyte retrieval and persisted until the day of the pregnancy test. In the presence of a positive result, progesterone treatment was extended for up to 10 weeks of gestation. Patients were divided into two groups, VAG and IMP, based on the type of LPS they received. VAG group consisted of patients given 8% micronized vaginal progesterone gel 180 mg daily (Crinone ®, Merck KGaA, Darmstadt, Germany) and the IMP group consisted of patients given intramuscular (injected into the gluteal muscle) progesterone in oil 100 mg daily for luteal support.
A positive pregnancy was defined by beta-human chorionic gonadotropin
(
Statistical analyses were performed using SPSS version 25.0 (IBM Corp, Chicago,
IL, USA). Descriptive statistics were presented as median (minimum to maximum
range), frequency, and percentage. Comparison of data was performed utilizing the
Chi-square test for nominal or ordinal scaled data, and Fisher exact test was
used in instances where the assumptions for the Chi-square test were not met due
to low expected cell counts. Parametric tests were used to analyze data sets
demonstrating a normal distribution, whereas nonparametric test sets were
utilized for data sets exhibiting deviation from normalit distribution. The
Mann-Whitney U test was employed where suitable. Whether there existed a
statistically significant difference between numerical variables in the two
groups, the Student’s t-tests were utilized to ascertain. p
The avarage age of patients in the VAG group was 31 years (range: 23–35), while it was 27 years (range: 23–35) in the IMP group. All demographic characteristics of the patients are summerized in Table 1.
| VAG Group (n: 116) | IMP Group (n: 144) | p value | ||
| Age (years) Ω | 31 (23–35) | 27 (23–35) | 0.319a | |
| Duration of infertility (years) Ω | 6 (2–8) | 5 (1–8) | 0.043a | |
| Atral follicle count on day 3 Ω | 8 (5–14) | 8 (5–12) | 0.743a | |
| D3 FSH (IU/L) Ω | 6 (4–10) | 6 (4–10) | 0.573a | |
| D3 E2 (pg/mL) Ω | 34.5 (5–74) | 33.0 (8–66) | 0.560a | |
| BMI (kg/m2) Ω | 22 (17–27) | 18 (17–27) | 0.062a | |
| Diagnosis of infertility. n (%) | ||||
| Unexplained µ | 76 (65.5%) | 98 (68.0%) | 0.779b | |
| Male factor µ | 34 (29.3%) | 37 (25.7%) | ||
| Tubal µ | 6 (5.2%) | 9 (6.3 %) | ||
Ω: data are presented as median (min–max); µ: data are presented as number (percent).
a: Mann-Whitney U test; b:
VAG, vaginal progesterone gel; IMP, intramuscular progesterone; FSH, follicle stimulating hormone; E2, estradiol; BMI, body mass index; D, day.
There was not any statistically significant differences between the two groups concerning ovarian stimulation parameters, including mean total gonadotropin doses, endometrial thickness, duration of stimulation, serum estradiol (E2) levels on HCG day, serum P4 levels on the day of HCG, total number of collected oocytes, total number of metaphase II stage (MII) oocytes, and fertilized oocytes (p = 0.456, p = 0.535, p = 0.626, p = 0.486, p = 0.522, p = 0.775, p = 0.937, p = 0.260, respectively) (Table 2).
| VAG group (n: 116) | IMP group (n: 144) | p value | |
| Total gonadotropin dose (IU) Ω | 3275 (2500–4250) | 3415 (2250–4900) | 0.456a |
| Endometrial thickness on the day of HCG (mm) Ω | 9 (7–16) | 10 (7–14) | 0.535a |
| Total duration of stimulation (days) Ω | 10 (8–13) | 10 (8–14) | 0.626a |
| Serum E2 on the day of HCG (pg/mL) Ω | 2100 (1600–2800) | 2200 (1750–2900) | 0.486a |
| Serum P4 on the day of HCG (ng/mL) | 0.8 (0.3–23) | 1 (0.3–9.6) | 0.522a |
| Total number of oocytes retrieved Ω | 7 (4–9) | 7 (4–9) | 0.775a |
| Total number of MII oocytes Ω | 6 (2–9) | 6 (2–9) | 0.937a |
| Number of fertilized oocytes (2PN) Ω | 6 (1–9) | 5 (1–9) | 0.260a |
| Clinical pregnancy rate/per transfer µ | 70.6% (n: 82) | 75.6% (n: 109) | 0.364b |
| Ongoing pregnancy rate/cycle µ | 36.2% (n: 42) | 39.5% (n: 57) | 0.577b |
| Live birth rate/cycle µ | 34.4% (n: 40) | 36.1% (n: 52) | 0.785b |
| Spontaneous abortion per pregnancy µ | 36.2% (n: 42) | 36.8% (n: 53) | 0.921b |
Ω: data are presented as median (min– max); µ: data are presented as number (percent).
a: Mann-Whitney U test; b:
VAG, vaginal progesterone gel; IMP, intramuscular progesterone; HCG, human chorionic gonadotropin; P4, progesterone; E2, estradiol; MII, metaphase II stage; 2PN, 2 pro nuclei on retrieval day 1.
No statistically significant differences were found between the two groups in terms of CPR per transfer (70.6% vs. 75.6%; p = 0.364), ongoing pregnancy per cycle (36.2% vs. 39.5%; p = 0.577), and LBR per cycle (34.4% vs. 36.1%; p = 0.785). Additionally, spontaneous abortion rates (36.2% vs. 36.8%; p = 0.921) were similar between the VAG and IMP groups (Table 2).
There was not any correlation between the pregnancy rate and several independent variables, including female age (odds ratio (OR): 0.907, 95% confidence interval (95% CI): 0.886–1.001, p = 0.218), duration of infertility (OR: 0.908, 95% CI: 0.873–1.028, p = 0.510), body mass index (BMI) value (OR: 0.615, 95% CI: 0.562–1.231, p = 0.425), or LPS type (OR: 0.718, 95% CI: 0.652–1.313, p = 0.451) (Table 3). No independent variable demonstrating statistically significant correlation with clinical pregnancy rates was identified (female age (OR: 0.768, 95% CI: 0.665–1.102, p = 0.321), duration of infertility (OR: 0.802, 95% CI: 0.783–1.117, p = 0.410), BMI value (OR: 0.508, 95% CI: 0.465–1.131, p = 0.366) and LPS type (OR: 0.598, 95% CI: 0.592–1.289, p = 0.562)) (Table 4). We found no independent statistically significant parameters which correlated with live births including age of women (OR: 0.957, 95% CI: 0.895–1.023, p = 0.199), duration of infertility (OR: 0.900, 95% CI: 0.885–1.019, p = 0.461), BMI value (OR: 0.996, 95% CI: 0.948–1.083, p = 0.699) and the types of LPS type (OR: 0.802, 95% CI: 0.661–1.202, p = 0.580) (Table 5).
| Independent variables | OR/95% CI (Lower–Upper) | p value |
| Female age | 0.907 (0.886–1.001) | 0.218 |
| Duration of infertility (years) | 0.908 (0.873–1.028) | 0.510 |
| BMI (kg/m2) | 0.615 (0.562–1.231) | 0.425 |
| Type of progesterone used for luteal support | 0.718 (0.652–1.313) | 0.451 |
Multivariate logistic regression analysis with live birth rate (LBR) as dependent (outcome) variable. Age, body mass index (BMI), infertility duration were controlled as confounding factors. OR, odds ratio; 95% CI, 95% confidence interval.
| Independent variables | OR/95% CI (Lower–Upper) | p value |
| Female age | 0.768 (0.665–1.102) | 0.321 |
| Duration of infertility (years) | 0.802 (0.783–1.117) | 0.410 |
| BMI (kg/m2) | 0.508 (0.465–1.131) | 0.366 |
| Type of progesterone used for luteal support | 0.598 (0.592–1.289) | 0.562 |
Multivariate logistic regression analysis with live birth rate (LBR) as dependent (outcome) variable. Age, body mass index (BMI), infertility duration were controlled as confounding factors. OR, odds ratio; 95% CI, 95% confidence interval.
| Independent variables | OR/95% CI (Lower–Upper) | p value |
| Female age | 0.957 (0.895–1.023) | 0.199 |
| Duration of infertility (years) | 0.900 (0.885–1.019) | 0.461 |
| BMI (kg/m2) | 0.996 (0.948–1.083) | 0.699 |
| Type of progesterone used for luteal support | 0.802 (0.661–1.202) | 0.580 |
Multivariate logistic regression analysis with live birth rate (LBR) as dependent (outcome) variable. Age, body mass index (BMI), infertility duration were controlled as confounding factors. OR, odds ratio; 95% CI, 95% confidence interval.
The application of logistic regression analysis aimed at evaluating the influence of confounding factors including age, BMI, and duration of infertility on pregnancy, CPR and LBR did not yield statistically significant findings (Table 6). The calculated odds ratios for these factors were 1.372, 1.232 and 1.098 (p = 0.452, p = 0.687 and p = 0.892, respectively). These results indicate a lack of statistically meaningful associations between the identified factors and the likelihood of pregnancy, clinical pregnancy and live birth.
| Outcome Measure | Coefficient (VAG group, n: 116) | Coefficient (IMP group, n: 144) | Odds ratio (95% CI) (Lower–Upper) | p value |
|---|---|---|---|---|
| Pregnancy rate | 84.4% (n: 98) | 81.2% (n: 117) | 1.372 (0.732–2.420) | 0.452 |
| Clinical pregnancy rate | 70.6% (n: 82) | 75.6% (n: 109) | 1.232 (0.629–2.308) | 0.687 |
| Live birth rate | 33.6% (n: 39) | 35.4% (n: 51) | 1.098 (0.591–1.949) | 0.892 |
In this study involving POSEIDON Group 1b patients undergoing fresh SET in IVF cycles, we found that providing LPS with daily 180 mg VAG yielded pregnancy outcomes such as CPR per transfer, ongoing pregnancy rates per cycle, and LBR per cycle were comparable to those given daily 100 mg IMP. Our study, conducted with a homogenous patient group in line with the nature of the POSEIDON classification, revealed that ovarian stimulation parameters such as mean total gonadotropin doses, endometrial thickness, duration of stimulation, serum E2 levels on the day of HCG administration, serum P4 levels on the day of HCG administration, total number of collected oocytes, number of MII oocytes, and fertilized oocytes did not differ between the groups utilizing vaginal or intramuscular progesterone. In our study, the rates of spontaneous abortion were comparable between the groups receiving VAG and IMP. As a secondary outcome of our study, we identified that the type of LPS in POSEIDON Group 1b patients, comprising suboptimal responders, did not correlate with pregnancy rate, CPR and LBR.
Addressing infertility in the presence of predictors indicating suboptimal
response continues to pose an unsolved challenge [15]. Polyzos and Drakopoulos
[11] stated that there are no established definitive treatment guidelines for
patients in POSEIDON Group 1, which includes unexpected suboptimal responders.
They emphasized the necessity for tailored management based on the underlying
pathophysiologic mechanisms responsible for these patients [11]. Presumably, all
these women respond suboptimally to ovarian stimulation, irrespective of age.
POSEIDON Group 1b encompasses individuals with good ovarian reserve, some of whom
exhibit follicle stimulating hormone receptors (FSH-R) and luteinizing hormone
receptors (LH-R) polymorphisms or variant luteinizing hormone beta-subunit
(LH
The administration of progesterone is standard practice for LPS [18, 19]. However, the full establishment of individualized LPS protocols remains to be achieved [19]. Recent study has highlighted the efficacy of progesterone in improving CPR regardless of the administration route [20]. For fresh embryo transfer cycles, various progesterone preparations, including vaginal, oral, and parenteral forms, have been recommended [21].
A meta-analysis by Abdelhakim et al. [22] found no significant differences between VAG and IMP in terms of clinical pregnancies, ongoing pregnancies, miscarriages, and live birth rates. Currently, no optimal route of progesterone administration for LPS following fresh embryo transfers has been universally recommended for patients in POSEIDON Group 1b [20, 21, 22].
Patil et al. [23] examined outcomes in poor responders classified by POSEIDON criteria, reporting a CPR of 39.1% and a LBR of 20.2% among 45 suboptimal responders. In their study, IMP was administered following fresh embryo transfer cycles [23]. Similarly, a recent large-scale retrospective cohort study involving POSEIDON patients reported a LBR per transfer of 44.4% in fresh embryo transfer cycles, with LPS provided by daily VAG administration [9]. However, the specific dose of vaginal progesterone was not clearly specified in that study [9]. Our findings suggest that daily administration of 180 mg vaginal progesterone gel in POSEIDON Group 1b patients undergoing fresh single embryo transfer is an effective LPS method that does not adversely affect IVF outcomes.
Administration of IMP during the luteal phase and early pregnancy necessitates
prolonged daily intramuscular injections, posing potential challenges related to
patient discomfort and adherence. A novel water-soluble progesterone formulation
has been formulated for subcutaneous administration but the mean satisfaction
scores of patients did not differ between subcutaneous group and VAG group (7.39
vs. 7.22, p = 0.892 and p
Previous studies have identified certain variables associated with live births
in fresh IVF cycles. Metello et al. [26] underscored the significance of
a woman’s age and serum AMH levels as robust predictors for live birth.
Similarly, Lee et al. [27] substantiated the value of AMH as a pivotal
biomarker for prognosticating both clinical pregnancy and live birth in women
It is noteworthy that advanced maternal age (
VAG 180 mg daily and 100 mg daily IMP have similar pregnancy outcomes in POSEIDON Group 1b patients undergoing antagonist fresh SET IVF/ICSI cycles. Future studies focusing on IVF outcomes in these young patients characterized by normal AMH levels might provide a clearer understanding of clinical factors influencing IVF success.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ART, artificial reproductive technology; P4, progesterone; CL, corpus luteum; IVF, in vitro fertilization; LBR, live birth rate; CPR, clinical pregnancy rate; VAG, progesterone vaginal gel; POSEIDON, Patient-Oriented Strategies Encompassing Individualized Oocyte Number; IMP, intramuscular progesterone; LPS, luteal phase support; AMH, anti-mullerian hormone; AFC, antral follicle counts; BMI, body mass index; FSH, follicle-stimulating hormone; HCG, human coryonic gonadotrophin; LH, luteinizing hormone; OS, ovarian stimulation; OHSS, ovarian hyperstimulation syndrome; rFSH, recombinant FSH.
HU and ŞH designed the research study. DG performed the research. HU and KB collected the data. MHD and AY contributed to the interpretation of the results. HU and AY wrote the manuscript. MHD and ŞH supervised the project. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Ondokuz Mayıs University Medical Faculty, Samsun, Turkey (approval number: 2017/121).
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. Michael H. Dahan is serving as Editor-in-Chief of this journal. We declare that Michael H. Dahan had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Panagiotis Anagnostis and Felix Wong.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.