1 Department of Obstetrics and Gynecology, College of Medicine, Mustansiriyah University, 10052 Baghdad, Iraq
2 Department of Obstetrics and Gynecology, Fatima Al Zahraa Maternity Hospital, 10052 Baghdad, Iraq
Abstract
Background: Preeclampsia (PE) is a major cause of maternal and neonatal
morbidity. Fetal growth restriction (FGR) shares many pathophysiological roles
with PE. Kisspeptin-10 is a peptide secreted by placental syncytium. It was
linked to many adverse pregnancy events. The current study aimed to examine
Kisspeptin’s-10 role in predicting FGR in PE pregnancies and to verify whether it
can predict its onset as early or late FGR. Methods: An observational
case-control study enrolled 120 eligible cases at matched gestational age (28–40
weeks) and body mass index (BMI); they were divided into 2-groups: (60) healthy
controls and (60) PE cases. PE cases were subdivided into early onset FGR
(28/60), who had a gestational age less than 34 weeks, and late-onset FGR (32/60)
with a gestational age equal to 34 weeks. A collection was made of the following
data: first: pregnant primary criteria [age, BMI, systolic and diastolic
blood pressure (BP), and urine for albumin], second: serum Kisspetein-10 was
evaluated via enzyme-linked immunosorbent assay
(ELISA), and third: ultrasonic criteria [estimated fetal weight, resistance, and
pulsatility index (RI, PI)] were recorded for all. Results: Serum
Kisspeptin-10 was significantly higher among the controls (309.56
Keywords
- early onset FGR
- estimated fetal weight
- fetal growth restriction
- Kisspeptin-10
- preeclampsia
Pregnancy is a unique state in a woman’s life; it is a physiological condition accompanied by many changes in the mother’s body to accommodate fetal growth. A good trophoblastic invasion by uterine spiral arteries is necessary for a healthy pregnancy and good neonatal outcome [1]. Defective placentation, therefore, will lead to multiple pregnancy problems such as abortion, preeclampsia (PE), fetal growth restriction (FGR), and gestational diabetes mellitus [2]. PE is considered one of the important disorders that increase global maternal and perinatal morbidity and mortality [3]. It can be presented in two stages according to its occurrence: early-onset PE occurring before 34 weeks and late-onset PE. Many theories tried to explain the exact pathophysiology of PE; some suggested maternal factors or other fetal causes, and some blamed the placenta [4]. FGR, on the other hand, occurs when the fetus fails to reach the normal growth process and is regarded as one of the most common pregnancy complications. FGR possesses multiple etiological and pathophysiological factors that are common to PE. Abnormal placental function is commonly considered the leading cause of fetal growth restriction and is more frequently encountered in PE cases [2]. There is a strong association between preeclampsia and perinatal disorders such as prematurity, maternal mortality, and even long-term implications for the neurological and cognitive development of the baby [5]. Many trials were conducted to improve the screening for FGR, especially among PE mothers, to improve the materno-fetal outcome.
Kisspeptin-10 is a peptide family member encoded by KISS1 gene that serves as an endogenous legend for Gq-protein-coupled receptors known as KISS-1R [6]. A growing body of evidence supports Kisspeptin-10’s ability to control and maintain the pulsatile effects of gonadotropin-releasing hormone level release, a critical step for organizing many functions of the female reproductive system [7]. Additionally, other studies have discussed Kisspeptin-10 involvement in early implantation and placentation [8]. Kisspeptin-10 displays its action via binding to KISS-1R receptors, shown by the placenta, testes, ovaries, umbilical cord, and smooth muscle fiber in the vascular system [9]. Earlier studies in the field discussed reduced concentration of pregnancy-associated plasma protein A and Kisspeptin-10 during the first trimester in pregnant women who later developed preeclampsia [10]. In normal, healthy pregnant women, syncytiotrophoblasts secret Kisspeptin-10 into circulation through all pregnancy trimesters, and the reduction of circulating Kisspeptin concentration immediately following childbirth supports its placental origin [7]. Kisspeptin-10 levels rise dramatically as gestation advances [11], making Kisspeptin-10 a valuable biomarker for placenta integrity and function. A growing body of research reported lower serum levels of Kisspeptins-10 in cases complicated by PE and FGR in addition to other adverse obstetrical complications such as abortion, preterm labor, and gestational diabetes [9, 10, 12, 13]. Dysregulation of the Kisspeptins signaling pathway seems to have a vital role in those adverse pregnancy outcomes. However, the result concerning its role in PE and FGR was presented with controversy and inconsistency [14]. The current study examined Kisspeptin-10’s role in screening for FGR among PE women and verified its performance in defining its onset, i.e., early vs. late onset FGR.
An observational case-control study was conducted at Al-Yarmouk Teaching Hospital from September 2022 to September 2023. After explaining the study goal and objectives, participants who attended our maternity clinic were invited to enroll. The ethics committee of Mustansiriyah Medical College/Department of Obstetrics and Gynecology issued the study approval, with IRB (Institutional Review Board) No.18 dated (1/9/2022). All participants gave signed consent; the whole study method was performed under the Helsinki umbrella.
Singleton pregnant mothers with maternal age between (20–40 years) and
gestational age of 28–40 weeks confirmed by early pregnancy dating scan or last
date of their menstrual cycle. Participants were matched regarding body mass
index (BMI), which was set below 30 kg/m
Cases complicated with preterm labor, active labor, or those who needed urgent
termination for impending eclampsia or fetal compromise. Premature rupture of membranes, and chorioamnionitis. Fetal problems other than FGR include fetal demise, twin pregnancy, chromosomal
abnormalities, and congenital malformation. Other maternal problems as: pregnancy-related diabetes mellitus, liver, kidney,
autoimmune or heart disorders.
The current study used a purposeful sampling method in which participants who
were eligible for the inclusion (n = 120) were divided into two main groups:
healthy pregnant controls, n = 60, and preeclampsia cases that were complicated
by fetal growth restriction (PE-FGR), where n = 60 cases. The PE-FGR cases were
further subdivided into early onset
Mean arterial pressure (MAP) (mmHg) = [Systolic BP + (2
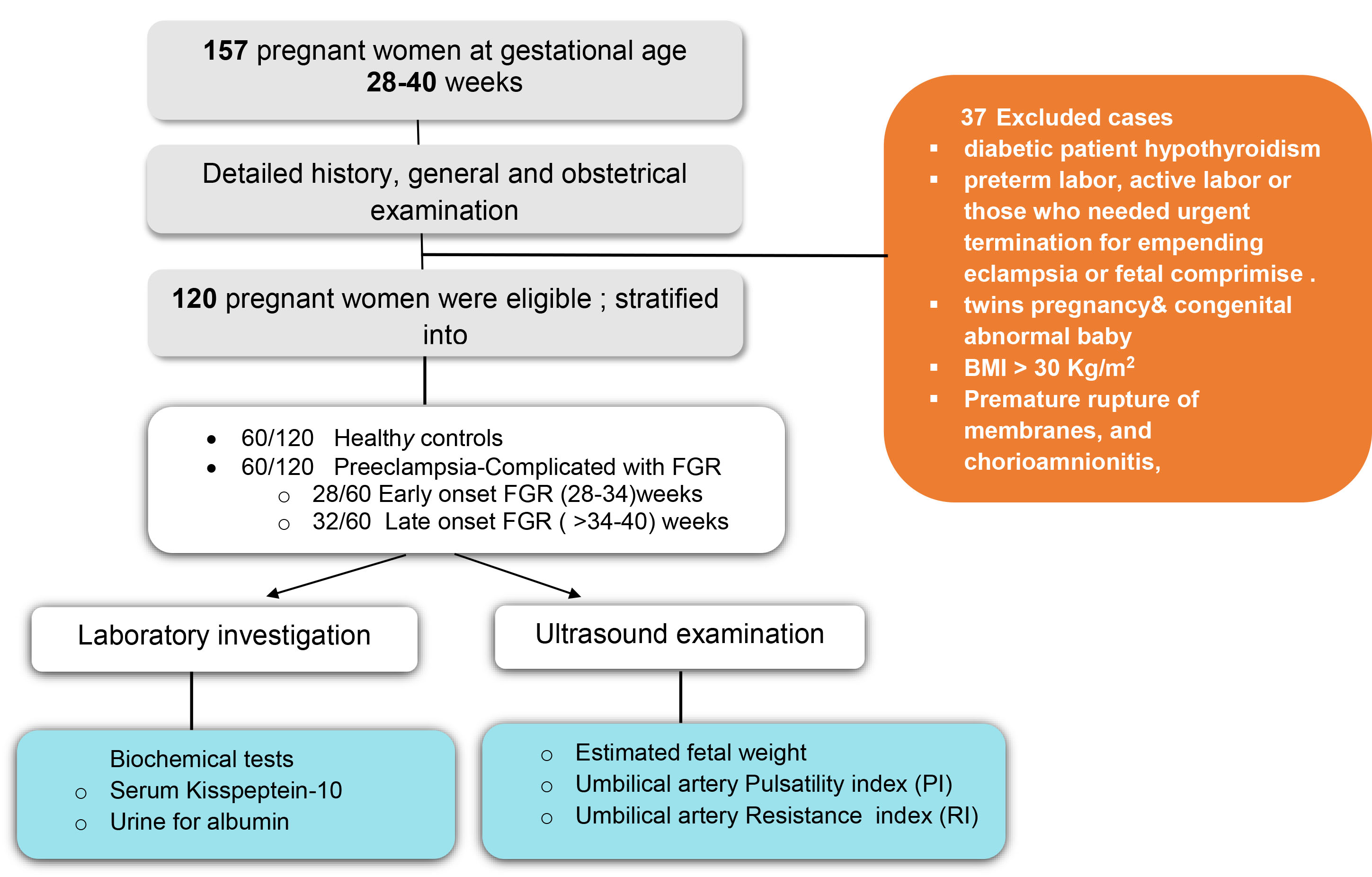
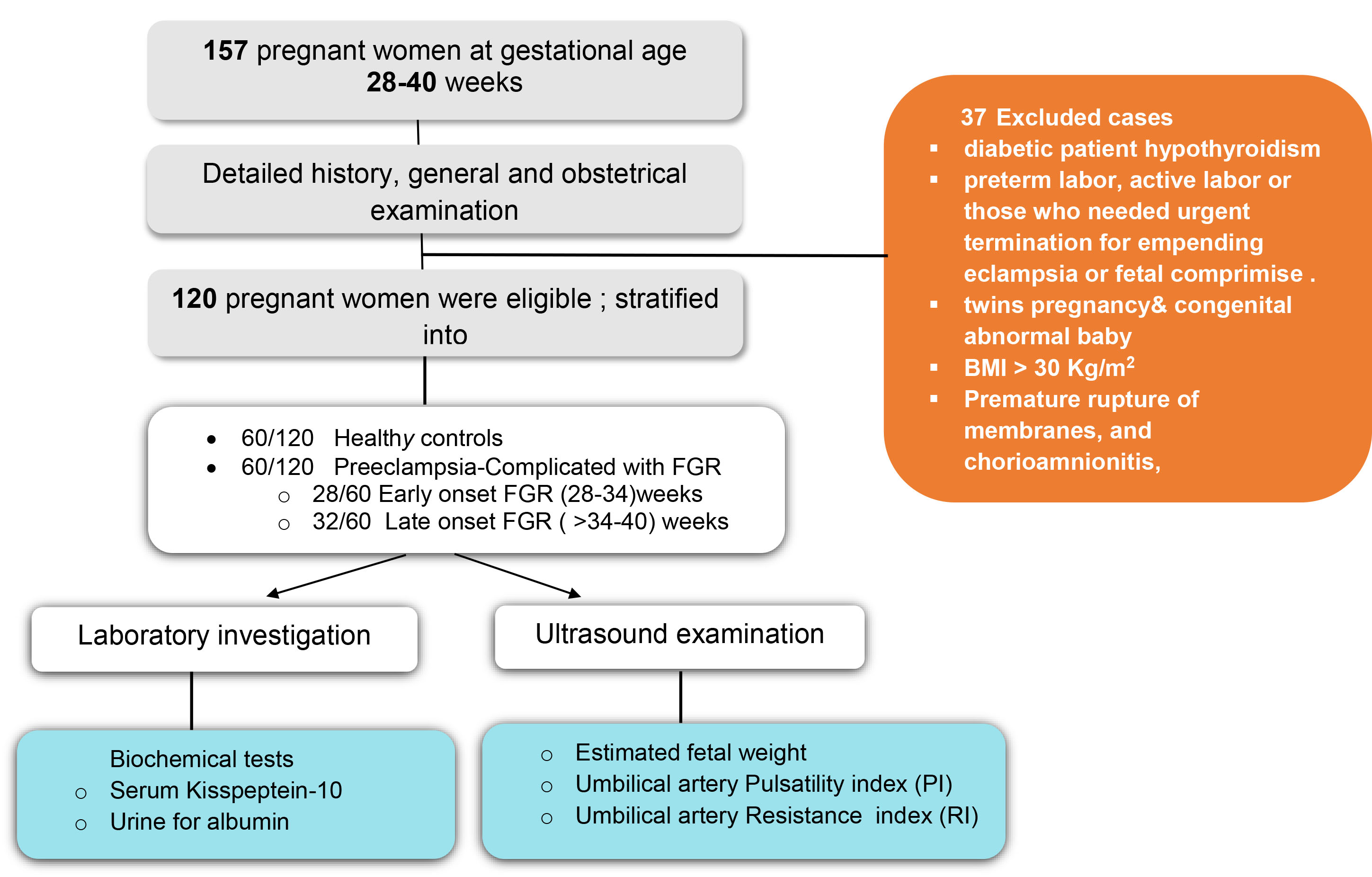 Fig. 1.
Fig. 1.
Study flowchart. BMI, body mass index; FGR, fetal growth restriction; PI, pulsatility index; RI, resistance index.
Patients were scheduled for the obstetrical ultrasound (US) via abdominal approach, where the gestational age, placental localization, estimated fetal weight, resistance, and pulsatility index (RI and PI) were recorded. The same operator made all assessments to eliminate intra-observational variation. Finally, the biochemical test was done, and 24-hour urine samples were taken in urine for albumin. A 5-cc blood sample was taken, centrifuged at (2500 rpm for 15 min), and stored at (–80 °C) for later analysis. Estimating serum levels of Kisspeptin-10 was done using an enzyme-linked immunosorbent assay (ELISA) kit, which is commercially available depending on the manufacturer’s instructions (Life Span Biosciences, Shirley, MA, USA).
Preeclampsia was defined based on the National institute for Health and Care
Excellence (NICE) guideline 2018 that defines PE as new-onset hypertension in a
pregnant above the 20th weeks of gestation (with a systolic and diastolic BP of
As for FGR, it was diagnosed based on serial US assessment of fetal weight, as an estimated fetal weight (EFW) and/or abdominal circumference (AC) were below the 3rd percentile or 10th percentile for their gestational age with associated problems in some Doppler parameters [17].
The prevalence rate of preeclampsia is 5 to 10 percent, corresponding to a 95% confidence level and 5% deviation from the populace, and it was found that 60 cases will be representative of the preeclampsia patients [18] with reasonable numbers of healthy pregnant.
The data was assessed by the Kolmogorov-Smirnov test and found to be normally distributed. Continuous data were expressed as means and standard deviations (SD). One-way analysis of variance (ANOVA) was used to compared different groups means since we have 3 groups. Pearson correlation tested the strength of the association between serum Kisspeptin and various study parameters. The odds ratio (OR) and 95% confidence interval (95% CI) for serum Kisspeptin were used to examine prediction ability to FGR. The receiver operator characteristic curve (ROC) examined serum Kisspeptin performance in discriminating FGR in PE cases and estimation of its cutoff value. The significance level was determined by a p-value less than 0.05 for all tests. The statistics were done through SAS 2018. (Version 9.6th ed., SAS. Inst. Inc., Cary, NC, USA).
In Table 1, the primary criteria of the study participants were described; none
of the maternal age, parity, and albumin urea were significant across the groups
as p
| Variable | Control | Early-FGR-PE | Late-FGR-PE | p-value |
| (n = 60) | (n = 28) | (n = 32) | ||
| means |
means |
means | ||
| Age (years) | 31.13 |
32.50 |
31.18 |
0.605 NS |
| Parity | 2.80 |
3.04 |
3.00 |
0.852 NS |
| Gestational age (weeks) | 36.23 |
31.96 |
36.47 |
0.0001 ** |
| Systolic BP (mmHg) | 114.31 |
155.35 |
156.25 |
0.0001 ** |
| Diastolic BP (mmHg) | 69.80 |
104.82 |
102.19 |
0.0001 ** |
| Mean arterial BP (MAP) | 92.05 |
130.08 |
129.22 |
0.0001 ** |
| Albumin in urine | - | 2.46 |
2.28 |
0.391 NS |
| Pulsatility index | 0.882 |
1.937 |
1.459 |
0.0001 ** |
| Resistance index | 1.453 |
1.09 |
0.939 |
0.873 NS |
| Weight of neonate (grams) | 2824.33 |
1005.58 |
1646.93 |
0.0001 ** |
| Kisspeptin-10 (ng/mL) | 309.56 |
212.09 |
235.46 |
0.0001 ** |
** high statistically significant p
In Table 2, Kisspeptin-10 correlations were described; it was correlated
negatively and significantly with diastolic blood pressure, mean arterial blood
pressure, and albumin in urine, with a correlation coefficient (r) of
(–0.48, –0.37, –0.28) respectively and a p-value of
| Variable | Pearson Correlation (r) | p-value |
| Maternal age (years) | 0.08 NS | 0.554 |
| Gestational age (weeks) | 0.39 ** | 0.002 |
| Parity | 0.09 NS | 0.475 |
| Systolic BP (mmHg) | −0.29 * | 0.021 |
| Diastolic BP (mmHg) | −0.48 ** | 0.0001 |
| Mean arterial BP | −0.37 ** | 0.0018 |
| Albumin in urine | −0.28 * | 0.028 |
| Pulsatility index | −0.14 NS | 0.288 |
| Resistance index | 0.03 NS | 0.843 |
| Weight of neonate (grams) | 0.27 * | 0.034 |
** high statistically significant p
| Groups | Odds ratio | 95% CI | p-value |
| Controls | 3.04 | 1.37–4.765 | 0.0001 ** |
| Early-onset PE-FGR | 2.18 | 1.06–4.08 | 0.0001 ** |
| Late-onset PE-FGR | Reference group | ||
CI, confident interval; ** high statistically significant.
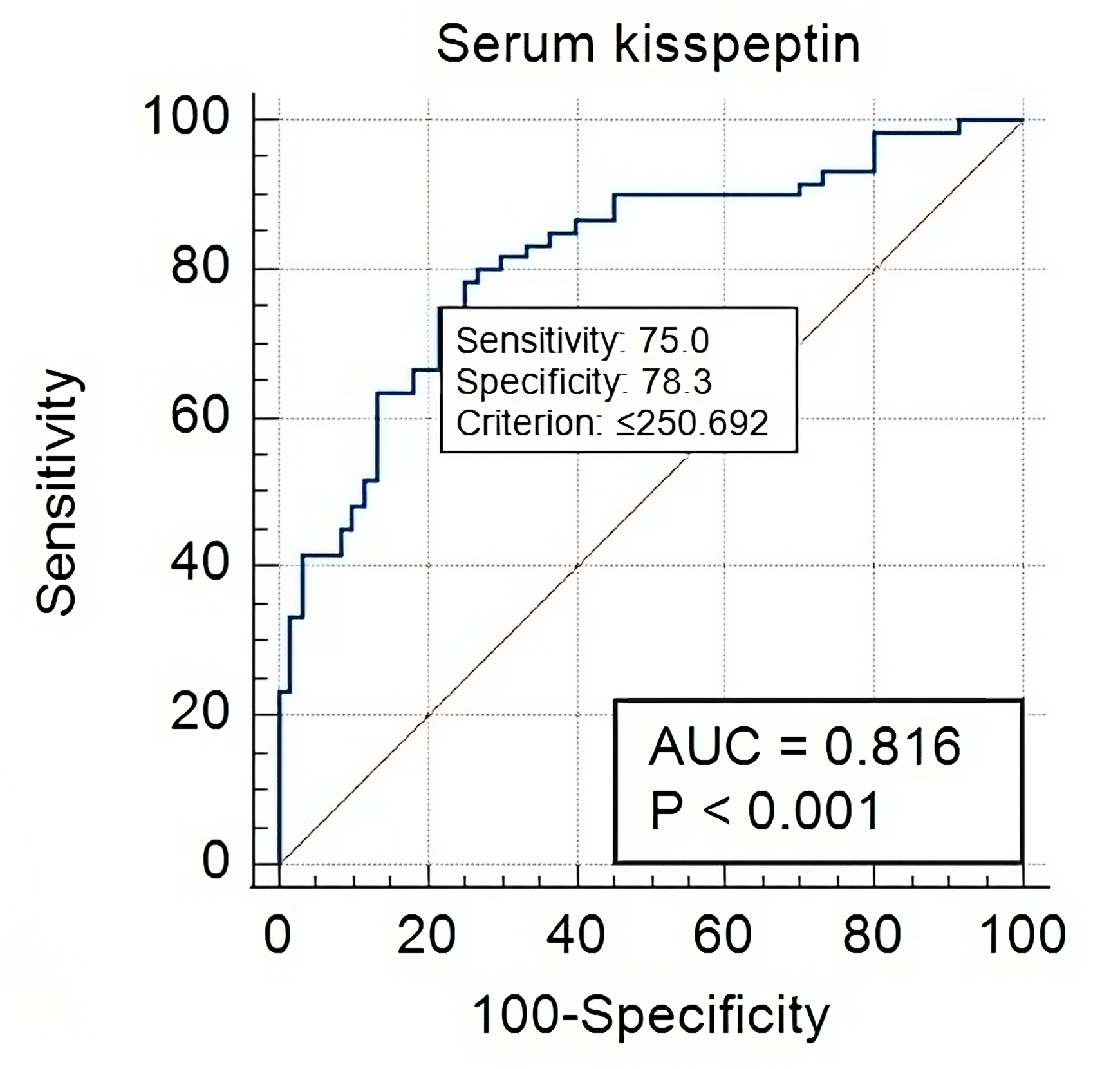
 Fig. 2.
Fig. 2.
Serum Kisspeptin-10 in healthy controls versus PE-FGR cases. At
a cut-off value of
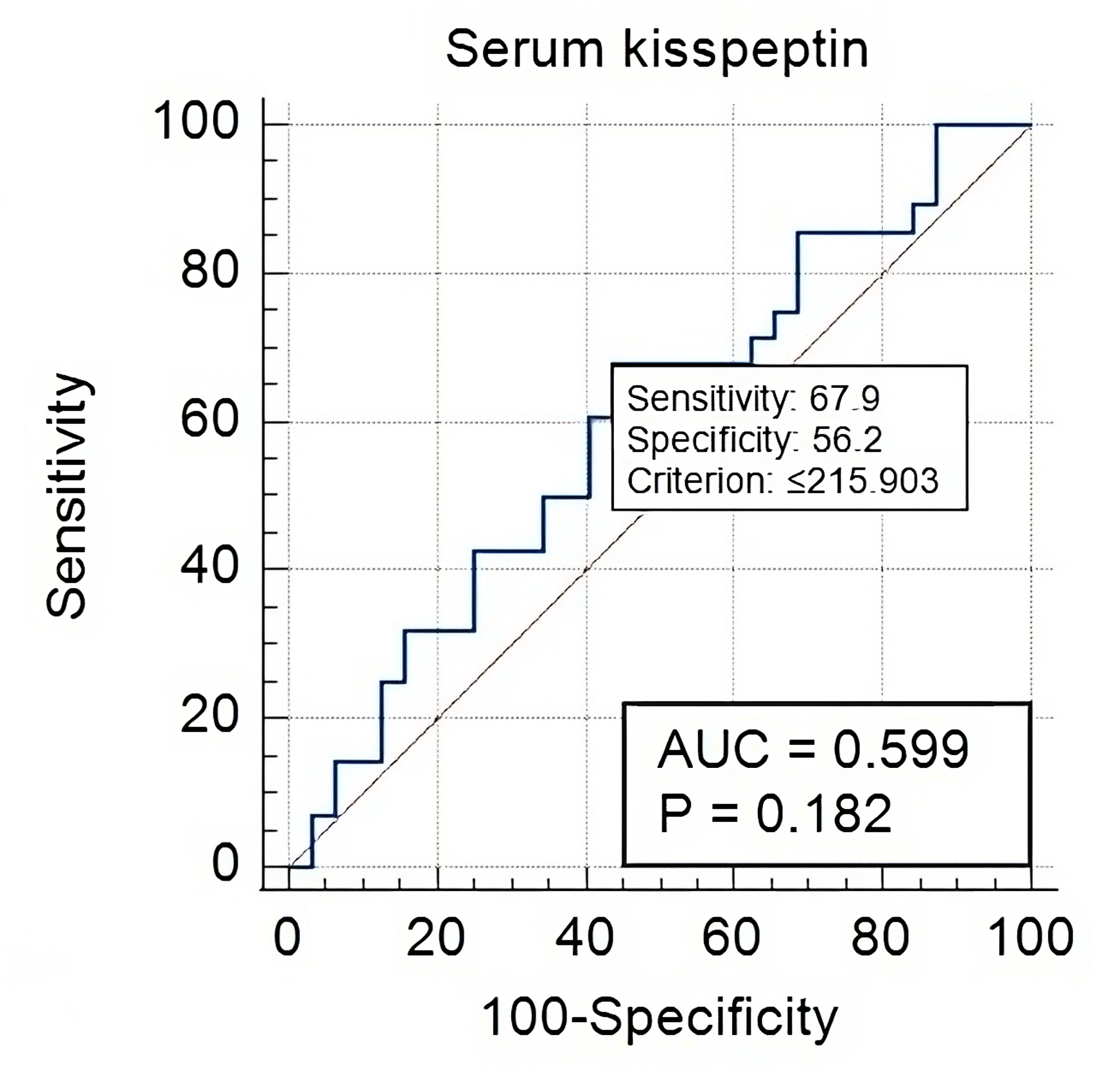
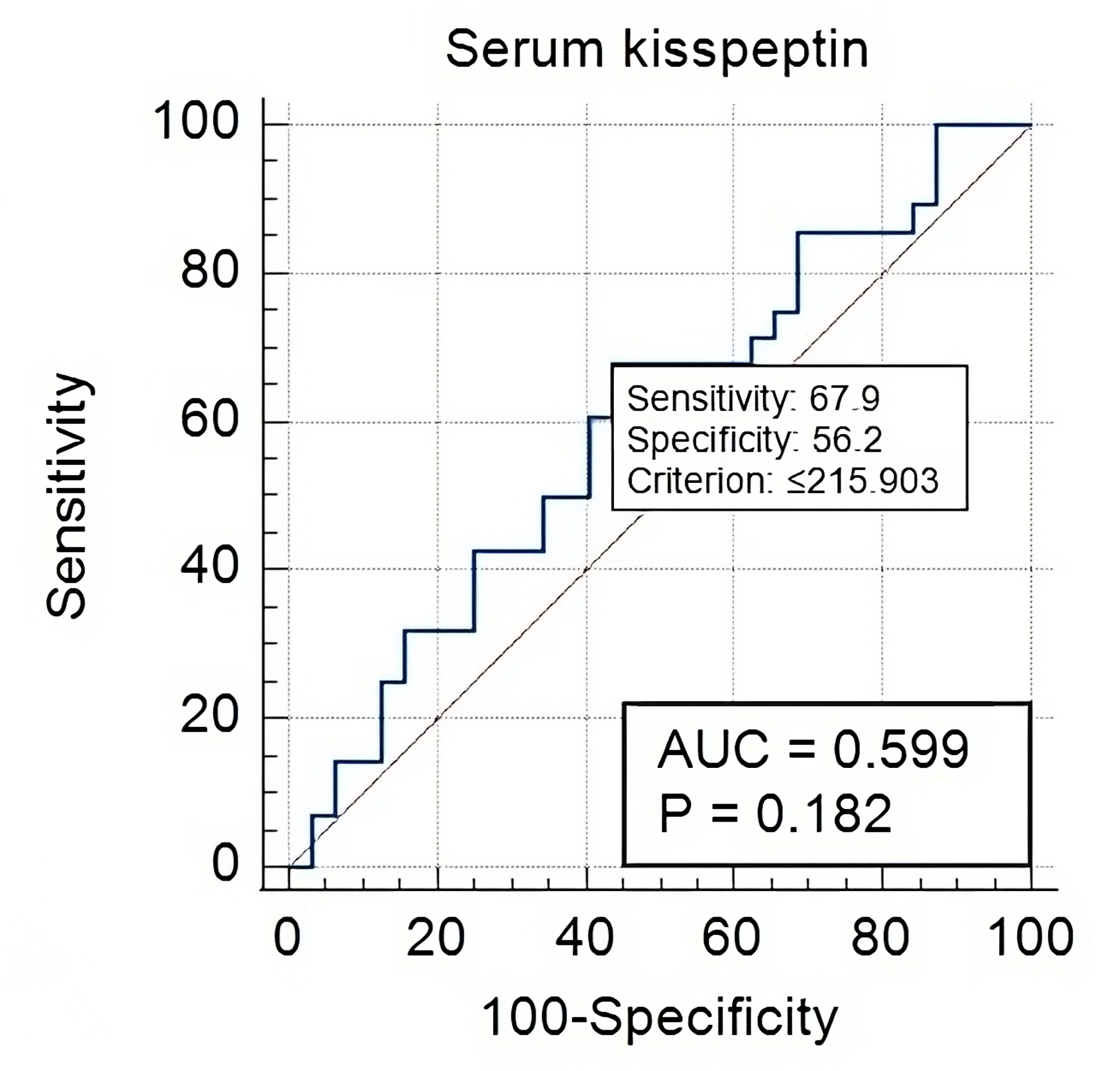 Fig. 3.
Fig. 3.
Serum Kisspeptin-10 in early versus late onset PE-FGR cases.
The current study showed significantly high levels of serum Kisspeptin-10 among healthy controls, while its levels were low in late-onset FGR and scored the lowest in early-onset FGR. It was negatively linked to systolic, diastolic pressure, and urine for albumin and was positively linked to estimated fetal weight; Kisspeptin-10 had an OR of 3.04; 95% CI (1.37–4.765); p = 0.0001 in discriminating healthy pregnancies from early onset FGR.
Placental insufficiency has been linked to diverse pregnancy problems; two of the most common are PE and FGR. Many acknowledge that both syndromes share common pathophysiological features, such as deficient trophoblast invasion that occurs early in pregnancy. The latter leads to a shallow insertion of placental spiral arteries and reduces the fetal-maternal exchange surface. The reduction in the oxygen and nutrient supply to the growing fetus prevents gaining its natural growth potential; this is the 1st stage of PE [19]. The 2nd stage of PE starts when the maternal body tries to overcome the increased local placental pressure, a defense mechanism to increase blood reaching the fetus. This stage is accompanied by a systemic inflammatory response, endothelial dysfunction, and an already compromised fetal growth aggravation [20].
Kisspeptin-10 is a peptide secreted by placental trophoblast cells. It has a major role in orchestrating early placentation, angiogenesis, and implantation, which are all defective steps in pregnancy cases complicated by PE and FGR [7, 11]. Normally Kisspeptin-10 secretion system is protective against adverse pregnancy problems. It is a deficiency that will render the odds of complications [10].
In agreement with our results, Al-Kaabi et al. [21] showed that Kisspeptin-10 levels were significantly low in the serum of PE moms. They postulated that it may play a critical role in PE pathophysiology. Moreover, they showed a trend reduction of Kisspeptin-10 levels with the pregnancy trimester; it was the lowest in 2nd followed by the 3rd trimester. The authors did not recommend using it since it had a low positive predictive value, making skipping the PE diagnosis high. However, it is useful for excluding PE development [21]. Another study looked into Kisspeptin concentration in PE vs. normotensive mothers by immune histochemistry in the maternal-fetal interface and the maternal vs. cord blood. They discussed that Kisspeptin levels were reduced in PE pregnancies, which mirrors defective trophoblastic invasion, and explained PE development at the molecular levels. They recommended using it as a marker of placental dysfunction [22].
Conversely, a study by Nijher et al. [23] found no difference in the role of circulating Kisspeptin in PE pregnant vs. normotensive and pregnancy-induced hypertension (PIH) cases. Furthermore, the systolic and diastolic blood pressure and heart rates were not linked to Kisspeptin-10, which contradicts our results. Their study recruited small numbers of PE moms (8 vs. 19) PIH cases compared to 78 normotensive females, which may have caused study bias. However, they introduce an interesting concept regarding the effect of BMI on circulating Kisspeptin-10 levels. The current study included match BMI cases, so we were unable to investigate this link further. Another study looked into the role of predicting multiple adverse pregnancy outcomes (PE, FGR, gestational diabetes mellitus (GDM), and preterm labor) [16]. Their results showed higher Kisspeptin-10 levels among late-onset PE vs. normotensive mothers, while early-onset PE had lower levels. The authors attributed this discrepancy to the fact that serum levels of the marker do not necessarily mirror the history of immune chemical expression of Kisspeptin-10 in placental tissues, which explains inconsistencies in published studies [24].
They extended their analysis into PE cases complicated with FGR, discussing that Kisspeptin-10 levels were significantly the lowest in FGR cases (without PE) compared to normotensive controls and for PE-associated FGR, respectively [14].
As a marker for FGR, many schoolers supported Kisspeptin-10’s role in
discriminating against FGR fetuses. Armstrong et al. [25] found a trend
reduction in cases with PE compared to FGR cases. Khaled et al. [26]
found significantly low serum Kisspeptin-10 in PE-FGR complicated cases, FGR
without PE compared to normotensive women. In line with the current study, Khaled
et al. [26] discussed a positive correlation between fetal weight and
albumin urea. However, they failed to link Kisspeptin-10 to arterial blood
pressure; having a small sampling power in their study could be the reason [14, 25, 26]. The current analysis supported serum Kisspeptin-10 capacity to predict
early-onset PE and to exclude normotensive cases while having the lowest
prediction for late-onset PE. This can be well comprehended, considering
Kisspeptin’s regulatory, anti-inflammatory role in early placentation and
angiogenesis [21]. What supports this is its role as a reliable maker of
miscarriage; moreover, its levels were reduced similarly to the beta-human
chorionic gonadotropin (
Kisspeptin-10 relationship to neonatal anthropometric parameters was tested by
Chen et al. [28] study, which confirmed an inverse correlation between
the two; especially when they added the BMI and baby sex as confounders, the
relationship remained stable, which added more reliability to the marker
performance. Our analysis showed that Kisspeptin-10 significantly discriminated
cases with early FGR-PE from normotensive cases at a cut-off value of
Improving our understanding of biomarkers’ role in the mechanism of FGR in PE cases may optimize interventional strategies and help implement new therapeutic approaches. Finally, the close association of Kisspeptin-10 to placental function throughout pregnancy allows long monitoring of affected cases to spot their progression and guide the obstetrician’s decision for a better outcome.
A large, multicentric study will help validate Kisspeptin-10’s role across ethnic groups and diverse BMI to elucidate its reliability and reproducibility. Such research may advance the current understanding and management and optimize the outcome for pregnant mums.
Many controversies exist in the literature regarding Kisspeptin-10’s role; a longitudinal study may perform best to unravel many of them. A multicentric study could have performed better rather than a uni-center study, which limits the globalization of the results. Preeclampsia syndrome is higher among pregnant with male fetuses, a confounder that was not addressed here [29]. One of the confounders that affect cytokine is obesity; the current study aimed to limit the impact of obesity on the analysis by excluding obese cases; future studies are recommended to see the impact of higher BMI on the marker performance.
The current study result could have clinical implications; many acknowledge the disadvantage of ultrasonic screening for FGR, which requires multiple examinations and patient compliance and may diagnose the case late when there is no time to interfere [30, 31, 32]. Conversely, biochemical screening for FGR may be more sensitive, allowing the opportunity for intervention, yet the exact timing cannot be determined for each marker [33, 34, 35]. Interestingly, Kisspeptin has gained attention for its diverse roles in human reproduction, endometriosis, and inflammatory diseases affecting women, which warrant further research [36].
The current study set the optimal time for using Kisspeptin-10 to screen for early onset FGR among PE women, which was the study’s strength. Moreover, the maker increased among many ominous pregnancy complications (GDM, preterm labor, miscarriage), which allowed risk stratification for patients when registered early in anti-natal care service [14].
Serum Kisspeptin-10 reliably predicted FGR associated with preeclampsia. It was significantly linked to preeclampsia parameters and ultrasonic markers of fetal weight with high sensitivity and specificity. However, it failed to discriminate early vs. late FGR. The current study validates Kisspeptin-10 role as a screening marker for FGR, especially for early onset FGR, which allows patient stratification, tailored therapeutic plans, and follow-up for cases deemed to suffer this ominous pregnancy outcome. It is worth exploring Kisspeptin-10’s reliability in first-trimester screening and among obese cases for future research.
The data supporting this study is available on reasonable request from the corresponding author.
Conceptualization: MMA, WN and SSH; methodology: MMA, WN and HFJ; software: WN; investigations and data curation: MMA and SSH; writing, reviewing and editing: WN and MMA; drafting and supervision: WN. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB). The ethics committee of Mustansiriyah Medical College/Department of Obstetrics and Gynecology issued the study approval, with IRB No. 18 dated (1/9/2022). All participants gave signed consent; the whole study method was performed under the Helsinki umbrella.
To Mustansiriyah University for continued support.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.