1 Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, 05505 Seoul, Republic of Korea
†These authors contributed equally.
Abstract
Background: This study aimed to assess the feasibility and efficacy of neoadjuvant chemotherapy (NACT) in treating patients with high-grade neuroendocrine carcinoma of the uterine cervix (HGNEC). Methods: We performed a retrospective case-control study at Asan Medical Center, Seoul, Republic of Korea, from January 1993 to December 2017, involving 60 patients with surgically treated HGNEC. Thirteen patients (21.7%) received NACT before undergoing surgery. Regarding the comparison between the group that underwent NACT and the group that did not, we used a propensity score-matched analysis, matching 22 patients in the primary radical surgery group with 11 patients in the neoadjuvant chemotherapy followed by radical hysterectomy group. Results: In the entire cohort, primary open surgery was more common in the primary surgery group compared to the NACT group (p = 0.004). After propensity score matching (PSM), the median tumor size was 3.5 cm in the primary surgery group and 2.4 cm in the NACT group (p = 0.078). After matching, there was no significant difference in the recurrence rate between the two groups (63.6% in the primary surgery group vs. 63.6% in the neoadjuvant chemotherapy group, p = 0.782). After PSM, the primary surgery group exhibited a lower intraoperative transfusion rate (10%) than the NACT group (45.5%, p = 0.052). Conclusions: While NACT was feasible in patients with HGNEC, it did not significantly improve the survival rate over primary radical surgery.
Keywords
- uterine cervical neoplasm
- neuroendocrine carcinoma
- prognosis
- neoadjuvant chemotherapy
Neuroendocrine tumors (NETs) of the gynecological tract are rare, constituting only 2% of all gynecologic cancers and roughly 1.2%–2.4% of all neuroendocrine tumors [1]. NETs are primarily found in the cervix among gynecologic organs, with about 0.9%–1.5% of cervical cancers having a neuroendocrine origin, followed by the ovaries, vulva, and endometrium in terms of frequency [2, 3]. Uterine cervical NETs can be categorized as carcinoid tumors, atypical carcinoid tumors, small cell carcinomas, or large cell neuroendocrine carcinomas. The latter two types are referred to as high-grade neuroendocrine carcinomas (HGNECs), known for their extreme aggressiveness, are associated with decreased survival rates, even among patients diagnosed in the early stages of the disease [4, 5, 6]. Therefore, HGNECs demand management strategy that is distinct from other histological types. However, owing to the lack of prospective studies on management approaches for HGNECs, standard treatment guidelines have not been established [7].
A recent meta-analysis investigating the response rates and long-term results of various cervical cancer histologies treated with neoadjuvant chemotherapy (NACT) followed by surgery indicated that squamous cell carcinomas achieved complete and partial clinical response rates of 21% and 59.2%, respectively, while nonsquamous cancers achieved rates of 32.2% and 42.9%, respectively. Interestingly, neither these differences in response nor long-term outcomes were found to be statistically significant when stratified by chemotherapy regimen or histology. Furthermore, none of these studies have evaluated the impact of NACT on HGNECs [8].
All published studies have included only a few cases of locally advanced-stage squamous or adenocarcinoma of the cervix underwent with NACT and surgery [9]. This study aimed to determine the survival and recurrence in 60 patients with histologically diagnosed HGNEC by comparing patients who received radical surgery after NACT with those who only underwent primary radical surgery.
We reviewed the medical records and pathological reports of 60 patients who were histologically diagnosed with HGNECs and had received treatment at Asan Medical Center (Seoul, Republic of Korea) between January 1993 and December 2017. Each patient received adjuvant treatment at the discretion of the clinician. The International Federation of Gynaecology and Obstetrics (FIGO) 2009 staging system was used to stage all patients. Information regarding the initial treatment was collected.
The inclusion criteria were as follows: (1) histologically confirmed diagnosis of HGNEC, (2) radical surgery (radical hysterectomy with bilateral salpingo-oophorectomy and pelvic and/or para-aortic lymphadenectomy), and (3) age between 18 and 80 years. The exclusion criteria were as follows: (1) unclear medical records and (2) previous radiotherapy. Due to the retrospective design, obtaining informed consents were waived. This study was approved by the Institutional Review Board of the Asan Medical Center (approval number: 2022-1406; approval date: June 28, 2022).
All the endpoints were computed from the first day of treatment until death, recurrence, or censoring at the final follow-up. Overall survival (OS) was determined from the date of surgery until either the date of death or the last follow-up. Disease-free survival (DFS) was defined as the time between surgery and the date of relapse, metastasis, or last follow-up. Operation-related indicators included intraoperative/postoperative transfusion rates and postoperative complications (e.g., hydronephrosis, voiding difficulty, lymphocele, and chylous ascites).
Propensity score matching (PSM) was used to minimize bias caused by an imbalance in the observed variables between the NACT and primary surgery groups. Five baseline characteristics (age, body mass index (BMI), surgical type, FIGO stage, and lymph node metastasis) were chosen as covariates in the PSM model, with a match tolerance set to a standardized mean difference of 0.1. Individual propensity scores were calculated using logistic regression analysis (SPSS version 22.0, version 26.0; IBM Corp, Armonk, NY, USA), and optimal 1:2 matching between the NACT and primary surgery groups was performed. Except for the initial squamous cell carcinoma-antigen (SCC-Ag), the distributions of the remaining observed variables were similar between the two groups after matching.
We employed independent Student’s t-tests or the Wilcoxon rank-sum test
to compare continuous variables, and the Fischer’s exact test for categorical
variables to assess the baseline characteristics of the two groups. Cox
proportional hazards model was utilized to examine OS and DFS. Statistical
significance was considered at p
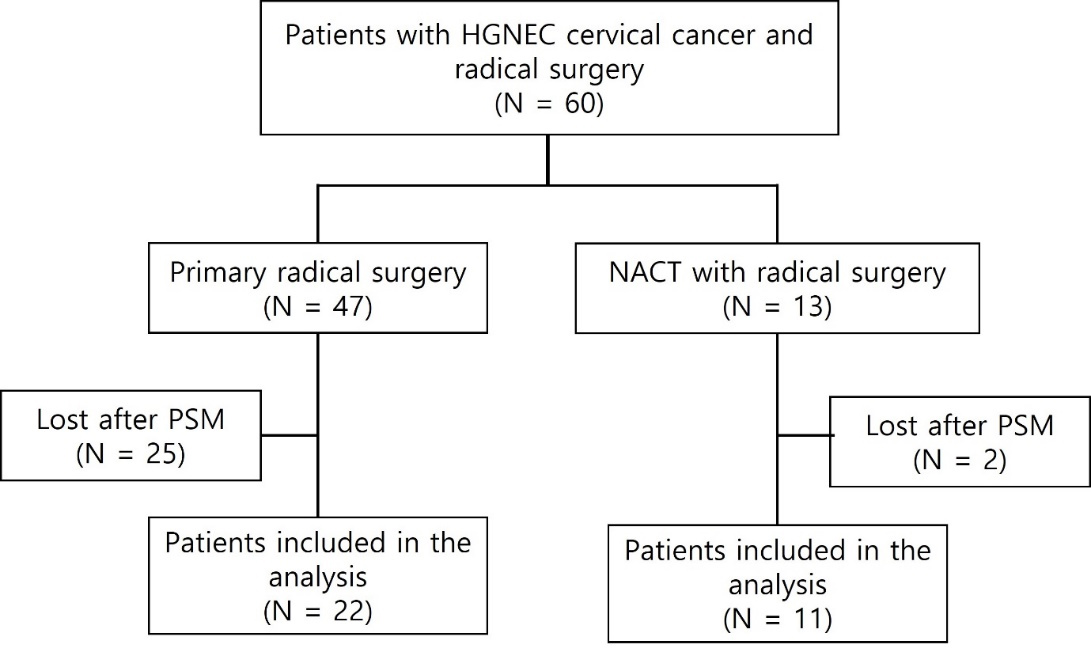
Sixty patients with HGNEC of the cervix who underwent radical surgery were included in this study (Fig. 1). Within this cohort, 47 patients underwent primary radical surgery (primary surgery group), and 13 patients underwent NACT with radical surgery (NACT group). The clinicopathological characteristics of the patients are listed in Table 1. Compared with the primary surgery group, the NACT group had a higher prevalence of open surgery among the surgical types (p = 0.004). After PSM, 22 patients in the primary surgery cohort and 11 patients in the NACT cohort were matched and their characteristics are shown in Table 1.
 Fig. 1.
Fig. 1.Study flow diagram. HGNEC, high-grade neuroendocrine carcinoma of the uterine cervix; NACT, neoadjuvant chemotherapy; PSM, propensity score matching; N, number.
| Characteristics | Before PSM | After PSM | |||||
| Primary surgery (n = 47) | NACT (n = 13) | p value | Primary surgery (n = 22) | NACT (n = 11) | Standardized mean difference | ||
| Age at initial diagnosis, years | 45.2 |
47.5 |
0.630 | 45.9 |
47.7 |
0.133 | |
| Median (range) | 45 (24–70) | 45 (27–77) | 47 (24–70) | 45 (31–77) | |||
| Follow-up, months | 59.1 |
49.3 |
0.219 | 66.0 |
52.4 |
–0.197 | |
| Median (IQR) | 42.3 (1.4–217.0) | 25.3 (9.25–233.40) | 33.5 (1.44–217.00) | 25.2 (9.3–233.4) | |||
| BMI, kg/m |
22.7 |
21.9 |
0.449 | 22.2 |
22.6 |
0.134 | |
| Median (range) | 22.8 (17.1–30.4) | 21.5 (17.4–30.3) | 22.8 (17.1–29.0) | 22.3 (19.1–30.3) | |||
| Initial SCC-Ag, units/mL | 0.82 |
1.04 |
0.126 | 0.84 |
1.00 |
0.331 | |
| Median (range) | 0.73 (0.29–2.00) | 1.00 (0.5–2.5) | 0.90 (0.3–2.0) | 1.00 (0.5–2.5) | |||
| Surgical type of RH | 0.003 | 0.000 | |||||
| MIS | 18 (38.3%) | 11 (84.6%) | 18 (81.2%) | 9 (81.8%) | |||
| Open | 29 (61.7%) | 2 (15.4%) | 4 (18.2%) | 2 (18.2%) | |||
| FIGO stage (2009) | 1.000 | 0.113 | |||||
| IB2 | 42 (89.4%) | 11 (84.6%) | 17 (77.3%) | 9 (81.9%) | |||
| IIA | 5 (10.6%) | 2 (15.4%) | 5 (22.7%) | 2 (18.2%) | |||
| Lymph node metastasis | 1.000 | –0.094 | |||||
| No | 34 (72.3%) | 9 (69.2%) | 13 (59.1%) | 7 (63.6%) | |||
| Yes | 13 (27.7%) | 4 (30.8%) | 9 (40.9%) | 4 (36.4%) | |||
| Adjuvant treatment | 0.638 | –0.125 | |||||
| No | 5 (10.6%) | 2 (15.4%) | 3 (13.6%) | 2 (18.2%) | |||
| Yes | 42 (89.4%) | 11 (84.6%) | 19 (86.4%) | 9 (81.8%) | |||
Data are mean
Abbreviations: BMI, body mass index; SCC-Ag, squamous cell carcinoma antigen; MIS, minimally invasive surgery; FIGO, the International Federation of Gynecology and Obstetrics; NACT, neoadjuvant chemotherapy; PSM, propensity score matching; IQR, interquartile range; RH, radical hysterectomy.
The pathological evaluations after surgery are shown in Table 2. The primary surgery group had a median tumor size of 3.5 cm, whereas the NACT group had a median tumor size of 2.4 cm; there was no statistically significant difference between the two groups (p = 0.078). Other characteristics such as resection margin involvement, parametrial invasion, lymphovascular space invasion, and vaginal extension, were not significantly different between the two groups. In primary surgery group, vaginal extension population was equal to 21 since 1 person was missing.
| Characteristics | Primary surgery (n = 22) | NACT (n = 11) | p value | |
| Tumor size, cm | 3.9 | 2.7 | 0.078 | |
| Median (range) | 3.5 (1.0–8.5) | 2.4 (0.6–3.8) | ||
| RM involvement | 0.534 | |||
| No | 19 (86.4%) | 11 (100.0%) | ||
| Yes | 3 (13.6%) | 0 (0.0%) | ||
| Parametrial invasion | 1.000 | |||
| No | 19 (86.4%) | 9 (81.8%) | ||
| Yes | 3 (13.6%) | 2 (18.2%) | ||
| LVSI | 0.391 | |||
| No | 4 (18.2%) | 4 (36.3%) | ||
| Yes | 18 (81.8%) | 7 (63.6%) | ||
| Depth of invasion | 1.000 | |||
| 3 (13.6%) | 1 (9.1%) | |||
| 19 (86.4%) | 10 (90.9%) | |||
| Vaginal extension | 1.000 | |||
| No | 18 (85.7%) | 10 (90.9%) | ||
| Yes | 3 (14.3%) | 1 (9.1%) | ||
Abbreviations: RM, resection margin; LVSI, lymphovascular space invasion; NACT, neoadjuvant chemotherapy.
Table 3 shows the survival outcomes of the two groups. The median follow-up duration was 33.5 months (range, 1.4–217.0) for the primary surgery group and 25.2 months (range, 9.3–233.4) for the NACT group. During follow-up, 14 patients (63.6%) in the primary surgery group experienced recurrence; among these patients, one had pelvic metastasis, 8 had extrapelvic metastasis, and 5 had combined (intra- and extrapelvic) recurrence. In the NACT group, seven patients (63.6%) experienced recurrence; among these patients, two had pelvic metastasis, four had extrapelvic metastasis, and one had combined (intra- and extrapelvic) recurrence. Furthermore, 12 patients (54.6%) died in the primary surgery group and seven patients (63.6%) died in the NACT group.
| Primary surgery | NACT | HR | 95% CI | p value | |
| Before PSM | n = 47 | n = 13 | |||
| Recurrence | 29 (61.7%) | 8 (61.5%) | 1.106 | 0.504–2.427 | 0.801 |
| Death | 23 (48.9%) | 8 (61.5%) | 1.653 | 0.735–3.717 | 0.224 |
| After PSM | n = 22 | n = 11 | |||
| Recurrence | 14 (63.6%) | 7 (63.6%) | 1.164 | 0.398–3.404 | 0.782 |
| Death | 12 (54.6%) | 7 (63.6%) | 1.591 | 0.505–5.007 | 0.428 |
Abbreviations: NACT, neoadjuvant chemotherapy; HR, hazard ratio; PSM, propensity score matching; 95% CI, 95% confidence interval.
The 5-year DFS and OS rates were 36.4% and 45.4%, respectively, in the primary surgery group, and 36.4% and 36.4 %, respectively, in the NACT group. In the PSM analysis, the 5-year DFS (hazard ratio (HR), 1.164; 95% confidence interval (95% CI), 0.398–3.404; p = 0.782) and OS (HR, 1.591; 95% CI, 0.505–5.007; p = 0.428) were not significantly different between the two groups.
Intraoperative transfusion was performed in five patients (45.5%) in the NACT group and two patients (10%) in the primary surgery group (p = 0.052) (Table 4). One patient in the NACT group who remained in the PSM analysis had postoperative complications (voiding difficulty and chylous ascites). In primary surgery group, population sums up to 20 since two people were missing in each category of characteristics.
| Characteristics | Primary surgery (n = 20) | NACT (n = 11) | p value | |
| Intraoperative transfusion rate | 0.067 | |||
| No | 18 (90.0%) | 6 (54.6%) | ||
| Yes | 2 (10.0%) | 5 (45.5%) | ||
| Postoperative transfusion rate | 0.999 | |||
| No | 19 (95.0%) | 11 (100.0%) | ||
| Yes | 1 (5.0%) | 0 (0.0%) | ||
| Postoperative complication | 1.000 | |||
| No | 18 (90.0%) | 10 (90.9%) | ||
| Yes | 2 (10.0%) | 1 (9.1%) | ||
Among 13 patients who received NACT, one received platinum-based chemotherapy. Seven patients (54%) received paclitaxel and cisplatin, three (23%) received etoposide + cisplatin, two (15%) received 5-fluorouracil + cisplatin, and one (8%) received paclitaxel + carboplatin. All patients exhibited a partial response to NACT according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria [10].
Neuroendocrine neoplasms are rare malignancies and are among the most aggressive types of gynecological cancers with limited therapeutic options [11]. Because patients are sometimes diagnosed in advanced stages with distant metastasis, curative surgery is not always the first treatment option. Instead, neoadjuvant therapy may be administered beforehand to reduce tumor burden [12]. A prior examination of six randomized trials, involving individuals with locally advanced cervical cancer who underwent NACT before undergoing surgery, found that NACT significantly reduced tumor size, invasion depth, and nodal metastasis [8]. However, studies on the effectiveness of NACT in patients with HGNECs of cervical cancer are lacking. Therefore, we aimed to share our single-center experience of treating patients with HGNEC who underwent NACT before radical surgery.
We found that the median tumor size decreased slightly in both groups, but the change was not statistically significant (Table 2). Furthermore, there were no significant differences in pathological risk factors between the two groups. However, we noted that the NACT group had a higher intraoperative transfusion rate (p = 0.052) because this group had some tissue changes caused by NACT, which resulted in a bleeding tendency during the operation (Table 4). In addition, since patients undergoing surgery after NACT are at risk of perioperative anemia due to myelosuppressive chemotherapy [13], they are more likely to receive transfusions during the operation.
Clinicians chose additional treatments depending on their decisions, and we found that the adjuvant treatment modality was not a significant prognostic factor for survival. Some retrospective studies comparing NACT followed by surgery with concurrent chemotherapy have shown improved survival outcomes; however, as these studies were retrospective or non-randomized, they cannot be suggested as a standard therapy for cervical cancer [14, 15, 16]. Another individual patient meta-analysis showed that there was an absolute benefit in overall survival in NACT followed by surgery; however, all of these groups received either chemotherapy or radiotherapy afterward, making it unclear which adjuvant treatment was superior [14]. Tumor size is another prognostic factor, with maximum benefits reported in patients with stages IB2 and IIB [17]. In our study, we found no significant difference in the overall survival rates between the two study groups, indicating that NACT followed by surgery did not yield favorable survival outcomes.
Data on the effect of NACT on HGNEC remains undetermined [18, 19]. Lee et al. [18] reported that patients with stage IB-IIA small cell neuroendocrine carcinoma (NEC) who underwent NACT followed by surgery had worse survival outcomes. This multicenter study included 11 patients who received neoadjuvant chemotherapy [18]. According to a study by Wang et al. [19], patients who received NACT tended to have worse failure-free survival (41.2% vs. 60.5%, p = 0.086) than those who underwent primary surgery, regardless of adjuvant therapy. This study included all disease stages. Six patients (16.7%) underwent neoadjuvant chemotherapy followed by surgery, while five patients underwent perioperative chemotherapy plus surgery [19].
According to a recent reviewer, the implementation of immunotherapy and targeted therapy has demonstrated advantages for individuals diagnosed with cervical cancer [20]. Research has indicated that human papillomavirus (HPV)-related cervical cancer demonstrates heightened expression of programmed cell death ligand 1 (PD-L1) [21], with HPV16 E7, in particular, possessing the capability to directly stimulate PD-L1 expression [22]. There have been reports suggesting that the majority of small cell neuroendocrine cervical carcinomas, a subtype of HGNEC, are associated with HPV infection [23, 24, 25]. Considering this, suggesting that the introduction of immunotherapy, such as pembrolizumab, may lead to favorable outcomes in patients with HGNEC. Further studies are required to validate these hypotheses.
This study has some limitations. First, although the current standard is the FIGO 2018 staging system, we used the FIGO 2009 staging system to stage all patients because the data were collected from 1993 to 2017. In the FIGO 2018 system, the stage is classified as IIIC if there is lymph node metastasis, which recommends concurrent chemoradiation therapy as the standard treatment. Fortunately, our study population did not show any lymph node metastases on preoperative images. Additionally, the treatment recommendation for FIGO staging is limited to cervical cancer of common cell types [26], which may not be applicable to neuroendocrine carcinomas. Secondly, although all NACT regimens were platinum-based, the lack of uniformity could be a potential limitation. Another limitation of our study is its small sample size, which was due to the rarity of neuroendocrine carcinoma in cervical cancer. However, it is evident that the groups receiving neoadjuvant chemotherapy were very small in previous studies [18, 19], and our study is among the larger ones.
Although neoadjuvant chemotherapy followed by surgery was feasible, it failed to improve survival outcomes. Further studies investigating more effective therapies are required to improve the DFS of patients with cervical HGNEC.
DFS, disease-free survival; HGNEC, high-grade neuroendocrine carcinomas; NACT, neoadjuvant chemotherapy; OS, overall survival; PD-L1, programmed cell death ligand 1; PSM, propensity score matching.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
HE and JuHK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: HE, JuHK, YKS. Acquisition, analysis, or interpretation of data: HE, YKS, JuHK, YMK, JongHK, DSS, DYK, JYP. Drafting of the manuscript: HE, JuHK, YKS. Critical revision of the manuscript for important intellectual content: HE, JuHK, YKS. Statistical analysis: HE, YKS. Administrative, technical, or material support: HE, YKS, JuHK, YMK, JongHK, DSS, DYK, JYP. Supervision: JuHK. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the institutional review board of Asan Medical Center (approval number: 2022-1406; approval date: June 28, 2022). The requirement of informed consent was waived because of the retrospective study design.
We express our gratitude to all those who helped us write this manuscript. We thank all peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflicts of interest. Jeong-Yeol Park serves as an Editorial Board member of this journal. Jeong-Yeol Park was not involved in the peer review of this article and had no access to information regarding peer review. Full responsibility for the editorial process of this article was delegated to Yasuhiko Ebina.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.