1 Department of Epidemiology and Biostatistics, School of Health, Social Determinants of Health Research Center, Birjand University of Medical Sciences, 9717853577 Birjand, Iran
2 Midwifery Department, Ministry of Health and Medical Education, 1467664961 Tehran, Iran
3 Department of Midwifery, Qom University of Medical Sciences, 1461965381 Qom, Iran
Abstract
Objective: Endometrial cancer is the sixth most common cancer in women. Extensive studies have been conducted around the world to determine the risk factors of endometrial cancer. Although each of these studies provides useful findings, review studies provide a clear view of the different aspects of a phenomenon. Therefore, this review study was conducted to determine the risk factors of endometrial cancer in the world. Mechanism: A comprehensive search was conducted in Medline, Web of Science Core Collection (Indexes = SCI-EXPANDED, SSCI, A&HCI Timespan), and Scopus databases with the keywords; “endometrial cancer”, “endometrial carcinoma”, “endometrial neoplasm”, “risk factor” and a combination of these words to find quality articles published from 2000 to 2024. Findings in brief: According to the literature, demographic factors, reproductive factors, gynecological factors, hormonal factors, lifestyle factors, medical conditions, and drugs can contribute to endometrial cancer. The study findings indicated that higher parity, oral contraceptive use, and regular physical activity may reduce the risk of developing endometrial cancer. Conversely, advanced age, prolonged reproductive period, infertility, polycystic ovary syndrome, postmenopausal hormone therapy, obesity, alcohol consumption, metabolic syndrome, and diabetes are associated with an increased susceptibility to this type of cancer. Conclusions: The findings of this study showed that reproductive factors such as early menarche, late menopause, nulliparity, and infertility increase the risk of endometrial cancer. Evidence shows that obesity, metabolic syndrome, and diabetes play a role in the occurrence of endometrial cancer. Although the use of menopausal hormone therapy increases the risk of endometrial cancer, the use of combined oral contraceptives is associated with a reduced risk.
Keywords
- endometrial cancer
- world
- endometrial neoplasm
- risk factor
Cancer ranks as the second leading cause of death worldwide, posing a significant burden on societies [1]. Endometrial cancer is the sixth most prevalent cancer in women, primarily affecting menopausal women [2]. In 2021, approximately 66,570 new cases of endometrial cancer were diagnosed, making up 7% of all cancer cases. The number of deaths attributed to endometrial cancer in 2021 was 12,940, accounting for 4% of all deaths that year [3]. Endometrial cancer is most prevalent in high-income countries, with the highest and lowest age-standardized incidence rate (ASIR) observed in Europe and Africa, respectively [4]. Factors such as reproductive and gynecological issues, anthropometric indicators, nutrition, physical activity, medical conditions like diabetes, hormone therapy, and smoking are all risk factors for endometrial cancer [5].
Despite the lack of screening methods, most women are diagnosed with endometrial cancer in the early stages, resulting in a relatively good survival rate. However, for those diagnosed with advanced or recurrent disease, the prognosis is poor [6]. Adenocarcinoma cancers make up over 90% of all uterine cancers, with 80% related to excess estrogen caused by metabolic syndrome and obesity (type I), and the remaining 20% of unknown causes (type II). These types of cancer are often diagnosed at an advanced stage and are associated with a high risk of metastatic recurrence and mortality. Unlike type I, which is linked to a high 5-year survival, type II endometrial cancer appears in more advanced stages and is associated with a moderate to low survival rate after 5 years [7].
Numerous global studies have been undertaken to identify the risk factors associated with endometrial cancer [8, 9, 10, 11]. While each study offers valuable insights, review studies are particularly valuable for informing program development and policy-making due to their comprehensive analysis of various aspects of the issue. Several review studies have been carried out in this field, some focusing on individual risk factors [12, 13] and others examining a group of causes [14]. Therefore, this review study was undertaken to investigate the risk factors of endometrial cancer in the world.
To investigate the risk factors of endometrial cancer, a thorough search was carried out in Medline, Web of Science Core Collection (Indexes = SCI-EXPANDED, SSCI, A&HCI Timespan) and Scopus databases using keywords such as “endometrial cancer”, “endometrial carcinoma”, “endometrial neoplasm”, “risk factor” and various combinations of these terms to identify high-quality articles published from 2000 to April 3, 2024. All keywords were cross-checked and assessed using PubMed Medical Subject Heading (MeSH). Additionally, a manual search was conducted in reputable journals to review the references of retrieved articles and related systematic review articles. All retrieved articles were organized using Endnote X7, following the PRISMA statement and guidelines recommended by Moher et al. [15].
Two researchers thoroughly reviewed the retrieved articles, using a two-stage screening process. The study included full-text quantitative articles in English with relevant keywords in their title or abstract published between 2000 and April 3, 2024. The research examined all risk factors except genetic causes.
Case reports, commentaries, letters to the editor, case series, systematic reviews, and animal studies were excluded from the study. Due to the retrieval of a large number of articles, if it was not possible to access the full text of the article, it was excluded from the study.
The researchers in this study were dedicated to upholding all ethical standards for conducting review studies. They ensured accuracy and reliability in every phase of data collection, analysis, and dissemination of results.
Following a thorough search of the databases mentioned above, a total of 681 articles published between 2000 and April 3, 2024, were initially identified for inclusion in the study. After removing duplicate articles using Endnote 21 software, 489 articles were selected for further review. Upon screening their titles and abstracts, 331 articles were excluded, leaving 158 for full-text review. Subsequently, 23 articles were removed from the study for scientific reasons (Commentary: 3, Case report: 1, Book chapter: 1, Review: 11, Editorial: 2, Not available full text: 5). After careful manual reference checking, 13 articles were ultimately included in the study, resulting in a total of 148 articles for analysis [8, 9, 10, 11, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159] (Fig. 1).
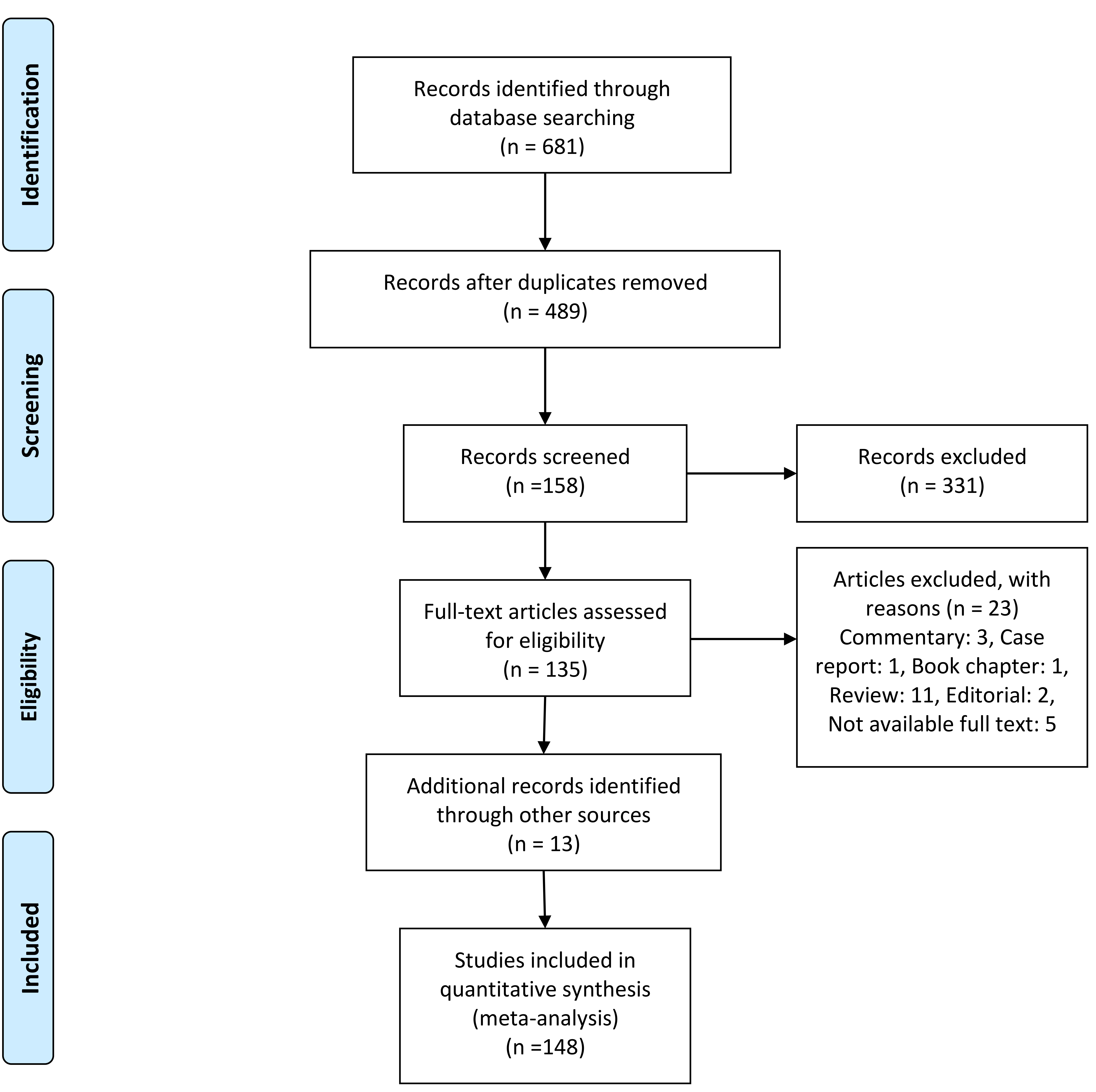 Fig. 1.
Fig. 1.PRISMA flowchart.
Table 1 shows factors related to endometrial cancer.
| Risk Factors | Protective | Predisposing | Controversial | |
| Demographic factors | Age | ✓ | ||
| Season of birth | ✓ | |||
| Blood group | ✓ | |||
| Reproductive factors | Menstrual history | ✓ | ||
| Parity | ✓ | |||
| Infertility | ✓ | |||
| Age at first delivery | ✓ | |||
| Pregnancy complications | ✓ | |||
| Gynecological factors | Endometriosis | ✓ | ||
| Polycystic ovary syndrome (PCOs) | ✓ | |||
| Pelvic inflammatory disease (PID) | ✓ | |||
| Uterine leiomyoma | ✓ | |||
| Hormonal factors | Postmenopausal hormone therapy | ✓ | ||
| Contraceptive methods | ✓ | |||
| Life style factors | Obesity | ✓ | ||
| Coffee and tea | ✓ | |||
| Alcohol consumption | ✓ | |||
| Smoking | ✓ | |||
| Diet | ✓ | |||
| Vitamins | ✓ | |||
| Physical activity | ✓ | |||
| Night work and duration of sleep | ✓ | |||
| Medical condition | Metabolic syndrome | ✓ | ||
| Diabetes | ✓ | |||
| Drugs | Non-steroidal anti-inflammatory drugs (NSAIDs) | ✓ | ||
| Ovulation-stimulating drugs | ✓ | |||
| Antidiabetic drugs | ✓ | |||
| Other | Circulating metabolites | ✓ | ||
| History of cancer | ✓ | |||
| Breastfeeding | ✓ | |||
| Stress | ✓ | |||
| Talcum Powder | ✓ | |||
Although older age is associated with an increased risk of endometrial cancer, the association of birth season and blood type with endometrial cancer is unclear.
Roughly 75% of endometrial cancer cases are diagnosed in postmenopausal women [158]. While it remains uncertain whether advanced age is an independent risk factor or a marker for other conditions that impact the disease [46], several studies have identified age as a potential risk factor for developing endometrial cancer [65, 88]. One study found that women over 50 years old (odds ratio (OR) = 3.064, 95% confidence interval (CI) = 1.945–5.931) are at increased risk for endometrial cancer if they have endometrial hyperplasia [65].
According to researchers, there is a belief that women born in summer and in higher geographical latitudes may have an increased risk of hormonal cancers like endometrial cancer. The exact reason for this connection is not fully understood, but it is thought to involve a complex interplay between light, hormones such as melatonin, and the individual’s circadian rhythm. However, a case-control study revealed that the season of birth is not a risk factor for endometrial cancer [157].
Based on previous research, there is no clear link between blood type and endometrial cancer [155, 156]. However, one study found that blood type “O” was the most common ABO blood type among patients with type I endometrial cancer, and was associated with a higher risk of developing this type of cancer. While ABO blood type may be linked to the occurrence of endometrial cancer, it does not appear to be significantly associated with the stage or type of the cancer [47]. Additionally, a case-control study found a significant dose-response relationship between the risk of endometrial cancer and levels of the “A” antigen [45].
Estrogens play a mitogenic role in the normal endometrium and cause endometrial growth during the menstrual cycle. During the follicular phase, estrogen is produced by the growing follicle, which leads to the growth of endometrium. Reproductive factors can change the risk of endometrial cancer by changing available estrogen and progesterone [44]. Studies indicate that increasing the length of the fertile period, reducing parity, and experiencing infertility can increase the risk of endometrial cancer. There are conflicting results when examining the relationship between age at first birth and pregnancy complications.
In many studies, early menarche [22, 26, 28, 30, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61] and late menopause [22, 28, 30, 52, 54, 57, 81] have been referred to as the risk factors of endometrial cancer. A case-control study showed that women with a menarche age of 17 years or more had a 45% [95% CI 0.36–0.83] lower risk of endometrial cancer compared to women with a menarche age of 12 years or less [30]. The result of another study showed a relationship between the years of menstruation in a person’s lifetime and the risk of endometrial cancer so every additional year increased the risk of endometrial cancer by 1.9%. According to the result of this study, this relationship was independent of factors including incomplete pregnancy, hormone therapy, menopause, diabetes and body mass index [43]. As mentioned earlier, the risk of endometrial cancer increases in late menopause. Accordingly, the results of a study showed that compared to people who experienced menopause before the age of 45 years, people whose menstrual cycles continued until the age of 55 years or over had a 5-fold increased risk of endometrial cancer [95% CI = 2.48–10.69] [30]. In addition to the duration of the menstrual period, the regularity and pattern of menstruation have also been discussed in some studies. According to the results of a study, irregular menstruation is related to the increased risk of endometrial candcer [87]. This is while Xu et al. [30] in a study argued that the duration and regularity of the menstrual cycle is not related to the risk of this cancer.
Nulliparous women have an increased risk of endometrial cancer [10, 27, 39, 48, 51, 58, 64, 66, 81, 87]. Childlessness is associated with an increased risk of type I and II endometrial cancer [66]. Studies have shown that each pregnancy is associated with a decrease in the risk of endometrial cancer [52, 56, 57, 59, 62]. Results of a study by Wernli et al. [59] showed that the hazard ratio does not decrease after the second pregnancy. Results of a cohort study revealed that first pregnancy is associated with a significant reduction in the risk of endometrial cancer, whether it is terminated by abortion or delivery. It also showed that each subsequent pregnancy is associated with a further reduction in the risk of this cancer, whether it is terminated by induced abortion or delivery. In this study, pregnancy duration, gestational age, spontaneous abortions, obesity, mother’s birth cohort, fertility and socio-economic factors did not change the results [42]. This risk reduction can be explained by a biological process that occurs during the first weeks of pregnancy, as pregnancies that are terminated by induced abortion are associated with a similar risk reduction as pregnancies that end in labor [42]. In this regard, some studies have shown that induced abortion reduces the risk of endometrial cancer [10]. Pocobelli et al. [16] states that giving birth is associated with a 35% reduction in the risk of endometrial cancer and a higher number of births is associated with a higher risk reduction [18, 20, 21, 24, 25, 27, 28, 52, 53, 54, 55]. Meanwhile, the results of a retrospective study showed that women with endometrial cancer had a higher number of childbirths in their postmenopausal age [17]. Contrary to parity, multiple births are associated with an increased risk of endometrial cancer [18, 19]. However, some studies have shown that parity is not correlated to the risk of endometrial cancer [88].
Research indicates that Infertility increases the risk of endometrial cancer [48, 49, 50, 51]. According to the results of a study, the incidence of endometrial cancer was significantly increased in women with hormonal infertility, women exposed to unopposed estrogen and women treated with clomiphene citrate (CC) and human menopausal gonadotropin (hMG). In a multivariate analysis, only women with uncontested estrogen exposure had an increased risk of endometrial cancer (hazard ratio (HR) = 1.4; 95% CI = 0.6–3.9) [137].
As a woman’s age at the time of her first live birth increases, her risk of endometrial cancer decreases [10, 16, 20, 21, 22, 23, 24, 25, 26]. Furthermore, the older age of the mother at her last delivery and the longer interval between her first and last delivery, the greater the protective effect against endometrial cancer [24]. Women who have their first childbirth at the age of 30 and older experience a 40% reduction in the risk of endometrial cancer compared to those who have their first childbirth at a teenage age [59]. While some studies have reported conflicting results, with some stating that older age at first delivery increases the risk of endometrial cancer [18], others have not found a relationship between the mother’s age at first delivery and the risk of endometrial cancer [27, 28, 29, 30].
No relationship has been found between the history of stillbirth or incomplete pregnancy, and the risk of endometrial cancer [16, 30]. Although there is no relationship between preeclampsia and the risk of endometrial cancer [31, 32], early preeclampsia significantly increases the risk of endometrial cancer [32]. Pre-pregnancy hypertension, gestational hypertension and pre-eclampsia are correlated to the increased risk of endometrial cancer [19, 25]. Hyperemesis gravidarum, postpartum hemorrhage, decollement, and history of intrauterine growth restriction (IUGR) do not affect the risk of endometrial cancer [25, 29]. However, although some studies revealed that the chance of endometrial cancer increases in women with a history of Gestational diabetes mellitus (GDM) or large for gestational age (LGA) [19, 29], the result of a study showed no relationship between gestational diabetes and the risk of uterine cancer [11].
Gynecological disorders are associated with endometrial cancer due to pelvic inflammation and increased available estrogen [159]. However, each of these disorders may change the risk of this cancer by different mechanisms. There is some evidence suggesting a connection between gynecological factors and endometrial cancer. However, there are conflicting results in research regarding the relationship between endometriosis, polycystic ovary syndrome, pelvic inflammatory disease, and uterine leiomyoma.
Endometriosis is a disease related to estrogen and is linked to the development of endometrial cancer due to the accumulation of estrogen and resistance to progesterone [33, 160]. Several studies have shown that endometriosis can increase the risk of endometrial cancer [34, 35], particularly type I endometrial cancer [36]. One study found that patients with endometriosis, especially those with long-term endometriosis, had a higher risk of developing endometrial cancer (adjusted hazard ratio, aHR = 2.92; 95% CI = 2.12–4.03). The highest risk of endometriosis-related endometrial cancer was observed in individuals who had endometriosis for 37–60 months (adjusted relative risk, aRR = 9.15, 95% CI = 4.40–19.02). Another study indicated that women aged 12–35 years were at the highest risk of developing endometriosis-related uterine cancer [37], although some studies did not find a link between endometrial cancer and endometriosis [49, 159].
The relationship between PCOS and the risk of endometrial cancer has been shown in many studies [38, 39, 40]. Women with PCOS were four times more likely to develop endometrial cancer than women without PCOS (OR = 4.0, 95% CI = 1.7–9.3). However, this association is reduced when the analysis is adjusted for body mass index (OR = 2.2, 95% CI = 0.9–5.7). PCOS, including hirsutism and very irregular periods, are significantly associated with the risk of endometrial cancer [41], and although this association seems to be limited to premenopausal women [71], it is higher in type I endometrial cancer [41].
Disorders of ovulation have been linked to a higher risk of endometrial cancer, especially in women who have not given birth and are experiencing infertility [49]. One cohort study found that the incidence of endometrial cancer in women with PCOS was 49.2 per 100,000 per year over a 5 to 10-year period, with no cases occurring in the comparison group during that time. However, after more than 10 years of follow-up, there was no statistically significant difference in the incidence of endometrial cancer between the two groups [68]. Another cohort study by Yin and colleagues [69] reported a risk ratio of 2.62 for endometrial cancer in women with PCOS, but when considering the variable of menopause, the risk ratio increased to 6.45. Interestingly, some studies have found no association between a history of ovarian cysts or PCOS and the risk of endometrial cancer [28, 70, 71].
Inflammation plays a crucial role in the endometrial remodeling cycle, with cytokines contributing to changes in the endometrial mucosa. Inflammatory cells have the potential to promote cell proliferation, inhibit apoptosis, and contribute to genetic changes [72]. However, contradictory results have been found in a literature review. Some studies have indicated no significant relationship between pelvic inflammatory disease, infection, and wound [37]. On the other hand, a study found that PID and fallopian tube or womb infection are not correlated with the risk of endometrial cancer [159]. Nevertheless, a population-based study revealed that the risk of endometrial cancer is higher in patients with PID, particularly in elderly patients or women with high blood pressure [72].
Uterine leiomyoma is correlated to an increased risk of endometrial cancer [56, 64]. Women with a history of uterine leiomyoma are at 42% increased risk of
endometrial cancer compared to women with no history of uterine leiomyoma. This
association is stronger when leukemia is diagnosed at a younger age. The
strongest association has been seen among women with a more recent diagnosis of
uterine leiomyoma. The incidence rate ratio of (IRR) at the time of uterine
leiomyoma diagnosis was 3.20, 0.95, 1.35, 3–9, and
Postmenopausal estrogen therapy has been found to elevate the risk of endometrial cancer. Conversely, oral contraceptive pills have been shown to decrease this risk.
Research indicates that the prolonged use of estrogen-only hormone replacement therapy (HRT) is linked to a higher risk of endometrial cancer [10, 21, 57, 60, 66, 74, 75]. Horn-Ross suggests that HRT for 5 years or longer is connected to an elevated risk of endometrial cancer [58]. Additionally, Faber et al.’s study [66] demonstrated that menopausal hormone therapy raises the risk of type I endometrial cancer. In contrast to estrogen-only hormone therapy, combined HRT has been linked to a lowered risk of endometrial cancer [53]. However, Lacey et al.’s cohort study [76] did not confirm this finding, stating that estrogen plus progesterone regimens neither increase nor decrease the risk of endometrial cancer. According to a study, continuous use of combination drugs was associated with a decreased risk of endometrial cancer, while the use of tibolone and estrogen-only drugs was linked to an increased risk. However, the use of cyclic combination drugs did not significantly change the risk. The study also found that a woman’s body mass index played a significant role in these associations, with non-obese women experiencing greater adverse effects from tibolone and estrogen-only HRT, and obese women experiencing greater benefits from combined HRT [77].
The reduced risk of endometrial cancer is most likely due to the prevention of ovulation among the users of oral contraceptives. The protective effect of these drugs is exerted by the reduction of ovulation and inhibition of Follicle-stimulating hormone (FSH) and Luteinizing hormone (LH) hormones [161]. According to studies, the use of oral contraceptive pills (OCP) is correlated to reduced risk of endometrial cancer [53, 64, 81] and this reduction increases with an increase in the duration of use and a decrease in time interval since the last use [28, 38, 48, 54, 64]. The risk of endometrial cancer in those who use birth control pills for more than two years is 50% lower compared to those who take no birth control pills [59]. This risk reduction is also greater in women who have used contraceptives for less than 5 years before their first live childbirth [64]. However, in another study, the results showed that OCP is not associated with a reduced risk of endometrial cancer [88]. Findings of some studies have also shown that in addition to OCP, the use of intrauterine device (IUD) is associated with a 50% reduction in the risk of endometrial cancer [48, 59], while other studies have not shown a relationship between tubectomy and the risk of endometrial cancer [59].
Obesity and alcohol consumption are the only lifestyle factors that increase the risk of endometrial cancer. The connection between endometrial cancer and tea and coffee consumption, alcohol intake, smoking, diet, vitamins, night work, and sleep duration is not well-defined. However, physical activity has been shown to protect against endometrial cancer.
The relationship between adult obesity and the risk of endometrial cancer has
been shown in many studies [38, 48, 51, 53, 56, 57, 58, 61, 64, 65, 66, 67, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91]. Endometrial
cancer is an estrogen-dependent disease, and obesity and fat accumulation are
related to increased levels of circulating estrogen, which justifies the
relationship between obesity and the risk of endometrial cancer [80]. In a cohort
study, a higher inflammatory and insulin potential of a diet was associated with
an increased incidence of endometrial cancer, and this association could almost
entirely be mediated by obesity [92]. Compared to women with a body mass index
(BMI) of less than 25, the adjusted IRR increases to 3.60 (2.24–5.78) with an
increased BMI of 40 and above [63]. Obesity is associated with a threefold
increase in the risk of endometrial cancer in nulliparous and multiparous women
[81]. For every 2 kg/m
Coffee consumption is correlated to the reduced risk of endometrial cancer [95].
A cohort study found that in women who drank 4 or more cups of coffee per day,
the RR for endometrial cancer risk reduction was 0.75 (95% CI = 0.58–0.97)
compared with those who drank 1 cup or less. This association appeared to be
largely restricted to overweight and obese women, who showed a risk reduction of
12% (95% CI = 0–20%) and 20% (95% CI = 7–31%) for each cup of coffee,
respectively. This risk reduction was not observed in normal-weight women [96].
This result was confirmed by the study of Giri and colleagues [97] who stated
that caffeinated coffee consumption may be associated with a lower risk of
endometrial cancer among obese postmenopausal women. The result of a cohort study
showed that compared with the non-daily drinkers (no or less than 1 cup per day),
the multivariable-adjusted hazard ratio for women who drank
The biological mechanism by which alcohol may increase the risk of endometrial cancer is related to alcohol’s effect on estrogen levels. Alcohol consumption increases the serum level of endogenous estrogen in women, which increases the risk of endometrial cancer by increasing mitotic proliferation and DNA replication errors. Although drinking less than one glass per day does not increase the risk of endometrial cancer, drinking higher amounts is associated with an increased risk of endometrial cancer. Compared to non-consumption, wine consumption of more than 2 glasses per day is associated with an endometrial cancer RR of 3.15 [98]. The result of a study showed that the effect of alcohol on endometrial cancer is age-dependent. According to this cohort study, women who were under 50 years old at the time of follow-up had a 70% or higher risk of endometrial cancer, while this risk was 40% lower in 50-year-old and older women [99]. The relationship between alcohol consumption and the risk of endometrial cancer is dose-dependent. In people who consume alcohol two or more times a day, the risk of endometrial cancer increases after menopause [98]. However, some studies have rejected the link between alcohol consumption and the risk of endometrial cancer [9].
The anti-estrogenic effects of smoking may decrease the risk of endometrial cancer. Additionally, smoking can indirectly lower estrogen levels by advancing menopause and promoting weight loss, further reducing the risk of endometrial cancer [100]. According to Viswanathan et al. [101], both current and past smoking are associated with a lower risk of cancer compared to non-smoking. Meanwhile, researchers showed that current smoking is associated with a reduced risk of endometrial cancer [9, 53, 89, 102, 103, 104] but previous smoking is not [104]. Smoking reduces the risk of endometrial cancer after menopause, and this protective effect disappears when smoking is stopped [103]. Research has shown that the number of cigarettes smoked per day and the duration of smoking are inversely related to the risk of endometrial cancer [101]. This protective effect of smoking may be attributed to the changes it induces in steroid production and metabolism [101]. Additionally, passive smoking has not been found to have any significant relationship with cancer [102]. A cohort study revealed that among premenopausal women, those who smoked more than 15 cigarettes per day or had smoked for 30 years or more at the time of entry to the study had a 2-fold increased risk of endometrial cancer compared to non-smokers [104]. However, it is important to note that some studies have not been able to confirm the relationship between smoking and the risk of endometrial cancer [55].
Diet by modulating the level of endogenous estrogen can affect the risk of
endometrial cancer [106]. Studies showed that higher intake of fat (p =
0.01) [105, 106] and decrease of soluble fiber [106] was correlated with the
increased risk of endometrial cancer, and also higher intake of sugar (p
= 0.009) and carbohydrates (p = 0.03) was correlated to decreased risk
of endometrial cancer [105]. Higher dietary calcium [106] and lignin intake are
associated with a reduced risk of endometrial cancer [107]. The result of a study
showed that high consumption of sugary foods and drinks is associated with an
increased risk of endometrial cancer and this risk is higher among people with
central obesity (waist-to-hip ratio (WHR)
Numerous studies have explored the impact of vitamin D on endometrial cancer, yielding varied results depending on the specific type of vitamin. While some studies have found no significant link between vitamin D consumption and endometrial cancer [112], one study reported a 28–38% decrease in the risk of developing endometrial cancer with a combination of dietary and supplemental vitamin D intake [106].
A study found that the daily intake of multivitamins, vitamin A, vitamins C and E, food carotenoids, and length of supplement treatment are not significantly correlated to the risk of endometrial cancer [79, 113]. However, another study showed that deficiencies in vitamin C, thiamine, and vitamin B6 are correlated to an increased risk of endometrial cancer [106]. Wang et al. [105] also confirmed this finding and stated that vitamin C levels are correlated to an increased risk of endometrial cancer (OR = 1.41; 95% CI = 1.16–1.72). Additionally, researchers in a study stated that riboflavin plays a role in reducing the risk of endometrial cancer [106].
The cause of endometrial cancer reduction through physical activity is not fully understood. However, it is believed that obesity is one of the main risk factors of endometrial cancer, and the lack of physical activity is associated with an increased risk of obesity, so this can explain the protective effect of physical activity against endometrial cancer. On the other hand, many effects of the known risk factors of endometrial cancer can be explained by the undisputed estrogen hypothesis. Physical activity helps to reduce the level of serum estrogen and therefore can reduce the risk of endometrial cancer [162].
According to studies, the risk of endometrial cancer decreases with physical
activity [55, 114] and with an increase in physical activity, this risk reduction
increases [114]. Compared to physical activity in women with normal weight (BMI
Night work reduces melatonin levels and in addition to its potential anti-estrogenic effects, it plays an important role in fat metabolism. Also, with increasing obesity, it increases the risk of endometrial cancer [116]. Working at night for a long time and more than 20 years increases the risk of endometrial cancer [116]. This risk is twice as high in obese women who work at night [116]. However, some studies have shown no statistically significant relationship between endometrial cancer and night shift or sleep duration [117, 118].
Metabolic syndrome and diabetes both increase the risk of developing endometrial cancer.
Many studies have shown that metabolic syndrome and its components are
associated with an increased risk of endometrial cancer. The risk of endometrial
cancer increases two-fold in people with metabolic syndrome (HR = 2.27; 95% CI =
1.67–3.09). A study showed that after removing waist circumference from the
definition, although this association was still positive, it was not
statistically significant. Also, waist circumference and hyperglycemia were
independently associated with the risk of endometrial cancer [79]. The result of
a case-control study showed that metabolic syndrome (62%) was significantly more
in the test group than the control group (38%). Also, a statistically
significant increased risk of endometrial cancer was observed in people with
metabolic syndrome (OR = 1.53; 95% CI = 1.17–2.00) and some of its components,
including waist circumference
Studies have demonstrated a link between high blood pressure and an elevated risk of endometrial cancer [38, 60, 63, 64, 65, 67]. In obese women, a blood pressure reading of 140/90 mm Hg or higher has been associated with a 3.5-fold increase in the risk of developing endometrial cancer compared to those with lower blood pressure [55]. Additionally, a retrospective study found that women with endometrial cancer were more likely to have post-menopausal hypertensive disorders [17].
Research has shown a clear link between elevated Triglyceride (TG) and TG/high-density lipoprotein (HDL) levels and an increased risk of endometrial cancer [120]. Furthermore, a high intake of dietary cholesterol has also been associated with a heightened risk of developing endometrial cancer [106]. Additionally, serum lipids are correlated with an increased risk of endometrial cancer, with total serum cholesterol, triglyceride, low-density lipoprotein cholesterol, and dyslipidemia all showing a positive correlation, while high-density lipoprotein cholesterol has shown a negative correlation [67]. Interestingly, some studies have suggested that hypercholesterolemia may be linked to a reduced risk of endometrial cancer [55, 56].
Research has found a strong link between waist and hip circumference, as well as waist/hip ratio, and a higher risk of endometrial cancer [121]. However, central fat, as measured by waist circumference and waist/hip ratio, is not a standalone predictor of endometrial cancer. In a multivariate analysis, hip circumference did not show a correlation with the risk of endometrial cancer [78]. Another study indicated that the risk of endometrial cancer increases in obese individuals with higher waist circumference [122].
Several biological mechanisms have been proposed for the increased risk of endometrial cancer in diabetic women. Hyperinsulinemia, obesity, and inactivity are among common features of diabetic people, and studies show that insulin stimulates the growth of endometrial stromal cells by binding to insulin receptors on endometrial cells. On the other hand, hyperinsulinemia may increase the level of bioactive estrogens by reducing the concentration of circulating sex hormone-binding globulin. Moreover, hyperinsulinemia leads to a decrease in insulin-like growth factor-binding protein 1 levels, and this increases circulating free Insulin-like growth factor 1 (IGF-1), which stimulates endometrial cell proliferation [163].
A history of type 2 diabetes is associated with an increased risk of endometrial cancer [39, 57, 60, 62, 63, 64, 65, 66, 67]. Diabetes increases the risk of type I and II endometrial cancer [66]. The result of a cohort study showed that diabetes increases the risk of endometrial cancer by three times [89]. The incidence of endometrial cancer in patients with diabetes for more than 8 years was higher than that of those who had diabetes for a shorter time [123]. Although in many studies a relationship was found between diabetes, duration of diabetes and the risk of endometrial cancer, the results of a study showed that this risk was not significant after adjusting the variables for BMI [124].
According to the studies, the link between drugs and the risk of endometrial cancer remains unclear.
NSAIDs are associated with a reduced risk of endometrial cancer [56]. The result of one study showed that at least weekly use of aspirin and NSAIDs was associated with an approximately 15% reduction in the risk of endometrial cancer among overweight and obese women [125]. Compared to no use, high aspirin use was also inversely associated with the risk of endometrial cancer (HR = 0.64, 95% CI = 0.41–1.01; p trend = 0.03). These findings were stronger for regular use than for low-dose aspirin [126]. The result of a study showed that the risk of endometrial cancer is reduced in people who are obese, currently using aspirin and have not used menopausal hormone therapy [127]. Although the result of a study showed that women who used any NSAIDs, including aspirin, did not have a lower risk of endometrial cancer compared to those who never used any NSAID, a study showed that this relationship did not exist regardless of duration of use, age at the first use, or time since first or last use of NSAIDs [128]. This result was confirmed in other studies [38, 126, 127, 129, 130, 131]. Other studies however have rejected the relationship between acetylsalicylic acid [38], acetaminophen [127], aspirin [132] and the risk of endometrial cancer.
Endometrial cancer is one of the hormone-related cancers. Although the relationship between endometrial cancer and ovulation-inducing drugs remains unclear, it appears that these drugs increase mitotic activity, DNA replication, mutation, and malignancy by increasing serum estradiol levels during the follicular phase [164]. Researchers in Norway conducted a cohort study and reported an increased risk of endometrial cancer in women treated with clomiphene citrate (HR = 2.91; 95% CI = 1.87–4.53), as well as nulliparous women (HR = 4.49; 95% CI = 2.66–7.60; p = 0.04). The risk was also higher among women with more than 6 cycles of treatment (HR = 4.68; 95% CI = 12.6–1.74) [133]. Parazzini and colleagues [134] reported the OR of endometrial cancer for the use of fertility drugs at 3.26. These researchers reported that the risk was higher in women who used fertility drugs for 12 months or more (OR = 6.10; 95% CI = 0.96–38.6) and for women who first used these drugs before the age of 30 years (OR = 5.14; 95% CI = 1.13–23.4) [134]. However, some studies have shown that the use of ovulation induction methods such as clomiphene citrate is not correlated with the increased risk of endometrial cancer [135, 136]. The result of a case-control study also showed that although infertility is associated with an increased risk of endometrial cancer, the use of fertility drugs is not associated with a greater risk of this cancer [137].
Based on the findings of a study, there is a correlation between the use of insulin and the risk of endometrial cancer, with the combined use of insulin and metformin further increasing this risk [123]. Brinton asserts that the use of metformin raises the risk of endometrial cancer, particularly among women under 30 years of age [138]. The use of metformin and other oral antidiabetic drugs has been linked to a higher incidence of endometrial cancer compared to non-use [123], although these findings have been contradicted by other studies. For example, some studies have found no correlation between metformin use and the risk of endometrial cancer [38, 124]. A case-control study also concluded that metformin, insulin, and other antidiabetic drugs do not impact the risk of endomfetrial cancer [139]. Additionally, a separate study indicated that only the use of statins at any time is associated with a lower risk of endometrial cancer [123]. Tseng’s study [140] reported mixed results, suggesting that the use of metformin may reduce the risk of endometrial cancer in a dose-dependent manner.
One study found no significant increase in the risk of endometrial cancer when
comparing prolactin-increasing antipsychotics to prolactin-sparing antipsychotics
(adjusted odds ratio [aOR] = 1.00; 95% CI = 0.68–1.48) [141]. However, another
study demonstrated that long-term use of thiazide for hypertension treatment
(
Sphingomyelin (SM) C18:0 is positively associated with the risk of endometrial cancer (OR1SD = 1.18, 95% CI = 1.05–1.33) and glycine, serine, and free carnitine (C0) be inversely associated with the risk of endometrial cancer (OR1SD = 0.89, 95% CI = 0.80–0.99; OR1SD = 0.89, 95% CI = 0.79–1.00 and OR1SD = 0.91, 95% CI = 0.81–1.00), respectively [143].
Higher levels of estrone, total estradiol and free estradiol are associated with the increased risk of endometrial cancer, while higher levels of sex hormone-binding globulin (SHBG) and SHBG-bound oestradiol have also been shown to be associated with the risk of endometrial cancer [144, 145].
In many cases, a history of cancer usually increases the risk of cancer in the same geographical region [146]. A family history of colon or uterine cancer is correlated to the risk of endometrial cancer [10, 64]. The result of a study showed that compared to women without a history of uterine cancer, the OR of endometrial cancer in women with a family history of endometrial cancer is 2.1 and for other types of uterine cancer is 1.8 [146]. A family history of intestinal cancer was associated with the risk of endometrial cancer in a study [146]. The result of a case-control study also showed that the history of reproductive cancer is associated with the 4.97 odds ratio of endometrial cancer [51].
An inverse association between breastfeeding and the risk of endometrial cancer is biologically possible. Breastfeeding can suppress ovarian follicle growth by inhibiting gonadotropin-releasing hormone and reducing estradiol levels. At this level of estradiol, mitosis of endometrial cells does not occur [147]. However, based on the results of some studies included in this review, there is no relationship between breastfeeding and the risk of endometrial cancer [28, 51, 54, 148, 149].
The risk of endometrial cancer is lower in people with higher levels of stress, which can be due to changes in gonadal estrogen synthesis, and also in uterine sensitivity to estrogen fluctuation [150]. Although Sun and colleagues [151] stated that major depressive disorder (MDD), anxiety and stress-related disorders (ASRD) are not genetically responsible for endometrial cancer, they argued that these conditions may indirectly affect endometrial cancer by affecting body mass index.
A study showed that the use of talcum powder was associated with a 13% increased risk of endometrial cancer for all women and a 21% increased risk of endometrial cancer for postmenopausal women. Regular use of talcum powder (more than once a week) was also associated with a 24% increased risk of endometrial cancer among postmenopausal women. Indirect use of talcum powder on sanitary napkins was not associated with the increased risk of endometrial cancer [152].
According to the findings of a study, the use of perineal powder is not associated with an increased risk of endometrial cancer. This study also found that using powder on the external genital area is not associated with an increased risk of endometrial cancer. However, it showed that using powder on the diaphragm for 20 years or more is associated with a threefold increase in the risk of endometrial cancer compared to no use [153]. This result was confirmed by another study [154].
Endometrial cancer ranks as the sixth most prevalent cancer in women globally, and its frequency has risen over the past twenty years [4]. Significant efforts to reduce the risk of this cancer concentrate on identifying and addressing key risk factors that contribute to its development. A thorough understanding of these risk factors and their role in tumor formation is crucial for the development of effective prevention methods. As such, this study aims to analyze the evidence on the primary risk factors associated with endometrial cancer. The results of this study showed that increasing parity, using oral contraceptive methods and physical activity can have a protective effect against the occurrence of endometrial cancer. Old age, longer reproductive period, infertility, polycystic ovary syndrome, hormone treatment after menopause, obesity, alcohol consumption, metabolic syndrome and diabetes predispose a person to the occurrence of this cancer.
Endometrial cancer is a type of cancer that is influenced by hormones, and fluctuations in hormone levels can impact the likelihood of developing this cancer [165]. The endometrium, or the lining of the uterus, changes its structure during the menstrual cycle in response to variations in estrogen and progesterone levels. Research indicates that excessive estrogen, without the balancing effect of progesterone, can lead to endometrial hyperplasia and an elevated risk of endometrial cancer. Prolonged and imbalanced exposure to estrogen may result from a deficiency in natural progesterone and elevated levels of estradiol. As a result, women with polycystic ovary syndrome (PCOS) and those who undergo estrogen therapy after menopause are at an increased risk of developing endometrial cancer [166]. Estrogen serves not only as a mitogen but also as a mutagen. Genotoxic metabolites of estrogen interact with DNA, leading to genetic instability through the accumulation of double-stranded DNA. Approximately one-third of endometrial cancers exhibit DNA mismatch repair defects, which can result from MLH1 somatic methylation or Lynch syndrome [167].
Reproductive factors, such as number of pregnancies and history of infertility, age of menarche and age of menopause, with differences in estrogen levels lead to changes in the risk of endometrial cancer. The result of a mata analysis including 4553 subjects showed that late menarche is associated with a lower risk of endometrial cancer [168].
Metabolic syndrome encompasses a range of metabolic abnormalities, such as obesity, insulin resistance, hypertension, and dyslipidemia. Research indicates that metabolic syndrome is a significant risk factor for endometrial cancer. Multiple meta-analyses have confirmed that conditions like obesity, diabetes, hypertension, and dyslipidemia are linked to a higher likelihood of developing endometrial cancer [169]. The exact connection between metabolic syndrome and endometrial cancer is not fully understood, but some hypotheses suggest abnormal fat metabolism, chronic inflammation, hyperglycemia, and hyperinsulinemia may be contributing factors [169, 170]. It is believed that elevated levels of leptin, a hormone encoded by the obesity gene, can promote the growth and spread of endometrial cells. Furthermore, inflammatory cytokines produced by fat cells are also thought to be involved in the development of endometrial cancer. An increase in serum glucose levels can increase glycolysis and thus increase the invasion of endometrial cancer cells. Glycolysis not only produces the energy required by tumor cells but also produces several metabolic intermediates that can be used by tumor cells in the synthesis of biological macromolecules [169, 171].
The correlation between obesity and the development of endometrial cancer is significant. Both type 1 and type 2 are linked to obesity [172]. Obesity leads to excessive conversion of androstenedione to estrone and decreased serum levels of sex hormone-binding globulin (SHBG), creating an estrogen-rich environment that promotes the growth of endometrial cancer cells. Additionally, obese women who lack ovulation may be exposed to elevated levels of estrogen, further increasing their risk of endometrial cancer. Furthermore, the environmental conversion of androgens to estrogen due to increased stored environmental fat inhibits apoptosis and stimulates the proliferation of endometrial cells. Insulin resistance and hyperinsulinemia, changes in sex hormone levels, and inflammatory factors and cytokines also play a significant role in this context [12].
A family history of endometrial cancer is associated with a two- to threefold increased risk of endometrial cancer. Although some of the association between family history and endometrial cancer risk may be attributable to environmental or lifestyle risk factors within a family, a genetic predisposition may be involved. Colorectal and endometrial cancer occurs in women with Lynch syndrome. Lynch syndrome is a hereditary cancer syndrome that accounts for 2 to 3% of all endometrial cancer cases [173]. Lynch syndrome is caused by mutations in the MLH1, MLH3, MSH2, MSH6, PMS2, TGFBR2, or EPCAM genes. Women with a first-degree relative who has had endometrial cancer also have a heightened risk of developing the disease, with a hazard ratio of 1.5 to 2.0. Cowden syndrome is a rare condition that arises from mutations in the tumor suppressor phosphatase gene. Women with Cowden syndrome face a significantly higher risk of developing endometrial cancer, about five times greater than the general population. Research has explored the connection between BRCA1 mutations and endometrial cancer in numerous studies [174, 175]. Furthermore, genetic variations in various genes have been found to impact the likelihood of developing endometrial cancer, with some polymorphisms influencing estrogen receptor transcriptional activity and, in turn, affecting the risk of developing the disease [176].
As the global burden of endometrial cancer continues to rise, there is a growing urgency to develop early preventive strategies for women at higher risk. Research indicates that as many as 90% of endometrial cancer cases can be detected in the early stages, underscoring the importance of informing women to promptly seek medical attention in the event of abnormal bleeding, particularly after menopause [177]. Alongside maintaining a healthy weight or achieving weight loss, the use of hormonal contraceptives, increased physical activity, and higher parity can also contribute to reducing the risk of endometrial cancer.
The use of English-language articles may limit the results of the study due to the omission of important data from other languages. On the other hand, due to the wide range of articles in the target field, qualitative evaluation of the articles was not done and all the studies that met the inclusion criteria were included in the study. However, a comprehensive review of endometrial cancer risk factors in the world is one of the strengths of the study.
This study identified the risk factors of endometrial cancer with a comprehensive review. Finding the factors related to endometrial cancer by identifying high-risk groups can formulate strategies to deal with the risk. The findings of this study showed that reproductive factors such as early menarche, late menopause, nulliparity and infertility increase the risk of endometrial cancer. Evidence shows that obesity, metabolic syndrome and diabetes play a role in the occurrence of endometrial cancer. Although the use of menopausal hormone therapy increases the risk of endometrial cancer, the use of combined oral contraceptives is associated with a reduced risk.
ZM, HS and LA designed the research study. ZM and HS performed the search. ZM, HS and LA analyzed the studies. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects.
Not applicable.
We would like to express our gratitude to all those who helped us while writing this manuscript. We are grateful to the Research Department of Qom and Birjand University of Medical Sciences for providing library facilities, online access to articles and other resources. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

