1 Center for Reproductive Medicine, The Third Affiliated Hospital of Sun Yat-sen University, 510630 Guangzhou, Guangdong, China
2 Department of Dermatology, The Third Affiliated Hospital of Sun Yat-sen University, 510630 Guangzhou, Guangdong, China
†These authors contributed equally.
Abstract
Background: Approximately 10% of women undergoing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment experience recurrent implantation failure (RIF). The causes for RIF are complicated. Inflammatory processes and thrombophilia play important roles in the development of RIF. This retrospective study was conducted to investigate whether there is an association between inflammatory parameters, including platelets (PLTs), plateletcrit (PCT) and lymphocytes in the complete blood count (CBC) and RIF. Methods: This was a single-center retrospective evaluation of 150 women who had a history of RIF and 163 controls who had a live birth after the first embryo transfer. Basal characteristics, CBC and coagulation parameters of both groups were compared. Results: Compared with the controls, the women with a history of RIF had significantly lower PLT, PCT and lymphocyte counts (p = 0.03, p = 0.019 and p = 0.012, respectively). Receiver operating characteristic curve analysis revealed that PLT had a sensitivity of 48.6% and a specificity of 66.4% with a cutoff value of 271.5 (area under the curve (AUC): 0.575); PCT had a sensitivity of 77% and a specificity of 38.9% with a cutoff value of 0.245 (AUC: 0.575); and lymphocyte count had a sensitivity of 49.3% and a specificity of 71% with a cutoff value of 2.015 (AUC: 0.577) for predicting RIF. The multivariant receiver operating characteristic (ROC) analysis revealed a cutoff value of 0.508 with a sensitivity of 70.3% and a specificity of 48.9% (AUC: 0.599) (p = 0.004). Conclusions: PLT, PCT and lymphocyte counts in patients with RIF are significantly reduced, although they are not effective parameters for predicting RIF.
Keywords
- complete blood count
- platelet
- plateletcrit
- lymphocyte
- recurrent implantation failure
In vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI)
technology has developed quickly, and success rates have gradually improved over
the past 40 years. However, approximately 10% of women undergoing IVF/ICSI
treatment still suffer from recurrent implantation failure (RIF) [1]. RIF is
defined by the European Society of Human Reproduction and Embryology (ESHRE) as
failure after
The causes of RIF are complicated as three different players are involved: female and male partners as well as the embryo. Maternal factors include age, chromosomal abnormalities, anatomical factors, endocrine factors, infectious factors and immunity factors [3]. In reproduction, the development of maternal immune tolerance is a key factor for embryo implantation, while inflammatory processes play important roles in the development of RIF [4]. The inflammatory reaction of the endometrium is characterized by lymphocyte infiltration through a mixture of lymphocytes and macrophages, which is a common pathologic mechanism for RIF [5, 6, 7]. Therefore, inflammatory markers of the endometrium may be used to predict RIF. However, endometrial biopsy is invasive. Thus, it is necessary to identify easily detectable inflammatory markers in patients with RIF. The platelet (PLT), plateletcrit (PCT), and lymphocyte counts along with the systemic immune-inflammation index (SII) are inflammatory markers for various diseases and are available directly from the complete blood count (CBC) [8, 9, 10, 11]. Since RIF is closely related to the inflammatory process, the PLT, PCT, and lymphocyte counts and SII may be used in the prediction of RIF.
Thrombophilia also plays a significant role in the development of RIF [12]. A retrospective study reported that coagulation parameters, such as platelet aggregation and prothrombin time, are predictive of RIF [3]. Moreover, a single-center prospective study showed that higher fibrinogen to albumin ratio (FAR) levels were related to adverse pregnancy outcomes such as recurrent pregnancy loss [13]. Therefore, based on previous studies, inflammatory parameters of CBC, coagulation parameters and FAR levels could be effective markers for predicting RIF. Clarification of the levels of these indicators in patients with RIF is essential.
To the best of our knowledge, inflammatory and thrombotic hematological markers such as the PLT, PCT, and lymphocyte counts, SII and FAR have not been previously investigated in RIF patients. In this study, we evaluated CBC parameters, fibrinogen and FAR changes in patients with a history of RIF, while the control group consisted of patients who had a live birth after the first ET.
The data of this retrospective study was obtained from the assisted reproductive
technology (ART) database at the Department of Assisted Reproduction of the Third
Affiliated Hospital of Sun Yat-sen University between March 2016 and May 2021.
The RIF group included women who had experienced
The inclusion criteria for the RIF group were: (1) individuals aged 22–38 years; (2) those experiencing infertility caused by tubal factors, endometriosis, anovulation, male factors or unexplained reasons; (3) those for whom karyotype analysis indicated that both partners were normal; (4) those for whom hysteroscopy was normal; (5) those with no reproductive tract infection or genital malformation [16, 17]. The inclusion criteria for the control group were: (1) individuals aged 22–38 years; (2) individuals with primary infertility; (3) healthy women without reproductive diseases [16].
A total of 152 patients were assigned to the RIF group and 166 patients were assigned to the control group. The exclusion criteria for both groups were: (1) a history of previous ovarian surgery, autoimmune or other serious medical illnesses [18] (determined as diabetes, hypertension, obesity, malignancy, chronic disease, chronic venous insufficiency); (2) the use of any drugs within one month that might affect the test results (hormone therapy, ovulation promoting drugs, nonsteroidal anti-inflammatory drugs, antiplatelet drugs or anticoagulants); (3) data unavailable [16, 18]. A total of 150 patients of the RIF group and 163 patients of the control group were analyzed (Fig. 1).
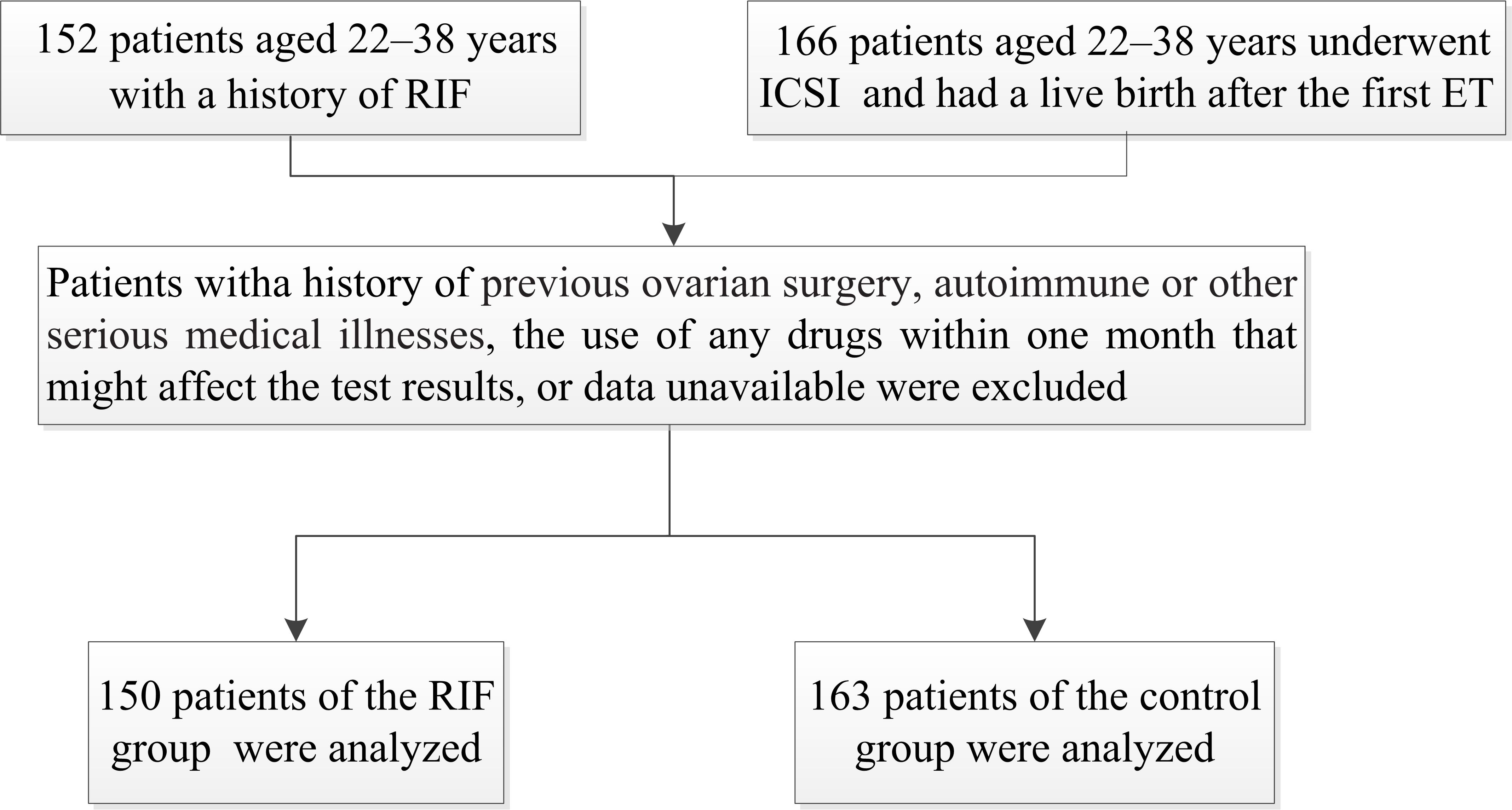 Fig. 1.
Fig. 1.The study flowchart. RIF, recurrent implantation failure; ICSI, intracytoplasmic sperm injection; ET, embryo transfer.
All patients had measures of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) on day two or three of the natural menstrual cycle. Blood samples for anti-Müllerian hormone (AMH), CBC and coagulation parameters detection were collected on any day of the natural menstrual cycle without acute infection [16, 18]. This study was approved by all patients who were provided informed consent for those undergoing ART treatments in our center. The anonymity of the patients has been strictly protected.
Body mass index (BMI) was calculated as the weight (kg) divided by the square of
the height (m
All data were analyzed using the SPSS statistical software package (version
23.0, IBM, Armonk, NY, USA). Numerical data are presented as median
(interquartile range). The Kolmogorov‒Smirnov test was used to determine the
distribution of the data. HCT in both groups was normally distributed, and the
difference in HCT between the two groups was measured by the continuous
t test. The other continuous variables in this study without a normal
distribution were analyzed using Mann‒Whitney U tests. An optimal cutoff value
for the significant parameters was determined using receiver operator
characteristic (ROC) curve analysis. The sensitivity and specificity, which were
evaluated by the area under the curve (AUC), were applied to determine the
accuracy of the test. p
No significant differences in age, BMI, duration of infertility, AMH, baseline
FSH and LH levels were detected between the two groups (p
| RIF (n = 150) | Control (n = 163) | p value | |
| Age (years) | 31.00 (29.00–33.00) | 30.00 (28.00–32.00) | 0.052 |
| BMI (kg/m |
20.50 (19.13–22.58) | 20.76 (19.23–23.13) | 0.337 |
| AMH (ng/mL) | 3.27 (1.95–6.13) | 3.87 (2.40–5.84) | 0.244 |
| Duration of infertility (years) | 3.00 (2.00–5.00) | 3.00 (2.00–4.00) | 0.465 |
| Baseline FSH (IU/L) | 6.74 (5.79–8.31) | 6.66 (5.93–7.77) | 0.161 |
| Baseline LH (IU/L) | 5.33 (4.22–6.98) | 5.32 (4.29–6.75) | 0.408 |
| Baseline E2 (pg/mL) | 38.99 (29.44–49.53) | 34.55 (26.06–44.12) | 0.045 |
| WBC ( |
5.79 (4.83–6.98) | 6.13 (5.24–7.06) | 0.226 |
| RBC ( |
4.51 (4.23–4.71) | 4.58 (4.35–4.79) | 0.109 |
| HGB (g/L) | 133.00 (125.00–138.75) | 134.00 (129.00–139.00) | 0.142 |
| HCT | 0.41 (0.39–0.42) | 0.41 (0.38–0.42) | 0.167 |
| MCV (fL) | 88.95 (86.03–91.88) | 88.80 (86.45–91.95) | 0.986 |
| MCH (pg) | 29.90 (28.53–30.80) | 29.60 (28.60–30.50) | 0.489 |
| MCHC (g/L) | 332.00 (326.25–337.00) | 331.00 (324.50–338.00) | 0.673 |
| PLT ( |
248.50 (218.50–296.25) | 263.00 (229.00–302.00) | 0.030 |
| RDW | 0.12 (0.12–0.13) | 0.12 (0.12–0.13) | 0.935 |
| PDW | 11.80 (10.73–13.00) | 11.70 (10.80–13.25) | 0.757 |
| MPV (fL) | 10.35 (9.80–11.00) | 10.30 (9.95–11.00) | 0.590 |
| PCT | 0.26 (0.23–0.30) | 0.27 (0.25–0.31) | 0.019 |
| Neutrophil ( |
3.27 (2.66–4.26) | 3.43 (2.75–4.26) | 0.497 |
| Lymphocyte ( |
1.86 (1.60–2.08) | 2.02 (1.67–2.44) | 0.012 |
| NLR | 1.74 (1.37–2.34) | 1.71 (1.32–2.27) | 0.294 |
| PLR | 138.33 (114.45–165.61) | 137.05 (106.48–167.92) | 0.524 |
| Fibrinogen (g/L) | 3.06 (2.73–3.39) | 3.04 (2.69–3.53) | 0.624 |
| Albumin (g/L) | 46.95 (45.15–48.55) | 47.10 (45.30–48.50) | 0.915 |
| FAR | 0.07 (0.06–0.07) | 0.07 (0.06–0.07) | 0.562 |
| SII | 447.74 (347.98–617.94) | 446.90 (329.69–642.29) | 0.980 |
Data are presented as median (interquartile range). CBC, complete blood count; RIF, recurrent implantation failure; BMI, body mass index; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet; RDW, red cell distribution width; PDW, platelet distribution width; MPV, mean platelet volume; PCT, plateletcrit; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte; FAR, fibrinogen to albumin; SII, systemic immune-inflammation index.
The RIF group had significantly lower PLT, PCT and lymphocyte counts than the
control group (p = 0.03, p = 0.019 and p = 0.012,
respectively) (Table 1). The ROC analysis showed that PLT had a sensitivity of
48.6% and a specificity of 66.4% with a cutoff value of 271.5 (AUC: 0.575); PCT
had a sensitivity of 77% and a specificity of 38.9% with a cutoff value of
0.245 (AUC: 0.575); and lymphocyte count had a sensitivity of 49.3% and a
specificity of 71% with a cutoff value of 2.015 (AUC: 0.577) for predicting RIF
(Fig. 2). The multivariant ROC analysis revealed a cutoff value of 0.508 with a
sensitivity of 70.3% and a specificity of 48.9% (AUC: 0.599) (p =
0.004) (Fig. 3). There were no significant differences between the RIF and
control groups in terms of other CBC parameters, including the WBC count, RBC
count, HGB level, HCT level, MCV, MCH level, MCHC, MPV, RDW, PDW, neutrophil
count, NLR and SII, or coagulation parameters, such as fibrinogen levels, the FAR
and albumin levels (p
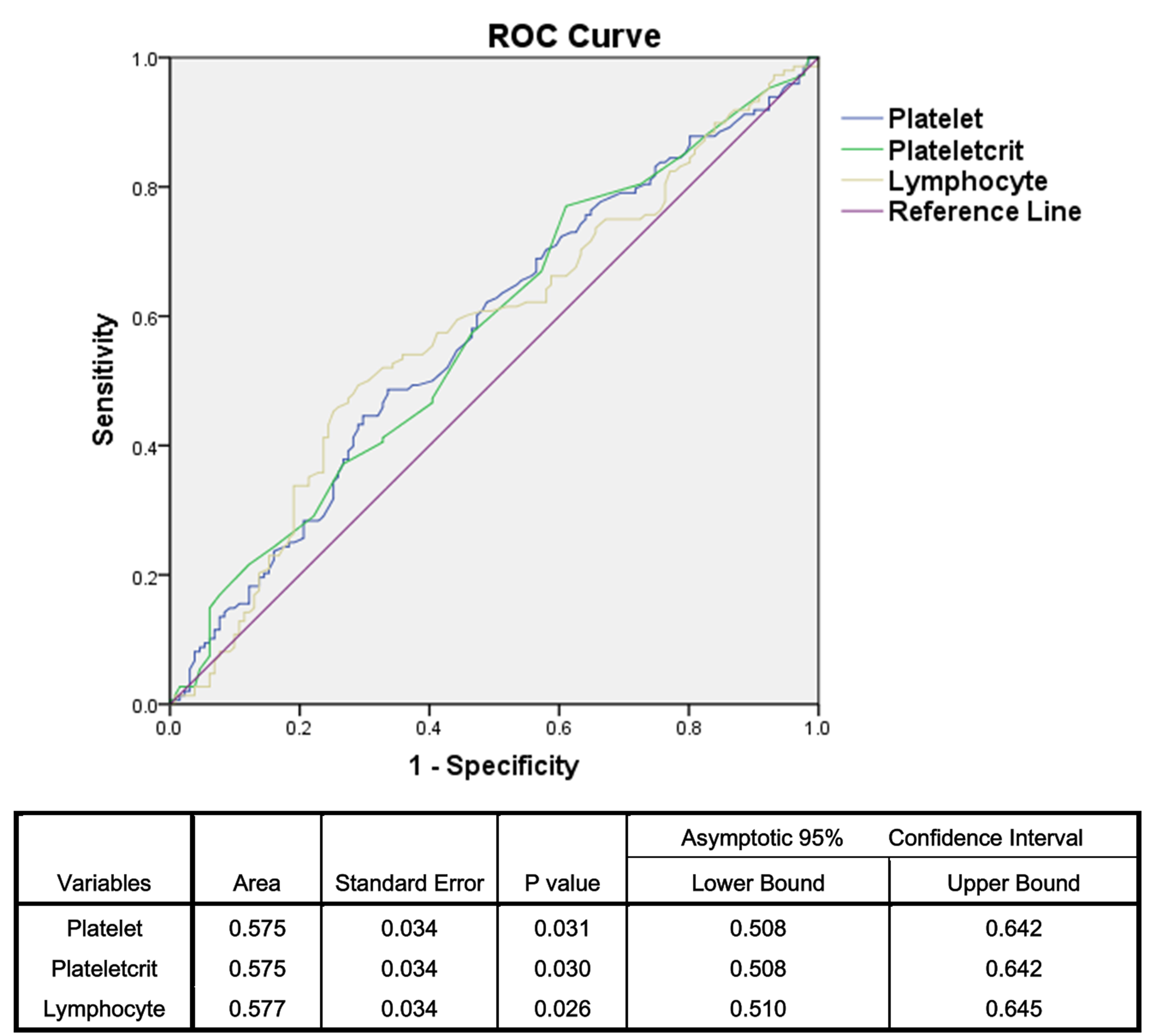 Fig. 2.
Fig. 2.Sensitivity and specificity of the platelet, plateletcrit and lymphocyte counts. ROC, receiver operator characteristic.
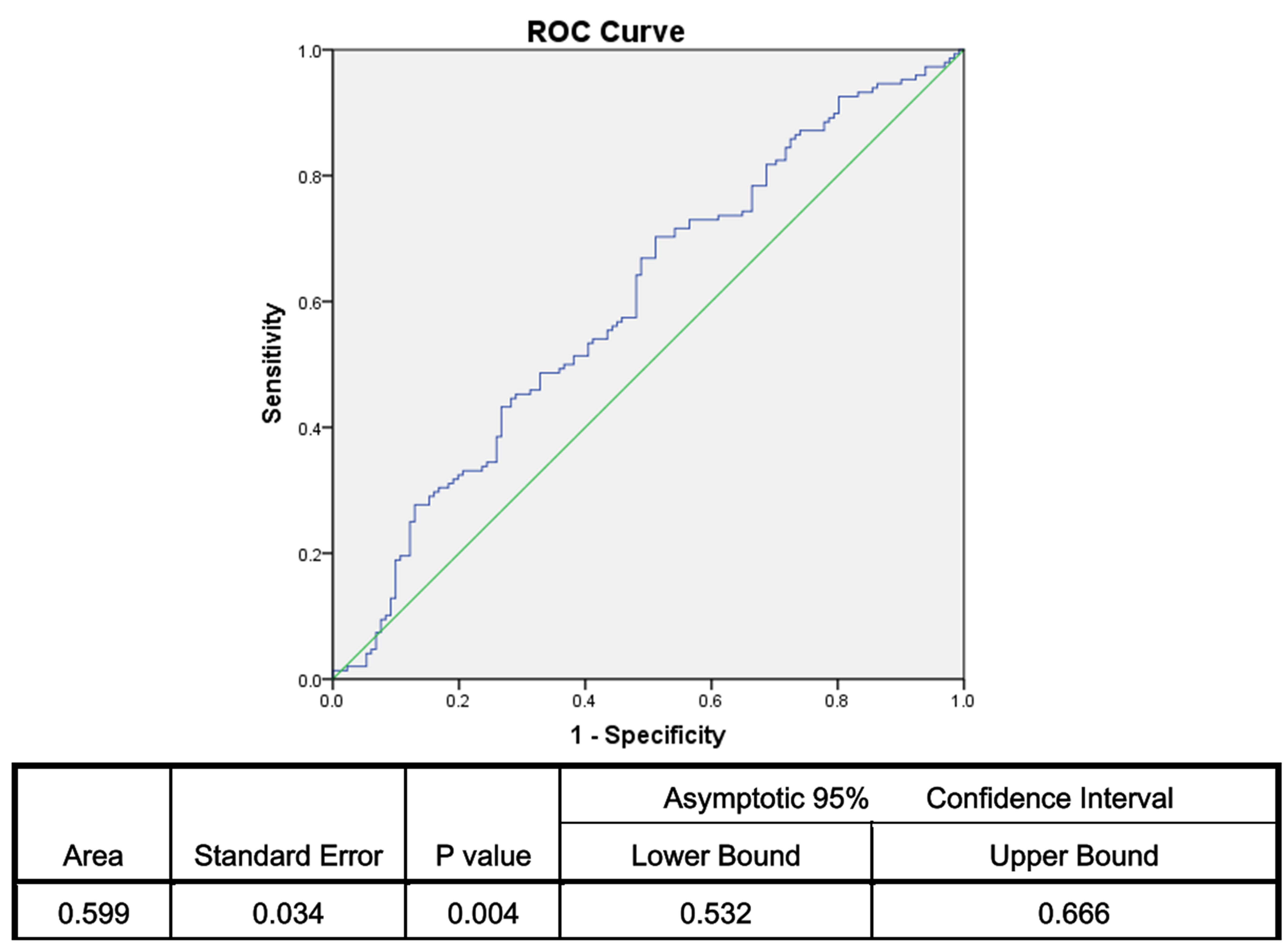 Fig. 3.
Fig. 3.Sensitivity and specificity of multivariant ROC of the platelet, plateletcrit and lymphocyte counts. ROC, receiver operator characteristic.
In this study, we found that PLT, PCT and lymphocyte counts were significantly lower in RIF patients than in women who had a live birth after the first ET. However, these parameters were not effective markers for predicting RIF risk.
Successful embryo implantation requires a favorable endometrium, good-quality embryos and delicate coordination between the embryo and endometrium [19]. The tightly controlled inflammatory response in the window of receptivity is essential for successful implantation and growing evidence suggests that chronic inflammation is associated with RIF [20, 21, 22]. In the pathogenesis of RIF, inflammation and coagulation disorders are suggested to have important roles, since immune hyperactivity damages trophoblast cells by increasing the inflammatory uterine environment and fibrin deposition affects endometrial blood flow, both of which adversely affect reproductive outcomes [3, 23].
Aynıoglu et al. [24] reported that lymphocyte counts were
significantly higher in patients with recurrent pregnancy loss than in controls.
However, experimental research on animals demonstrated that heifers with
low-grade inflammation (lymphocyte count
PCT is one of the PLT indices which shows the percentage of blood occupied by platelets and is thought to be a potential marker of inflammation, which is significantly negatively related to the degree of inflammation [9]. Studies have shown that activated platelets allow the delivery of bioactive molecules to target tissues at high concentrations, resulting in the stimulation of angiogenes [27]. A double-blind randomized controlled trial demonstrated that the intrauterine infusion of platelet-rich plasma could improve the implantation rates in women with a history of RIF [28]. Therefore, certain PLT and PCT levels are necessary for the successful implantation of embryos and low PLT and PCT levels as present in the CBC may lead to an excessive inflammatory reaction and angiogenesis disorder from the blood system to the endometrial environment, which is not suitable for embryo implantation. We showed that PLT and PCT counts were dramatically decreased in patients with RIF. However, some studies reported that the PLT and PCT counts were significantly higher in patients with recurrent pregnancy loss as compared to controls [24]. This may be because RIF and recurrent abortion occur at different time periods and their inflammatory mechanisms are different. However, as the AUC was less than 0.7 [26], PLT and PCT counts may not effective markers for predicting RIF risk.
A recent study assessed the value of inflammatory hematological markers such as the WBC count, NLR, MPV, and PDW and concluded that these markers were not effective in predicting IVF success [29]. Another study found that none of the inflammatory markers, including the WBC count, neutrophil count and the FAR, as demonstrated by the CBC were predictive of clinical pregnancy, take-home baby, and clinical and biochemical abortion rates among nonobese patients with unexplained infertility [16]. Additionally, the RBC count, HGB level, HCT level, MCV, MCH level, and MCHC were statistically similar in pregnant patients, women with a history of recurrent pregnancy loss and healthy controls [30]. In accordance with these studies, our data also showed that the WBC count, RBC count, HGB level, HCT level, MCV, MCH level, MCHC, RDW, PDW, MPV, neutrophil count and NLR were not effective parameters for predicting RIF.
The SII is a new inflammatory marker that has been reported to better reflect systemic inflammation than the NLR alone in many diseases [31, 32], but it has never been studied in people with RIF. In the current study, no predictive value of the SII for RIF was found, which may be due to the fact that the systemic immune-inflammation of peripheral blood is not evident in patients with RIF.
The prethrombotic state is a state of hypercoagulability, which can lead to embryo implantation failure [33]. Recent studies seem to indicate a close relationship between the prethrombotic state and unexplained RIF [3, 34]. Kuroda et al. [35, 36] reported that 20%–30% of patients with RIF had thrombophilia and they believed that the treatment of thrombophilia is a predictive factor for RIF. A recent review suggested that the optimization of thyroid function, thrombophilia, immunity, and uterine milieu treatment strategy can improve pregnancy outcomes in women with RIF, except for advanced age women with embryonic factor-induced reproductive failure [37]. Moreover, it was found that patients with RIF have reduced plasma fibrinolytic potential [38]. Recent retrospective studies found a significantly elevated level of fibrinogen in Chinese women with RIF or unexplained recurrent pregnancy loss as compared to controls (2.65% vs. 2.47%) [3, 39]. However, fibrinogen and albumin levels and the FAR were similar in the two groups in the present study. Although the sample size of the current study is the largest thus far, the fibrinogen content and the FAR among RIF patients need further investigation.
The limitations of our study include the use of single-center data and the small-scale retrospective design. To minimize the risk of bias in the control patient selection, we strictly followed the inclusion and exclusion criteria. Despite these limitations, this was the first study using PLT, PCT and lymphocyte count as markers to determine their associations with RIF.
A decline in PLT, PCT and lymphocyte counts were found to be associated with RIF. However, these parameters were not effective markers for predicting RIF risk. Our findings should be supported by further prospective studies involving a larger population to clarify the relationship between these blood cell function markers and RIF.
The raw data generated in this study are available upon reasonable request from the corresponding authors.
Conceptualization: TX, HH, JO. Data curation: XL, TX. Formal analysis and investigation: XL, TX. Methodology: TX, HH, JO. Resources: TX, XL. Supervision: HH, JO. Writing – original draft: XL, TX. Writing – review & editing: HH, JO, TX. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Institutional Review Board of the Third Affiliated Hospital of Sun Yat-sen University [2022]02-084-01. All patients in this study signed informed consent forms to undergo IVF/ICSI treatments in our center, and the anonymity of the patients has been strictly protected.
Not applicable.
This project was supported by the Medical Scientific Research Foundation of Guangdong Province of China (Grant No.: A2020575).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



