1 Department of Maternal and Child Health and Urological Sciences, Sapienza University of Rome, Policlinico Umberto I, 00161 Rome, Italy
2 Department of Gynecologic Oncology, IRCCS National Cancer Institute, 20133 Milan, Italy
3 Obstetrics and Gynecological Unit, Department of Woman's and Child's Health, San Camillo-Forlanini Hospital, 00152 Rome, Italy
4 Department of Medical and Surgical Sciences and Translational Medicine, PhD Course in “Translational Medicine and Oncology”, Sapienza University of Rome, 00185 Rome, Italy
5 Unit of Gynecology, Department of Surgical and Medical Sciences and Translational Medicine, Sant'Andrea Hospital, Sapienza University of Rome, 00189 Rome, Italy
Abstract
Objectives: Until 2018, cervical cancer (CC) was clinically staged; however, it was frequently under-staged. For this reason, in 2018, the International Federation of Gynecology and Obstetrics (FIGO) incorporated the imaging assessment into the staging of this malignancy. The aim of this review is to discuss available data regarding the role of imaging in the diagnosis, pretreatment staging, and how an adequate radiological evaluation could assist in the treatment planning for CC. Mechanism: An extensive literature search was conducted to identify relevant studies across various databases, including articles addressing topics related to imaging used in CC. The selected articles underwent thorough examination and evaluation to identify studies that met the objectives of this review, taking into account the specified inclusion and exclusion criteria. Subsequently, relevant data were extracted and analyzed for each article. Findings in Brief: Transvaginal ultrasound (TVS) and transrectal ultrasound (TRUS) have been shown to be accurate diagnostic tools to assessing the local spread of CC disease. Currently, magnetic resonance imaging (MRI) appears to offer the highest sensitivity, specificity, and accuracy in detecting parametrial and stromal invasion, as well as tumor size. Computed tomography (CT) and contrast-enhanced (CE)-CT are considered the best imaging modalities for the detection of lymph node metastases. However, positron emission tomography (PET) has demonstrated notable precision and exhibited high negative predictive value in predicting the pelvic nodal status during the early-stage diagnosis of CC diagnosis. Radiomics represents a newly introduced field of translational research with the potential to predict several clinically and pathological relevant variables in cervical carcinoma patients. These variables include disease staging, histological type, lymph node status, probability of recurrence, and estimated survival. Conclusions: Imaging plays an indispensable role in diagnosis, tumor staging, and monitoring the evolution of pathology in response to therapies over time. It provides physicians with the indispensable tool for optimal regulation of therapeutic strategy.
Keywords
- cervical cancer
- staging ultrasound
- MRI
- PET-CT
- radiomics
- gynecological oncology
Cervical cancer (CC), according to the Global Cancer Statistics of 2020, remains the second most-diagnosed cancer and the fourth leading cause of cancer death among women. It is estimated that there were 604,000 new cases and 342,000 deaths worldwide from CC in 2020 [1].
Until the revision of staging by the International Federation of Gynecology and Obstetrics (FIGO) in 2018 [2], CC was clinically staged based on gynecologic examination, colposcopy with biopsy, and, if necessary, cystoscopy or proctoscopy [3]. However, the underestimation of the tumor extent occurred in approximately one-third of early-stage disease cases and up to two-thirds in advanced disease cases, representing a significant limitation of clinical-staging examination [4, 5]. Assessing the craniocaudal extension of tumor volume, parametrial involvement, and pelvic lymph node metastasis remains a challenge during clinical examination. However, evaluating these parameters is crucial for determining the optimal therapeutic approach for patients, including options such as neoadjuvant chemotherapy (NACT), radical surgery, and/or external radiotherapy. For this reason, imaging and pathology assessments were incorporated in the recently revised FIGO staging system (2018) of CC, recognizing the prognostic significance of nodal status and tumor size.
The main diagnostic methods used for CC include magnetic resonance imaging (MRI), ultrasound (US), including transvaginal US (TVS) or transrectal US (TRUS), computed tomography (CT), positron emission tomography (PET), as well as combined PET-CT and PET-MRI.
The introduction of MRI has been recommended for detecting tumor size, defining the depth of stromal invasion, assessing parametrial invasion, and evaluating pelvic node involvement [6]. Furthermore, scientific evidence indicates that TVS or TRUS could be capable staging techniques for assessing the local extension of the disease [7]. Indeed, data available in literature demonstrated that TVS and TRUS are both highly accurate, with results comparable to those obtained through MRI in evaluating the local extension of the disease [8, 9, 10]. In addition, the US offers potential advantages such as being faster, less expensive, more readily accessible, and requiring no patient preparation before the exam, in comparison to MRI. On the other hand, CT and PET were adopted for the assessment of metastatic CCs [11, 12]. The aim of this review is to discuss the available data regarding the role of imaging in diagnosis, pretreatment staging, and how an adequate radiological evaluation could help assessing the treatment planning of CC.
In August 2023, the present narrative review was performed. Different authors performed an extensive literature search to identify relevant trials across various databases, including MEDLINE, Embase, PubMed, and Cochrane. We selected all articles meeting the following key criteria: “cervical cancer”, “cervical cancer staging”, “ultrasound cervical cancer”, “magnetic resonance imaging”, “computed tomography (CT)”, “positron emission tomography (PET-CT) “, “radiotherapy cervical cancer”, “image-guided brachytherapy in cervical cancer”.
No filter on the year of publication was applied. The selected articles underwent rigorous review and evaluation to identify studies that potentially meet the aims of this review.
Key criteria for inclusion were: (1) articles in English, (2) original studies addressing the role of radiological imaging in CC, (3) studies evaluating the limitations and strengths of various radiological imaging in staging CC, (4) studies comparing different radiological techniques in staging of CCs patients.
Regarding studies examining the role of PET-CT and PET-MRI, no specific selection criteria were applied for the radiotracers used.
Letters, editorials, and case reports were excluded from this review. The studies that met the inclusion criteria were further analyzed, and relevant data were extracted and analyzed for each paper. Any discrepancies between the investigators were resolved through a consensus approach.
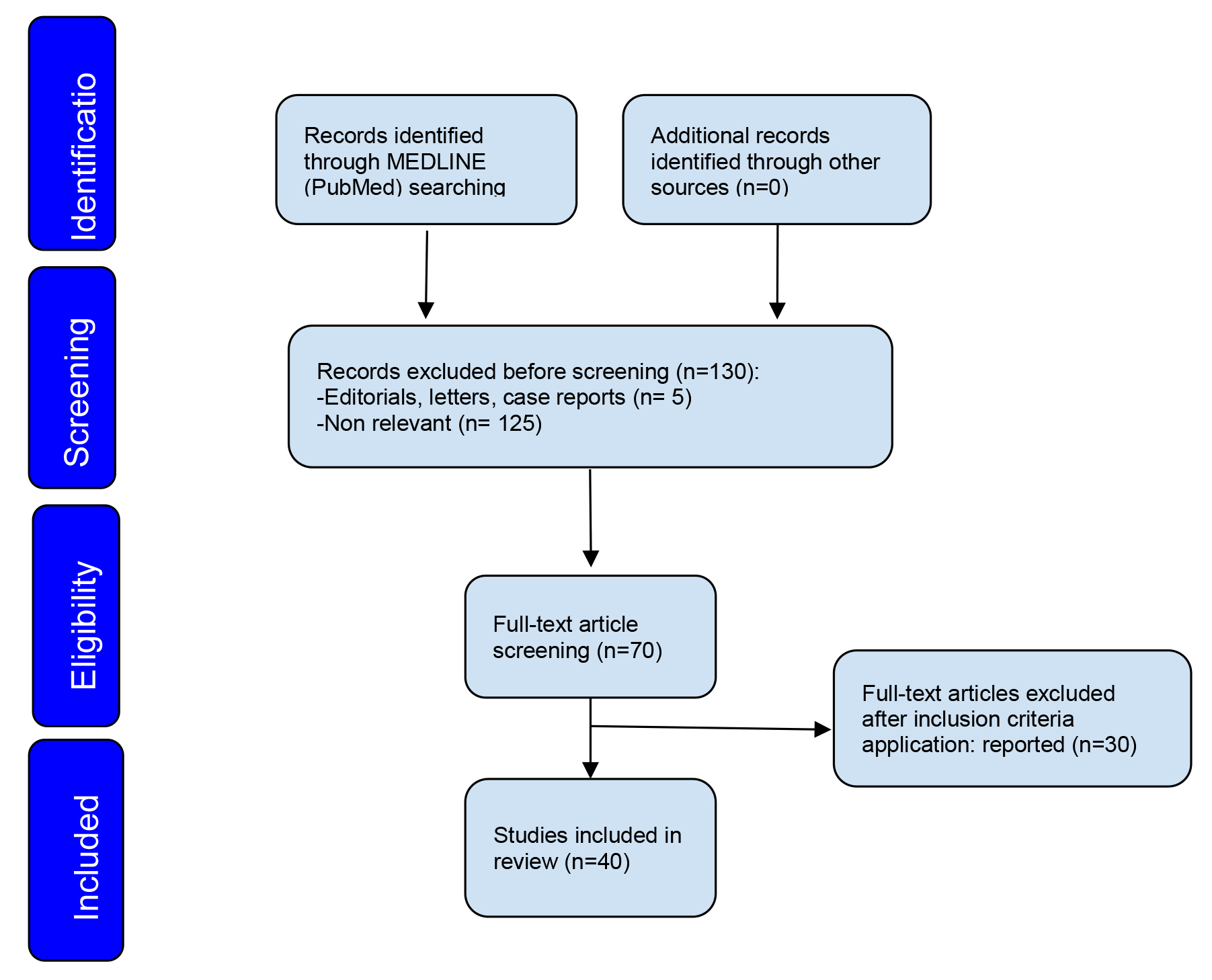
A search of the MEDLINE (PubMed) database resulted in 130 relevant articles. The search was narrowed to article between 1998 and 2023. A total of 70 materials were initially identified to be potentially relevant for the review. Finally, 40 articles were included, and they were found to match the inclusion criteria [9, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51].
All the included studies related to PET-CT and PET-MRI utilized
 Fig. 1.
Fig. 1.Flow diagram illustrating the study selection process.
In recent years, there has been a growing interest in the utilization of US for
diagnosis and preoperative staging of CC. The CC tissue is typically represented
as hyper- or isoechoic with respect to the surrounding stroma. The use of a
transrectal probe is an optional choice in case of bulky, exophytic and necrotic
friable tissue to reduce the risk of bleeding and to limit artifacts related to
the presence of blood and clots. Fischerova et al. [8], analyzed 95 CC
patients with TRUS and demonstrated that TRUS had good sensitivity, specificity,
and accuracy for tumor detection of 93.4%, 94.7%, and 93.7%, respectively.
Similar results have also been confirmed regarding the detection of small tumors
(
The parametrial infiltration is presented as an irregular extension of hypoechogenic prominences into the paracervical tissue. A prospective multicenter trial of 209 patients with CC demonstrated excellent agreement between US findings and pathology for detecting deep stromal invasion and parametrial invasion [10].
Finally, Alcazar et al. [14] in a meta-analysis including 9 studies, demonstrated not only high sensitivity and specificity for the diagnosis of parametrial infiltration, but also non-inferiority to MRI.
Recent evidence compared the detection power between US and MRI in the evaluation of these CC prognostic factors. Findings demonstrated similar accuracy, sensitivity, and specificity between the two radiological approaches, except for the detection of residual tumors following conization [9, 10]. The superiority of the US accuracy in revealing residual disease after treatment compared to MRI is still debated. Pinkavova et al. [15], in a prospective study of 42 women with locally advanced CC histologically treated with NACT, demonstrated a lower sensitivity of TVUS/TRUS than MRI in detecting residual tumors.
Additionally, TVUS and TRUS, which establish with high accuracy the distance from the tumor to the cervical canal and the remaining cervical length after conization, could make an important contribution to determining the eligibility of CC patients for the fertility-sparing approach. In the future, intraoperative US could help the surgeon in determining the level of excision during conization by ensuring a tumor-free cervix length for women desiring pregnancy. Moreover, a relevant study evaluated the utility of the US in predicting the response to NACT in terms of tumor volume reduction and lesion vascularization (VI) reduction [16].
The potential of 3D power Doppler US in predicting response to chemotherapy was also investigated in another study. This study concluded that the flow index (FI) is the only marker useful in predicting both clinical and histological responses to chemotherapy in patients with laparoscopic approach to locally advances cervical cancer (LACC), with values higher observed in responders compared to non-responders [17].
In addition, Alcázar and Jurado [18], using Doppler examination, tested possible variations in vascular indices and showed an increase of the pulsatility and resistance index in patients with a response to chemotherapy.
Given the evidence, considering the role of US in diagnostic study and in assessing the local spread of CC may be reasonable. Moreover, the US is faster, cheaper, and readily available, requiring no patient preparation compared to other radiologic imaging techniques. Therefore, the ultrasonography in CC demonstrated good accuracy in tumor detection and in assessing tumor size and the local extent of disease. US imaging is characterized by its low cost and high reproducibility. However, disadvantages may be related to limited penetration of deep tissue and dependence on operator experience, in contrast to other no-operator related techniques, such as MRI or CT.
MRI is the preferred imaging modality due to its capability to provide comprehensive insights into the anatomical morphology and specific characteristics of CC. This diagnostic tool plays an important role in the diagnosis and formulation of therapeutic strategies. MRI is extensively applied in the staging process, allowing for precise determination of the tumor’s size and enabling a meticulous estimate of tumor spread into the adjacent parametrial tissues and location within the cervix. Moreover, MRI is indispensable for the examination of pelvic lymph nodes, identifying minimal variation of dimension, form, or another anomaly. MRI also plays a crucial role in monitoring the efficacy of treatments by assessing the tumor’s response to ongoing therapeutic interventions [19].
The high-resolution T2-weighted images (T2WI) in MRI are commonly used for detection and analysis of cervical carcinoma [19, 20].
The measurement of the tumor must be done on all three orthogonal planes to effectively determine the largest diameter. The possible therapeutic strategies depend on the maximum size of the tumor, which is determined through this fundamental process. The main imaging techniques most widely used are dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI). The first involves the use of a gadolinium-based contrast agent to obtain detailed information on tissue perfusion, while the second generates contrast in images by measuring the diffusion of water in tissues [20, 21].
In DCE-MRI, smaller tumors typically exhibit a uniform enhancement pattern, while larger tumors frequently display necrotic areas and demonstrate variations in enhancement levels [20]. In clinical scenarios where a uterine tumor mass is identified, and uncertain clinical or histological assessments, the use of MRI could help to differentiate primary CC from primary endometrial cancer. This differentiation is most pertinent in cases where the lesion involves both the endometrium and the endocervix [22].
DWI represents a functional imaging modality based on the contrast disparity arising from the stochastic movement of water molecules within tissues. Although challenges associated with motion and artifacts have historically limited its use in abdominopelvic imaging, advancements in techniques have significantly facilitated data acquisition faster and reduced artifact occurrence.
Cervical tumors display restricted diffusion, leading to increased signal intensity in both the primary tumor and metastatic lymph nodes in DWI modality. Moreover, CC presents markedly lower apparent diffusion coefficient (ADC) values compared to normal cervical tissue [23, 24].
Central to the pretreatment evaluation of CC is the assessment of cervical stroma integrity. This appears as a hypointense ring on T2WI in the para-axial plane. The main sign of stromal infiltration is the presence of a T2-hyperintense image within the hypointense ring.
MRI can detect the superficial and deep invasion of the cervical stroma by the tumor, with good sensitivity (81%), specificity (95%) and accuracy (93%).
Multiple studies have provided compelling evidence of MRI’s high accuracy (88%) and high negative predictive value (94–95%) in detecting parametrial invasion. Parametrial involvement can be identified by the absence of integrity in the interface between the cervix and parametrial fat, as well as by observing irregular and spiculated cervical-parametrial interface in early infiltration, or by the presence of tissue mass within the parametrial fat in cases of deep infiltration [25]. In the study by Woo et al. [26], which analyzed more than 1000 patients, MRI demonstrated notable effectiveness in the detection of parametrial invasion in CC is highlighted.
Data available in the literature highlight conflicting results regarding the accuracy of detecting lymph node metastasis in CC patients [27, 28]. MRI detects additional suspicious characteristics such as rounded shape, nonuniform signal intensity, and spiculated margins, which could enhance the sensitivity and specificity of MRI in detecting advanced CC. Indeed, as reported in recent studies, sensitivity, specificity, and accuracy of conventional MRI present among 80%, 84%, and 40–50%, respectively [28].
Mitchell et al. [29], in a study involving 208 patients with biopsy-proven invasive CC who underwent MRI and CT before attempted curative radical hysterectomy, demonstrated that MRI findings could help predict the presence of histologic lymph node involvement in these patients. This offers valuable prognostic information, particularly in the decision-making process regarding fertility-sparing approach.
MRI in CC offers advantages, including superior soft tissue resolution, the ability to assess the local stage of the disease, and the lack of ionizing radiation. Furthermore, the MRI is the radiological technique with a highest negative predictive value in detecting parametrial invasion, which is a crucial prognostic factor influencing both prognosis and the appropriate therapeutic management of these patients. However, disadvantages may include its high cost, the need for immobile patients during the examination for a long time, and the possible need for contrast medium, which can lead to adverse reactions in some individuals. In addition, MRI methodology may not provide optimal accuracy in evaluating the extent of the retroperitoneal disease, often requiring the use of complementary imaging approaches.
CT and CE-CT have long been considered inferior to MRI and TVS/TRUS, especially in local staging, primarily because of their lower contrast resolution [30, 31]. Cervical tumors typically appear as iso-dense on CT imaging compared to adjacent structures, regardless of their size. In order to detect parametrial invasion on CT, the observations of soft tissue density projections extending into the fatty parametrial tissue can serve as a useful indirect sign in the evaluating CC. In 2021, Zhu et al. [30] conducted a study involving 196 CC patients and found that CT accuracy in detecting vaginal invasion, uterine invasion, bladder invasion and cervical invasion were 55%, 41.6%, 66.6%, and 20%, respectively. These values were lower compared to the accuracy obtained with MRI, PET-CT, and PET-MRI. Consequently, the principal limitations of CT and CE-CT lie in defining pelvic cervical tumor extension. With suboptimal sensitivity (14% and 31%) and specificity (55% and 58%) in detecting parametrial invasion and pelvic lymph node metastasis, respectively [32, 33, 34, 35], the guidelines from the European Society of Gynecological Oncology (ESGO), the European Society of Radiotherapy and Oncology (ESTRO), and the European Society of Pathology (ESP) recommend CT (or PET-CT) for the detecting lymph node metastases and distant spread in locally advanced CC or in early-stage disease with suspicious lymph nodes on imaging [36].
Despite the controversy over the relative sensitivity and specificity of CT in detecting distant metastatic lymph nodes, in the investigation conducted by Choi et al. [37], CT imaging showed an overall sensitivity of 50% and a specificity of 92%. Simultaneously, PET imaging alone or in combination with CT (PET-CT) recorded sensitivity values of 82% and 95%, respectively. On the other hand, MRI exhibited a sensitivity of 56% and a specificity of 91%. In summary, CT showed an overall inferior diagnostic performance compared to PET, PET-CT, or MRI in identifying metastatic lymph nodes in CC patients [37].
CT imaging in CC allows for rapid and comprehensive evaluation, with a good resolution of anatomical structures, and the ability to identify any retroperitoneal and distant metastases. However, limitations include suboptimal ability to define the extent of pelvic tumors, with poor sensitivity in analyzing soft pelvic tissues and these disease-related features. In addition, X-ray exposure may be a concern, particularly in women of childbearing age.
PET-CT is an advanced medical imaging technique that combines two types of
imaging: PET and CT. PET-CT is used to determine the extent of a gynecologic
tumor, such as CC, and to evaluate whether the cancer has spread to other parts
of the body, such as lymph nodes or distant organs. PET-CT can be used to
evaluate how the tumor is responding to therapy. After treatment, PET-CT can be
used to detect any recurrence of gynecological cancer [32, 34, 38, 39, 40]. PET-CT uses
a small amount of a radioactive substance, such as
PET-CT has demonstrated notable precision in diagnosing early-stage CC and
exhibited a substantial negative predictive value in anticipating the pelvic
nodal status in early-stage CC. The accuracy of PET-CT for early-stage cervical
carcinoma was 95.45% [43]. The negative predictive value of PET-CT imaging in
predicting pelvic nodal involvement was 93.75% [44].
In early-stage CC, the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) may indicate the presence of lymph node metastasis [47]. Notably, the absence of pathological cervical uptake on PET-CT could be a promising prognostic factor [48]. Additionally, considering a recent meta-analysis, PET-CT has been shown to be a valuable method for evaluating recurrent CC [49].
PET-CT in CC has good sensitivity in early detecting metabolically active lesions, even when small, by globally analyzing body areas. The combination of two types of imaging allows for overcoming diagnostic doubts related to each of the two methods, thereby optimizing the sensitivity, specificity, and accuracy in evaluating the disease in abdominal, retroperitoneal, and distant regions, as well as detecting tumor responds to therapy. High cost, radiation exposure, and use of radiotracers, as well as the needs to use other imaging modalities to assess local extension, are major limitations.
PET-MRI is an innovative medical imaging technique that combines two distinctive imaging modalities: PET and MRI. This conjunction allows high-resolution data on the morphology and physiology of biological tissues to be acquired in a single imaging session, producing a multi-parametric imaging picture. An important benefit associated with the use of this methodology is the lower exposure of the patient to ionizing radiation, in contrast to other imaging procedures such as PET-CT. This new method does not differ from MRI in the correct identification of tumor T-stage. However, in detecting positive lymph nodes in patients with CC, PET-MRI exhibited higher sensitivity, specificity and accuracy of compared to MRI alone. Moreover, this success of PET-MRI was also found in the evaluation of distant metastases. This finding has a positive impact on treatment planning, allowing for better-individualized treatment for the patient. Unfortunately, due to the lengthy time required to complete the examination, which can take up to an hour and a half, this radiological technique is reserved only in selected cases, especially for the detection of distant metastases [47].
The great potential of PET-MRI is strengthened by the investigation conducted by Grueneisen et al. [50]. In this research, the imaging technique in question was able to determine the disease grade T in all 27 patients examined and accurately detect the occurrence of regional metastases in 80% of the cases, as well as and non-regional lymph node metastases in 100% of the patients [50].
Other studies confirm these results, where sensitivity, specificity, and accuracy for nodal metastasis were 92.3%, 88.2%, and 90.0% for fused PET-MRI and PET/CE-CT; 84.6%, 94.1%, and 90.0% for non-fused PET-MRI; and 69.2%, 100%, and 86.7% for MRI [51].
In addition, it is possible to use PET-MRI biomarkers such as the minimum and
average ADC (ADCmin and ADCmean, respectively), maximum standardized uptake value
(SUVmax), MTV, and TLG to obtain meaningful prognostic information in CC
patients. This association was highlighted in a prospective analysis conducted on
a sample of 54 patients, where expert radiologists measured specific parameters,
including ADCmin and ADCmean, maximum SUVmax, MTV, and TLG in primary tumors. The
results of this analysis demonstrated, at a statistically significant level
(p
By providing metabolic and morphologic information in the same examination, PET-MRI offers advantages over PET-CT, including better pelvic anatomic resolution, ensuring good sensitivity and specify, as well as improved detection of small lesions. The lower exposure to ionizing radiation determines the safest radiological modality. However, limitations are related to high cost, limited availability in clinical practice, and the long examination time compared to other imaging modalities.
Radiomics represents a recently emerging field of translational research that focuses on establishing networks between quantitative information derived from radiological imaging and clinical data. The objective of innovative methods is to provide valuable support for optimal clinical decision-making. It is a methodological approach based on mathematical and statistical analyses for acquiring information from diagnostic images within the medical domain. This methodology holds the potential to predicts several clinically relevant variables in cervical carcinoma patients, including disease staging, histological tumor type, regional lymph node status, probability of recurrence, and estimated survival.
Through the application of radiomics, it is feasible to generate nomograms and derive RAD (radiomics) scores, enabling predictions regarding the invasion of the lymphovascular space. A study by Xiao et al. [53] demonstrated the feasibility of this methodology by developing a radiomic nomogram using multi-parametric MRI in patients with early-stage cervical carcinoma. This approach facilitates therapeutic decision-making for the physician and offers a significant advantage in the management of patients with this disease [53].
The high predictive efficacy and strong stability of nomograms in predicting recurrence and disease-free survival were evaluated in a retrospective study conducted in a sample of 115 patients with locally advanced cervical carcinoma. Specifically, the analysis revealed that the area under the curve (AUC) in the context of the predictive model for cervical carcinoma recurrence was 0.977, while the AUC for the predictive model of disease-free survival at 1, 3 and 5 years was 0.895, 0.888 and 0.916, respectively. These results tangibly highlight the significant intrinsic clinical value of radiomic nomograms predicting recurrence and disease-free survival in patients with advanced cervical carcinoma following their exposure to concomitant chemoradiation therapy [54].
Radiomics could also help in the lymph node metastases detection [55]. The predictive model for lymph node metastases achieved an AUC of 0.95 in the training group and an AUC of 0.88 in the test group, indicating significant potential for discriminating lymph node metastasis expression through a non-invasive approach.
Radiomic analysis enables the extraction of quantitative and qualitative features from radiological images, allowing a more detailed tumor tissues characterization. Radiomics in CC management could contribute to tailored treatment and improve prognosis. However, the significant lack of homogeneity in the software used among the various centers, requires standardization of procedures and validation of developed radiomic models.
The 2018 FIGO staging system integrates information on tumor size and lymph node involvement, which are important prognostic determinants in the context of CC. The primary purpose of cervical imaging is to provide anatomical and functional data in order to facilitate accurate diagnosis and a highly effective treatment strategy. A major obstacle in clinical examination is the underdiagnosis of patients, which arises from inaccurately assessing parameters such as the invasiveness of lateral structures and the presence of lymph node metastases. Such scenario could result in unnecessary surgery, the patient might benefit more from primary therapy based on chemoradiation therapy.
The high accuracy by contrast-use, multiparametric complexity, and multiplanar versatility of MRI configure it as the optimal imaging modality for the study of CC. Considering the remarkable consistency between MRI findings and histologic examination specimens, MRI currently serves as the main radiological imaging modality for the assessment of tumor size and local extent of CC, demonstrating superior results in term of accuracy, sensitivity, and specificity.
Tumor size analysis plays a crucial role in the preoperative setting. As
highlighted by Kato et al. [56], patients with tumor size
In particular, for locally advanced CC, prediction of lymph node involvement on the basis of lymph node diameter detected by MRI shows moderate sensitivity (41.7%) and high specificity (88.4%) when a threshold value of 17.0 mm is adopted [58]. Furthermore, in a recent meta-analysis involving 1021 patients, Shen et al. [28] demonstrated an improvement in sensitivity and specificity of 86% and 84%, respectively, in detecting pelvic lymph metastases with the use of DWI-MRI [59]. Advances in MRI technology have optimized the definition of the characteristics of CC, establishing it as a highly effective diagnostic methodology capable to providing accurate assessments of the specific peculiarities of the malignancy.
Although MRI is the gold standard for assessing the T-factor of tumors, PET with whole-body CT, where both imaging techniques, PET and CT, are performed simultaneously on an integrated PET-CT scanner, is the preferred modality for the detection of lymph node metastases in the context of CC. PET may not be able to detect small lesions, resulting in false negative due to the limited spatial resolution of small lesions [60]. Therefore, PET-CT plays a complementary role with MRI in analyzing the local extent of disease [61].
Several radioactive tracer options can be used to detect metabolic activity,
including
Despite the wide range of imaging methodologies available, there has been a significant increase in the development and adoption of radiomics. However, it is imperative to emphasize that radiomics is still an evolving field that requires further investigation and validation before it can be fully integrated into routine clinical practice. In this context, a meta-analysis conducted on a sample of over 2000 patients revealed that the ADC parameters exhibit considerable clinical potential. They are more readily available and commonly utilized, although they show a non-statistically significant tendency to outperform radiomic analysis [64]. Bizzarri et al. [65] showed the valuable contribution of the radiomic method in predicting pathological and oncological outcomes, especially when integrated with clinical variables. These interesting results could help clinicians to assess personalized treatment options for each patient with CC [65].
In recent years, although artificial intelligence has made significant progress in the field of precision oncology, relatively less attention has been paid to the gynecological field. The implementation of this methodology could play a decisive role in enhancing the screening process for CC through image analysis, allowing the detection of abnormal cells with greater accuracy and efficiency compared to human analysis [66, 67, 68].
The main strengths of this study lie in the aggregation of the most recent scientific contributions in the literature concerning the use of imaging in the context of CC. This study performs a detailed analysis of the existing data, highlighting the essential role played by the imaging approach in the diagnosis, staging, and planning of treatment strategies. It should be noted, however, that the present study examined various types of research, albeit not all of them, which is one of its limitations. Indeed, our work can be considered a comprehensive review that encompasses the main methodologies used in the field of CC imaging. Future prospects look highly promising, with numerous research and development directions showing the potential to lead to significant improvements in the management of this disease. The present study could provide an important basis for further investigations, in particular randomized studies, aimed at comparing different imaging techniques. The aim of such studies will be to establish an optimal imaging approach that not only meets the requirements of localized phase disease but is also adequate assesses distant metastatic lesions.
In conclusion, while the staging system primarily remains rooted in the clinical approach, the use of advanced radiological techniques emerges as a useful component in preoperative evaluation. This practice aims to precisely direct patients toward the most appropriate treatment plan. The extraordinary ability of these radiological techniques to accurately identify the extent of the primary lesion and the presence of any metastases directly influences the selection of treatment options, which may range from surgery to radiotherapy and chemotherapy.
Finally, imaging plays an indispensable role in supervising the evolution of pathology in response to therapies over time, providing physicians with the indispensable tool for optimal regulation of therapeutic strategy.
In parallel, radiomics is emerging as a revolutionary new field destined to chart the course of precision medicine. Radiomics grants physicians the ability to accurately predict the stage of the disease, the histological subtype of the tumor, the analysis of the status of regional lymph nodes, the risk of recurrence, as well as the estimation of survival. Its main objective is to create a tailor-made therapeutic approach for each individual patient, promoting personalized treatment based on specific radiomic data.
Drafted and edited the review, and analyzed data: EDA, NT, IC. Made substantial contributions to conception and design of the study: AG, VDD, GP, GB. Performed data acquisition: TGD’A, AM, RA, ODO. Data mining, analysis and presentation: LM. Edited the review: All authors. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Ludovico Muzii and Andrea Giannini are serving as Editorial Board members and Ilaria Cuccu, Ottavia D’Oria, Violante Di Donato and Andrea Giannini are serving as Guest editors of this journal. We declare that Ludovico Muzii, Ilaria Cuccu, Ottavia D’Oria, Violante Di Donato and Andrea Giannini had no involvement in the peer review of this article and have no access to information. Full responsibility for the editorial process for this article was delegated to Michael H. Dahan.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.