1 Korean Mother-Safe Counselling Center, Pregnancy and Breastfeeding Medicines Information Center, 10380 Goyang, Republic of Korea
2 Department of Obstetrics and Gynecology, Inje University Ilsan Paik Hospital, 10380 Goyang, Republic of Korea
3 Department of Obstetrics and Gynecology, CHA Gangnam Medical Center, CHA University, 13496 Seoul, Republic of Korea
4 Division of Biology and Public Health, Mokwon University, 35349 Daejeon, Republic of Korea
Abstract
Background: Despite South Korea’s Ministry of Food and Drug Safety
(MFDS) risk management program (RMP) since June 2019, isotretinoin prescriptions
have surged 2.5 times from 0.39 million in 2017 to 0.97 million in 2021. This
study assesses pregnancy termination risk perception and termination following
periconception isotretinoin exposure in South Korea. Methods: A dataset
of 1785 pregnancies from January 2001 to September 2022 was collected by Korean
Mother Safe Counselling Center, which is a call center for teratogenicity
information. A semi-structured questionnaire was sent to 1107 women to evaluate
the rate of pregnancy termination, trends of their risk of perceptive
malformation and termination of pregnancy following isotretinoin exposure in
periconception before and after getting the counselling on teratogenic risk from
our center. Results: There were 317 respondents from the total 1107
women (28.6%). The termination rate was 29.0% (92 out of 317). The perception
of major malformation risk decreased from 77.1%
Keywords
- isotretinoin
- teratogens
- pregnancy
- termination of pregnancy
- maternal exposure
Isotretinoin (13-cis-retinoic acid) is commonly used to treat acne worldwide, including North America, the European Union, and Korea [1, 2, 3]. Isotretinoin was originally approved by the United States FDA (Food and Drug Administration) for treatment of cystic and recalcitrant acne in 1982, with pregnancy category X for human fetal risk [4]. Isotretinoin was first approved in Korea by the Ministry of Food and Drug Safety in 1997. Since then, the Nimegen Soft Capsule (10 mg isotretinoin) has been publically available [5]. The number of prescriptions for it in Korea was 0.39 million in 2016 according to the Korean Health Insurance Review and Assessment Service [3]. In 2021 there were 0.97 million prescriptions identified in the electronic medical record systems, according to the unpublished report of Ministry of Food and Drug Safety (MFDS), presented at the Retinoid Risk Management Improvement Council meeting November 2022. This 2.5 times increase in prescriptions for isotretinoin occurred despite the MFDS having launched a risk management program (RMP) for the cautious use of isotretinoin in 2019.
There have been several reports on the teratogenicity of isotretinoin. On and Zeichner [6] reported that the use of isotretinoin during pregnancy may result in congenital anomalies in ~20–30% of exposed fetuses. These anomalies include brain, cardiovascular, thymus and some miscellaneous anomalies such as limb reduction [7].
Drawing from both animal and human research, it has been suggested that teratogenicity arises from a shared mechanism, potentially involving factors that inhibit normal activity and the interactive effects on neural crest cells [8].
Retinoic acid receptors and retinoid X receptors, which are grouped in nuclear ligand-induced receptors, have exerted important roles in retinoid teratogenicity. It has been suggested that Hox genes expression causes the adverse effects of retinoic acid on the development of neural crest [9].
However, our recent findings indicate that there is no notable contrast in neonatal outcomes, such as major malformations, between pregnant individuals who were exposed to isotretinoin from the periconception period and those who were not exposed [10]. Additionally, our systematic review and meta-analysis showed that 85% of the fetuses exposed to isotretinoin were likely to be delivered without major malformations [11].
Besides the issues of malformations following isotretinoin exposure in the periconception period, another serious adverse health problem is the high rate of pregnancy termination. In Canada, France and Berlin, the pregnancy termination rates of women who became pregnant while on isotretinoin were 45%, 40% and 76%, respectively [12, 13, 14]. A study in the Korean population reported that the pregnancy termination rate was 26.6% [15]. In Korea, isotretinoin exposure during pregnancy is a problematic and complex reproductive health issue, encompassing birth defects and termination of pregnancy. This is due to the fear of its teratogenicity, and it’s over prescription, even though the RMP for isotretinoin was introduced in 2019. At the Korean Mother Safe Counselling Center, 1.43% (120/8394) of the patients were exposed to isotretinoin as the introduction of the isotretinoin RMP has not changed this exposure rate [16]. Despite the ongoing debate, the introduction of iPLEDGE’s (https://ipledgeprogram.com/#Main) rigorous contraceptive protocol in 2006 has been autonomously linked to a reduction in pregnancies. The yearly count of isotretinoin prescriptions remained consistent before and after iPLEDGE’s implementation, indicating a genuine decline in reported pregnancies compared to prescriptions [17].
We aimed to evaluate the risk perception of pregnancy termination, major malformation before and after getting the counselling on teratogenic risk from our center and the rate of pregnancy termination following periconception exposure of isotretinoin.
Our study utilized data in the Korean Mother Safe database on pregnant women exposed to isotretinoin who voluntarily contacted the Mother Safe Call Center to seek information after the use of various medicines during pregnancy (http://mothersafe.co.kr/).
This study encompassed a cohort of 1785 pregnant individuals who were exposed to isotretinoin during the periconceptional period, enrolled in a database from January 2001 to September 2022. Of this group, 1107 women were selected after eliminating those with no valid contact information. Since they had voluntarily contacted Korean Mother Safe Counselling Center, which is a call center for teratogenicity information, to seek information on the teratogenicity of isotretinoin exposure during this period, a follow-up study was conducted to examine pregnancy outcomes including the rate of pregnancy termination.
Information was available on teratogenic risk of isotretinoin according to their
exposure time such as preimplantation (
A semi-structured questionnaire was sent to the women to evaluate pregnancy outcomes including spontaneous abortion, pregnancy termination, gestational age, birth weight, structural birth defects, and neurodevelopmental delay. Furthermore, the questionnaire includes respondents’ perception of the risk for congenital malformations and the likelihood of considering termination of pregnancy before and after teratogen counselling during the observation period using a visual analog scale (VAS). As shown in Fig. 1 (Ref. [18]), the VAS for perception of the risk for major malformations ranged from 0% to 100%, whereas the scale for consideration of termination of pregnancy ranged from 0 to 10. Thus, the higher the score, the greater the perceived risk or likelihood.
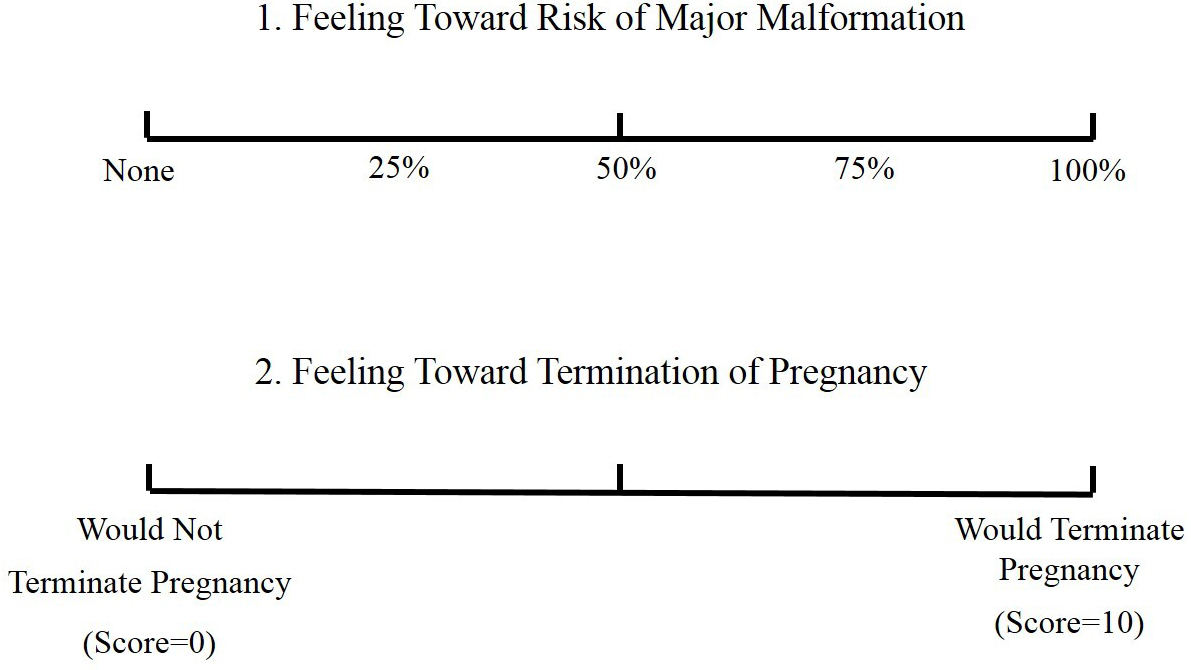 Fig. 1.
Fig. 1.Visual analog scale. Reproduced with permission from American Journal of Obstetrics and Gynecology; published by Elsevier, 1989 [18].
The questionnaire’s final response rate was 28.6% (n = 317).
The assessment of the risk perception regarding major congenital malformations
and the propensity to contemplate pregnancy termination before and after
teratogen counseling among pregnant women exposed to isotretinoin was evaluated
utilizing the paired t-test. A p-value
In total, 317 respondents were included in the analysis. The mean age was 29.4
years (19–46), gravidity 1.3 (1–8), and body mass index (BMI) 20.0 kg/m
| Variable | Mean (SD) | Range | n (%) | |
| General information | ||||
| Age (years) | 29.4 (4.4) | 19.0–46.0 | - | |
| Gravidity | 1.3 (0.7) | 1.0–8.0 | - | |
| Parity | 0.1 (0.4) | 0.0–3.0 | - | |
| Spontaneous abortion (%) | 0.0 (0.2) | 0.0–2.0 | - | |
| Induced abortion (%) | 0.1 (0.4) | 0.0–7.0 | - | |
| Body mass index (kg/m |
20.0 (2.2) | 14.9–28.8 | - | |
| Co-exposures | ||||
| Number of other medications | 1.1 (2.4) | 0.0–14.0 | - | |
| Alcohol | - | - | 184 (16.6) | |
| Cigarette smoking | - | - | 37 (3.3) | |
| Radiation | - | - | 30 (2.7) | |
| Isotretinoin exposure information | ||||
| First exposure in prohibited period (days) |
3.8 (28.2) | –28–99 | - | |
| Last exposure (days) |
21.5 (22.5) | –28–110 | - | |
| Dose/day (mg) | 10.7 (3.0) | 10–40 | - | |
| Total cumulative dose (mg) | 252 (232) | 10–1650 | - | |
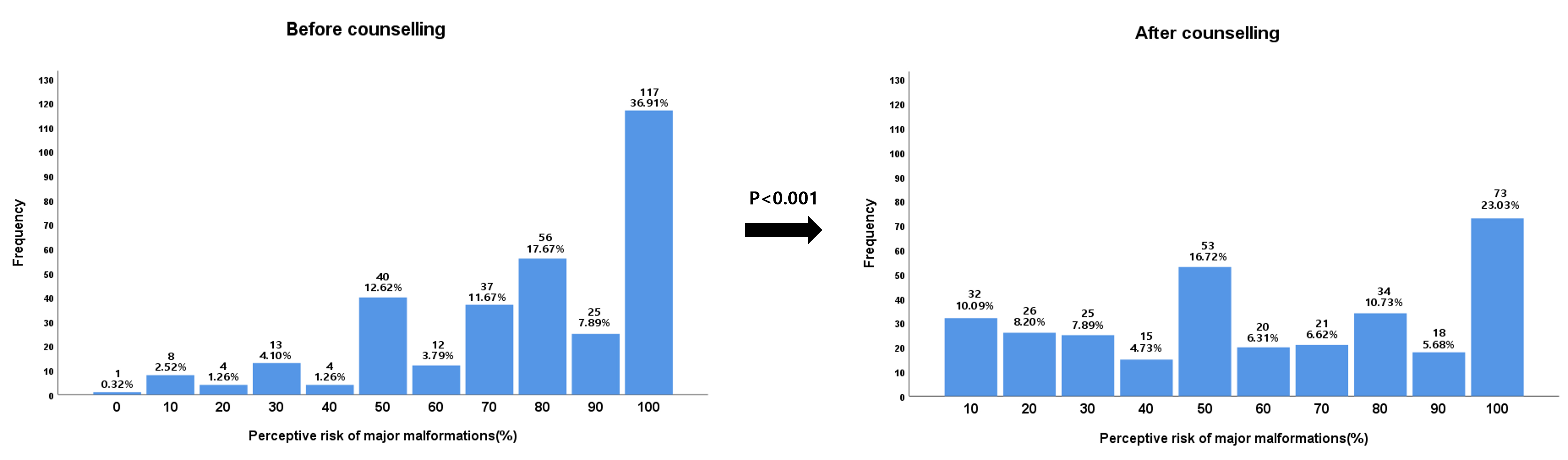
Pregnant patients exposed to isotretinoin assigned themselves a risk of 77.1%
(standard deviation [SD]: 24.5%) for major malformations before counselling and
60.4% (SD: 31.0%) after counselling (p
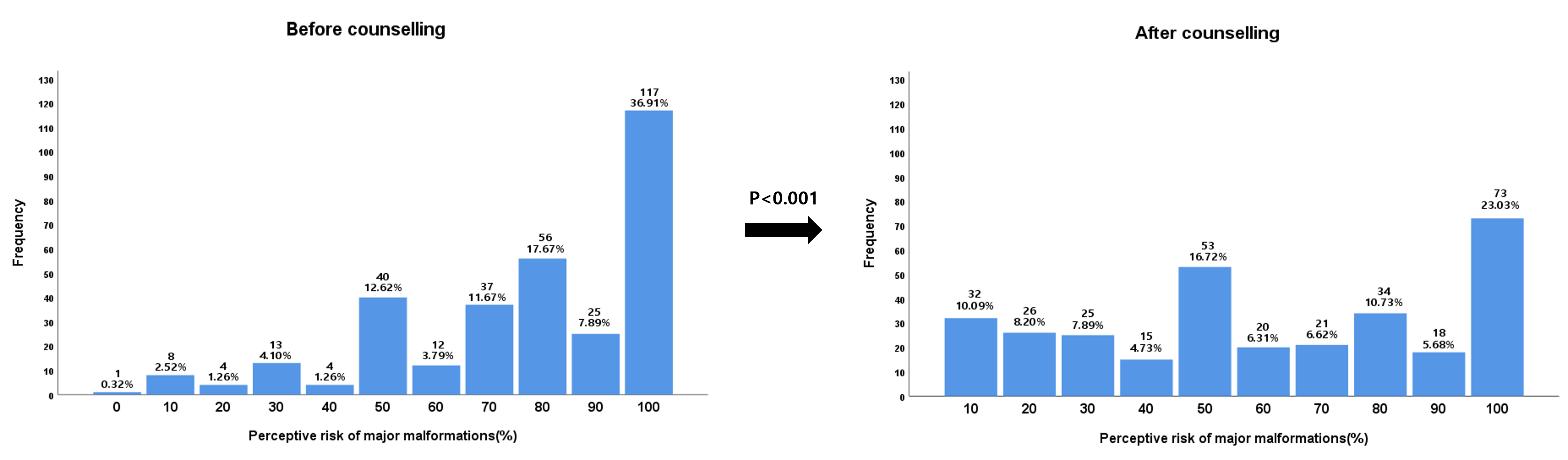 Fig. 2.
Fig. 2.The perception of the risk of malformation after isotretinoin exposure during pregnancy before and after teratogenicity counselling.
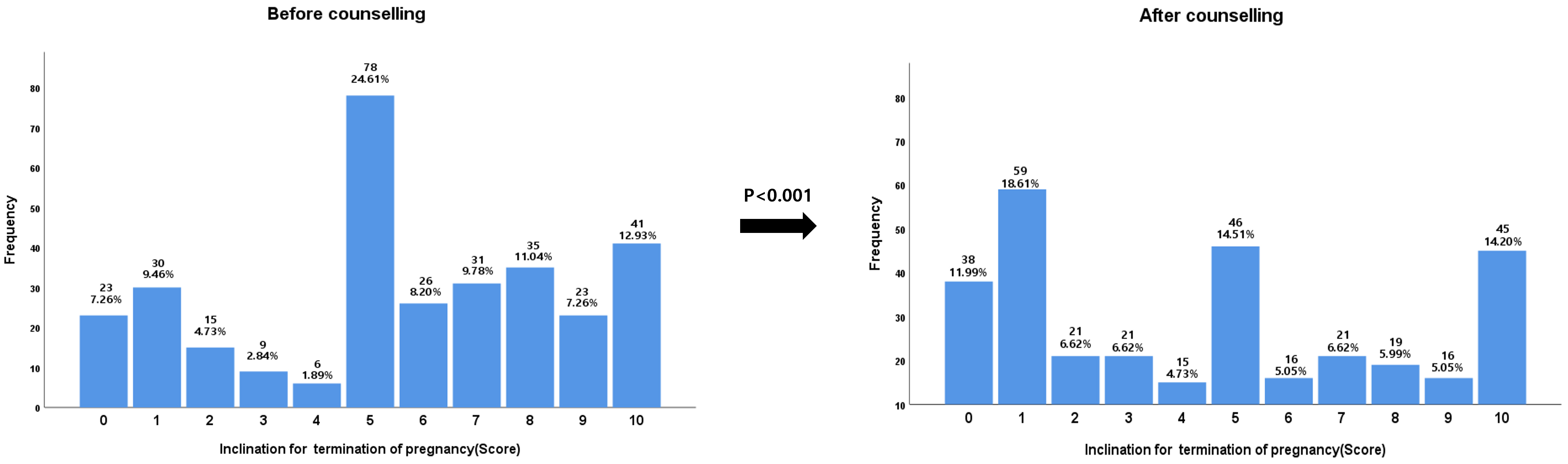 Fig. 3.
Fig. 3.The inclination for termination of pregnancy following isotretinoin exposure during pregnancy before and after teratogenicity counselling.
As shown in Table 2, the rate of termination of pregnancy was 29.0% (92/317).
| Pregnancy progress | Frequency | Percent |
| Continuing pregnancy | 214 | 67.5 |
| Spontaneous abortion | 11 | 3.5 |
| Termination of pregnancy | 92 | 29.0 |
| Total | 317 | 100.0 |
Table 3 displays other pregnancy outcomes including congenital malformations, preterm birth, low birth weight, and neurodevelopmental delay.
| Variables | Mean (SD) | Range | % (n) | |
| Birth outcomes | ||||
| Delivery weeks of gestation | 38.6 (1.37) | 32.0–42.0 | - | |
| Birth weight (kg) | 3.1 |
2.0–4.1 | - | |
| Preterm birth ( |
- | - | 4.7 (10) | |
| Low birth weight ( |
- | - | 6.5 (14) | |
| *Congenital malformations (%) | - | - | 0.9 (2) | |
| Neurodevelopmental outcomes | ||||
| Period of follow up (months) | 41.5 |
0.5–180.0 | - | |
| Neurodevelopmental delay | - | - | 4.7 (10) | |
| **Suspected autism spectrum disorder | - | - | 1.9 (4) | |
* Hydrocephalus (1), biliary obstruction (1). ** Interaction with others - about 5 years and language-about 2–3 years delay (1), interaction with others - about 5 years delay (1), interaction with others - about 3 years delay (1), and interaction with others - about 2–3 years delay (1).
On average, the gestational age at delivery was 38.6 weeks (range 32 to 42 weeks), with a mean birth weight of 3.1 kg (range 2.0 to 4.1 kg). The incidence of preterm birth was 4.2 while 58% of infants were born with low birth weight.
Among the 214 infants examined, 2 (0.9%) were identified to have major malformations, comprising cases of hydrocephalus (1) and biliary obstruction (1). With an average follow-up duration of 41.5 months post-birth (range 0.5 to 180 months), 10 out of 214 infants (4.7%) exhibited neurodevelopmental delays, with 4 infants (1.9%) displaying symptoms suggestive of autism spectrum disorder.
Our study found significant reduction in the perception of the risk of congenital malformations and the inclination to terminate the pregnancy after our teratogen counselling in women exposed to isotretinoin during pregnancy. However, the risk is still high. The resultant rate of termination of pregnancy was approximately 30%.
Our previous study showed that the termination rate was 1.1% (12/1068) among Korean pregnant women who non-exposed to isotretinoin [20].
The perception of the risk for major malformations and the inclination to
pregnancy termination in this study can be compared with a previous report by
Koren et al. [18]. The perception of the risk for major malformation
when exposed to isotretinoin is 3.2 times higher than those of pregnant patients
exposed to agents not known to be teratogenic (77.1
In this study, we did not evaluate the effect of advice on determination of termination of pregnancy when in their pregnancy the exposure occurred.
According to our previous report, the average rate of pregnancy termination
after isotretinoin exposure is 30.1%. The rate of pregnancy termination was
different according to exposure time in the periconception period:
The frequency of pregnancy terminations in Canada, France and the Berlin far exceeds that in Korea. In these nations, 45% [12], 40% [13], 76% [14] of women who conceived while using isotretinoin opted for termination of their pregnancies, respectively.
The Korean RMP was implemented in 2019 to prevent pregnancy during isotretinoin use. However, compliance with our RMP was poor. In our previous study, 1.43% (120/8394) of patients at the Korean Mother Safe Counselling Center were exposed to isotretinoin, with this proportion remaining stable after RMP implementation. Additionally, during the study duration, the average rate of physician-provided information on isotretinoin’s teratogenicity, obtaining acknowledgment (by signature) of knowledge regarding its teratogenic nature, guidance on effective contraception, administration of a pre-isotretinoin pregnancy test, and dispensing the drug within seven days were 84.7%, 15.3%, 39.8%, 0.8%, and 99.2%, respectively. Compliance with the RMP recommendations did not exhibit any discernible trends or alterations during the 12-month post-implementation period [16].
Conversely, according to the US FDA, there were 6740 reported pregnancies among women exposed to isotretinoin from 1997 to 2017, with a peak of 768 out of 117,784 pregnancies (0.65%) occurring at the initiation of the iPLEDGE program in 2006. Since then, rates of pregnancies, abortions, and fetal defects associated with isotretinoin have decreased. However, it’s essential to interpret the decline in isotretinoin-related pregnancies within the broader context of national pregnancy and contraception practices.
We suggest that the Korean RMP should consider implementing more stringent measures, such as those in the iPLEDGE program, for the effective prevention of pregnancy exposure to isotretinoin [17].
Our data showed that other pregnancy outcomes, including the rates of spontaneous abortion, preterm birth, low birth weight, and congenital malformation, were not significantly different from pregnancy outcomes of the general population. Additionally, there were 4 children with suspected autism spectrum disorder, 1.9% (4/214).
According to findings by Adams and Lammer [22, 23], a subgroup of children,
consisting of 44 who were exposed to isotretinoin and 40 control subjects,
underwent longitudinal follow-up to assess their neuropsychological profiles. At
the age of five, 13.6% (6 out of 44) of the exposed children were observed to
function within the mildly or moderately impaired or delayed range of general
intelligence (
Additionally, Alay et al. [24] stated that the risk of neurocognitive disorder after isotretinoin exposure in pregnancy is 30%.
This abnormal neurodevelopment is consistent with observations from studies in neurobehavioral teratology involving rodents exposed to retinoid [25].
Olson and Mello [26] reviewed studies that have implicated retinoid in learning and memory deficits of post-embryonic and adult rodent and songbird models. The studies revealed significant insights into the role of vitamin A in maintaining neuronal plasticity and cognitive function in adulthood.
Our study had some limitations. First, the collected data were based on self-reported outcomes, particularly neurodevelopmental delays. In addition, the response rate was 28.6% (317 out of 1107 women). In other words, about 70% fail to the response may be associated with several reasons including change of phone number, and uncomfortable of uninterested in the questionnaire regardless their pregnancy outcomes. The low rate of response may make undetected bias, such as those women who were more likely to terminate the pregnancy or those women with children with malformations may not have been as willing to respond to the questionnaire. Therefore, our findings cannot be generalized. Second, the perceived risk of congenital malformations and inclination to terminate pregnancy termination before and after counselling was biased due to incomplete recollection. Lastly, our long-term observational study did not include a comparative analysis of pregnant patients unexposed to isotretinoin. Therefore, these findings should be interpreted with caution. Despite these limitations, this study has several strengths and implications. The major strength of our study was the source of the data; pregnant patients exposed to isotretinoin during the periconception period, prospectively enrolled at Korean Mother Safe Database over more than 20 years. Our data demonstrated that the rate of perception of risk for major malformations and likelihood of consideration of pregnancy termination in the women exposed to isotretinoin during pregnancy even though they received teratogen counselling was still high. Hence, the termination rate of pregnancy was approximately 30%.
Our findings confirm that isotretinoin exposure during pregnancy increases the perception of the risk of major malformations and the likelihood of pregnancy termination due to fear of teratogenicity even after teratogen counselling. As a result, periconception isotretinoin exposure results in a high rate of pregnancy termination, therefore, the RMP for isotretinoin exposure should be strictly applied in Korea.
The dataset used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
YRL, DWH: acquired patient data, drafted the manuscript, created figures and tables; JYH & HYK: conception, design, statistical analysis, responsible for the accuracy and integrity of the work presented here; JWK & JSC: statistical analysis, created figures and tables; YAK & KCC: interpretation of data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final version of the manuscript.
This study was conducted with ethical approval from the Institution Review Board (IRB) of Ilsan Paik Hospital, Inje University College of Medicine (ISPAIK 2020-04-013-001). All participants provided their written informed consent after receiving a complete explanation of the study protocol.
We would like to express our gratitude to all those who helped us during the writing of this manuscript and the study participants.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.