1 Department of Family Planning, Women's hospital, School of Medicine, Zhejiang University, 310006 Hangzhou, Zhejiang, China
Abstract
Background: This study aimed to evaluate the effectiveness of different
treatments for cesarean scar pregnancy (CSP) and to identify key factors
influencing treatment selection, in order to help standardize CSP management.
Methods: We retrospectively analyzed data from 220 CSP patients at the
Family Planning Department of the Women’s Hospital, School of Medicine, Zhejiang
University, from January 2019 to December 2019, adhering to the Strengthening the
Reporting of Observational studies in Epidemiology (STROBE) guidelines. Treatment
methods included dilation and curettage (D&C), curettage after uterine artery
embolization (UAE+C), hysteroscopy plus curettage (H/S+C), H/S+C following UAE
(UAE+H/S+C), and hysteroscopy combined with laparoscopic resection (L/S+H/S). We
assessed treatment outcomes by evaluating the normalization of serum
Keywords
- cesarean scar pregnancy
- uterine artery embolization
- curettage
- hysteroscopy
- laparoscopy
- surgery
Cesarean scar pregnancy (CSP) occurs when an embryo implants in the lower uterus at the site of a previous cesarean section (CS) scar, affecting women with a history of CS [1]. It represents a specific type of ectopic pregnancy and is considered a long-term complication of CS. Globally, CS rates have been increasing, with over 30% of deliveries conducted via CS in 15 countries as of 2008. In China, the CS rate has consistently remained high, reaching 34.9% according to the 2014 National Maternal and Child Health Annual Report [2]. Due to persistently high CS rates, resulting in the likelihood of experiencing CSP in subsequent pregnancies for women with a history of CS estimated at 1 in 531 [3], and factors such as the relaxation of birth policies, the incidence of CSP in China is on a clear upward trend.
Due to the lack of large-scale studies on the outcomes of continuing pregnancies in patients with CSP, accurately classifying these patients remains challenging. As the pregnancy progresses, the risks of spontaneous bleeding, placental implantation, and uterine rupture significantly increase, especially during the mid to late stages. These complications can require a hysterectomy, eliminating the chance of future pregnancies and potentially endangering the patient’s life. Therefore, it is widely recommended to terminate a CSP as soon as it is diagnosed [4].
To date, no standardized treatment protocol exists for CSP. Treatment plans are
typically tailored based on the patient’s overall condition, including clinical
symptoms, gestational age (GA), serum
This study adopts a retrospective design. Considering the nature of the retrospective approach and the anonymization of patient data, approval (IRB-20220061-R) for the study protocol has been granted by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University. Consequently, the necessity of obtaining informed consent from individual patients has been waived. This exemption is due to the low risk posed to participants by the retrospective analysis of anonymized data, which ensures that patient confidentiality and privacy are maintained.
We adhered to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guidelines [7] for our methodology, ensuring comprehensive and transparent reporting of our study design and findings.
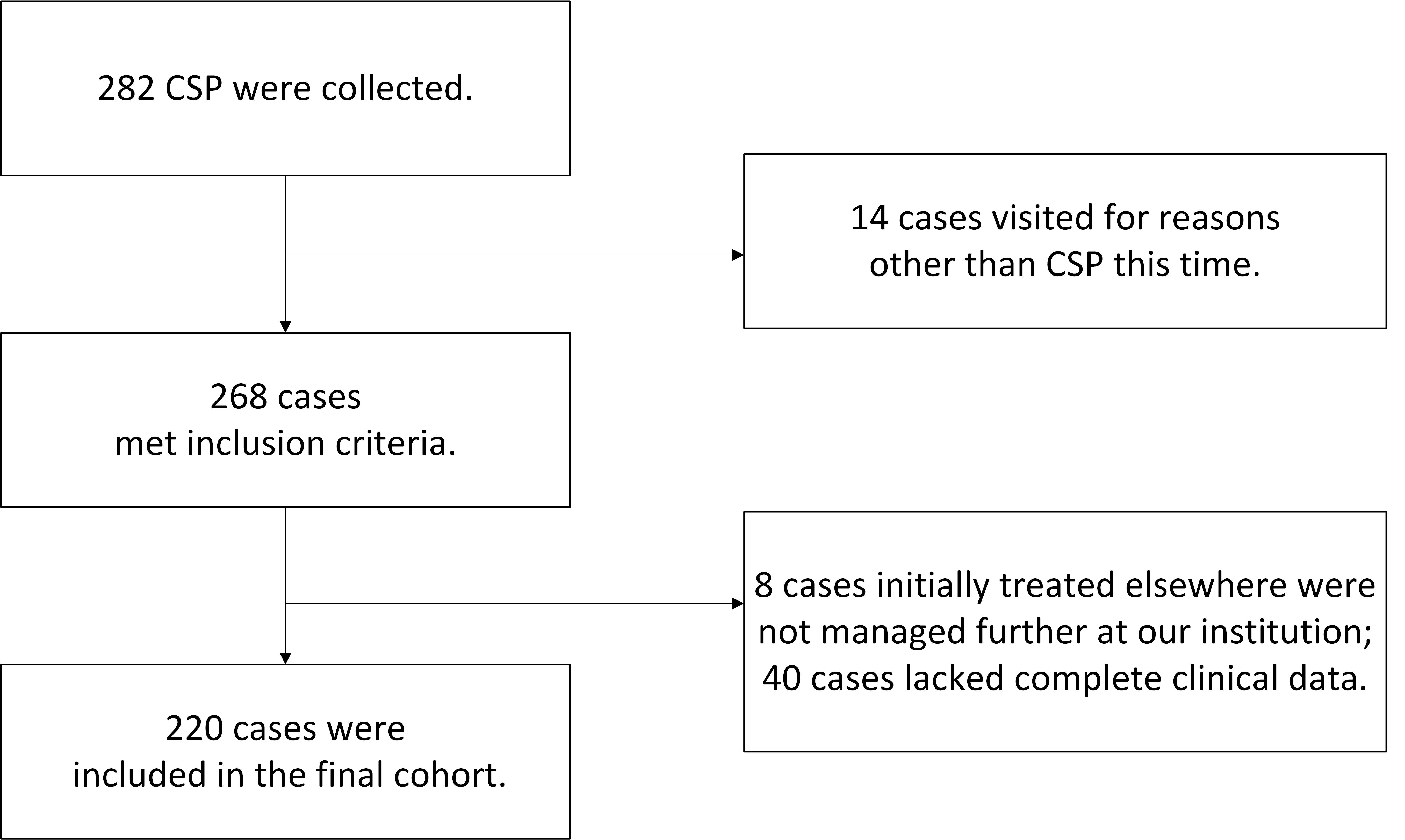
A total of 282 first-trimester patients diagnosed with CSP were initially identified using the keyword ‘CSP’ from the hospital records database at the Family Planning Department of Women’s Hospital, School of Medicine, Zhejiang University, covering the period from 1st January 2019 to 31st December, 2019. After applying the inclusion and exclusion criteria, 220 patients were ultimately included in the study (Fig. 1).
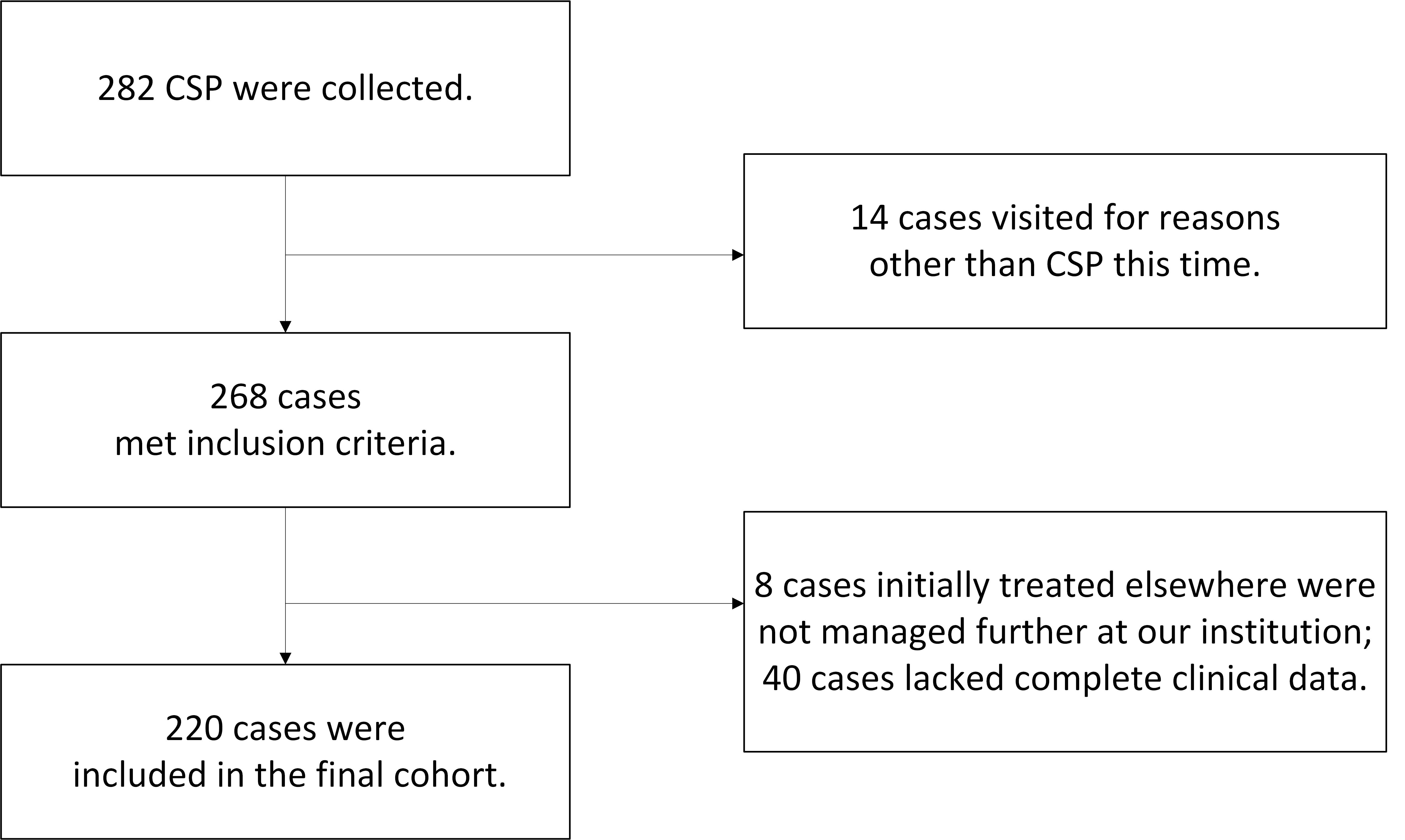 Fig. 1.
Fig. 1.Study flowchart. CSP, cesarean scar pregnancy.
The inclusion criteria were: (1) a history of cesarean delivery, (2) a history of amenorrhea and a positive pregnancy test, (3) a color Doppler pelvic ultrasound (WS80A, manufactured by Samsung Medison Co., Ltd., Kangwon-do, South Korea) indicating a uterine scar pregnancy, based on the diagnostic criteria recommended by Timor-Tritsch et al. [8], (4) postoperative pathology confirming the presence of chorionic villi.
The exclusion criteria included: (1) lack of complete clinical data, (2) patients initially treated elsewhere and not managed further at our institution.
The patients were categorized into three types based on the “Expert Consensus on
the Diagnosis and Treatment of CSP 2016”, formulated by the Family Planning Group
of the Chinese Medical Association Obstetrics and Gynecology Branch [9]. Type Ⅰ:
(1) the gestational sac (GS) partially implants in the uterine scar; (2) the
myometrial layer between the GS and the bladder becomes thin, with a thickness
The study collected data by reviewing medical records, including information on
maternal age, gravidity, parity, previous D&C, previous CSP, CS frequency,
interval since the last CS, GA, presenting symptoms, gestational sac diameter
(GSD), embryonic length (EL), fetal heartbeat, myometrial thickness, serum
The treatment approach for CSP primarily relies on the attending physician’s expert opinion and may include D&C, UAE+C, H/S+C, UAE+H/S+C, or L/S+H/S. The objective of treatment is to effectively manage CSP, minimize associated trauma, and preserve fertility.
Patients scheduled for uterine artery embolization (UAE) were transferred to a radiological intervention treatment center. Experienced radiologists performed super-selective UAE using gelatin sponge powder containing 60 mg of methotrexate (MTX). Gynecological surgery was performed 24–48 hours post-embolization.
Patients were administered 150 mg of mifepristone orally the day before surgery and 400 µg of misoprostol sublingually 2 hours before the procedure.
D&C was conducted by an experienced gynecologist under ultrasonographic guidance. If necessary, apply iodine-soaked gauze might have been applied to the isthmus for 24 hours postoperatively to minimize bleeding.
The H/S+C procedure involves using a 10 mm hysteroscope. It began with a hysteroscopic examination to locate the GS, followed by vacuum suction aspiration of the GS. A final hysteroscopic check ensured the complete removal of any residual pregnancy material, with minimal use of energy instruments to prevent damage to the thin-walled uterine diverticulum. Iodine-soaked gauze compression at the isthmus might have been applied for 24 hours to reduce postoperative bleeding, if required.
L/S+H/S were utilized when the GS significantly protrudes towards the bladder. Preoperative UAE minimized surgery-related bleeding risk. The procedure involved adhesion separation, incision of the vesicouterine peritoneal fold, bladder retraction, and exposure of the isthmus. Vasopressin (6 U in 20 mL saline) was injected to reduce bleeding before incising the previous cesarean scar. Excised scar and pregnancy tissues were followed by intermittent suturing of the uterine muscle layer with 1–0 absorbable thread and continuous suturing of the serosa. A hysteroscopic examination ensured no residual pregnancy or bleeding in the isthmus.
Primary treatment success is defined by the normalization of serum
We performed data statistical analysis using Statistical Package for Social
Sciences (SPSS; IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version
22.0. Armonk, NY, USA). Descriptive statistics for the collected research data
were presented as mean
The baseline clinical characteristics of patients undergoing different
strategies for treating CSP are detailed in Table 1. The L/S+H/S group, which
consisted of only one individual, was not included in the statistical analysis.
Significant differences were observed in CS frequency (p
| Overall | D&C | UAE+C | H/S+C | UAE+H/S+C | L/S+H/S | p-value | ||
| (N = 220) | (N = 100) | (N = 19) | (N = 54) | (N = 46) | (N = 1) | |||
| Maternal age (years) | 34.2 |
33.9 |
36.6 |
33.3 |
34.9 |
32 | 0.057* | |
| Gravidity | 4 [2–10] | 5 [2–9] | 5 [2–7] | 4 [2–8] | 5 [2–10] | 6 | 0.178** | |
| Parity | 1 [1–4] | 1 [1–4] | 2 [1–2] | 1 [1–3] | 2 [1–2] | 2 | 0.052** | |
| Previous D&C | 2 [0–8] | 2 [0–7] | 2 [0–5] | 2 [0–5] | 2 [0–8] | 3 | 0.549** | |
| Previous CSP | 0 [0–2] | 0 [0–2] | 0 | 0 [0–1] | 0 [0–1] | 0 | 0.514** | |
| CS frequency | 1 [1–3] | 1 [1–3] | 2 [1–2] | 1 [1–3] | 2 [1–2] | 2 | 0.045** | |
| Interval since the last CS (years) | 6.0 [1.0–21.0] | 6.0 [1.0–21.0] | 6.0 [1.0–19.0] | 5.5 [1.0–20.0] | 6.0 [1.0–18.0] | 5 | 0.741** | |
| GA (days) | 48 [34–98] | 45 [34–77] | 52 [36–81] | 44 [35–98] | 55 [39–92] | 40 | ||
| GSD (cm) | 2.7 [0.4–10.2] | 2.2 [0.6–5.7] | 4.2 [1.7–6.4] | 2.4 [0.4–5.4] | 4.5 [1.8–10.2] | 4.8 | ||
| EL (cm) | 0.2 [0.0–4.5] | 0.0 [0.0–2.3] | 0.4 [0.0–4.5] | 0.0 [0.0–1.3] | 0.5 [0.0–3.0] | 0.0 | ||
| Fetal heartbeat n (%) | 93 (42.3) | 37 (37.0) | 14 (73.7) | 16 (29.6) | 26 (56.5) | 0 | 0.001*** | |
| Myometrial thickness (mm) | 1.9 [0.1–7.5] | 2.5 [0.6–7.5] | 1.5 [0.1–3.8] | 1.8 [0.1–4.0] | 1.0 [0.1–3.1] | 0.1 | ||
| Subtype n (%) | ||||||||
| Type I | 43 (19.5) | 36 (36.0) | 2 (10.5) | 4 (7.4) | 1 (2.2) | 0 | ||
| Type II | 137 (62.3) | 58 (58.0) | 13 (68.4) | 39 (72.2) | 27 (58.7) | 0 | ||
| Type III | 40 (18.2) | 6 (6.0) | 4 (21.1) | 11 (20.4) | 18 (39.1) | 1 (100) | ||
| Pre-treatment serum |
28,451.5 [334.0–391,077.0] | 21,059.0 [1857.0–142,596.0] | 73,056.0 [1022.0–391,077.0] | 19,961.0 [354.9–150,524.0] | 77,214.0 [334.0–343,187.0] | 13,874 | ||
| Reduction in |
68.1 |
64.0 |
79.5 |
63.3 |
77.5 |
79.1 | ||
| Blood loss (mL) | 20 [10–1600] | 10 [10–100] | 30 [10–1600] | 20 [10–400] | 50 [10–300] | 600 | ||
| Post-treatment hospitalization duration (days) | 2 [1–7] | 2 [1–4] | 3 [2–6] | 2 [2–6] | 2 [2–7] | 6 | ||
| Cost (Yuan Renminbi) | 4970.4 [2184.3–32,266.8] | 2832.9 [2184.3–4926.5] | 12,877.9 [11,763.6–32,266.8] | 5500.3 [4280.8–7520.6] | 14,846.4 [13,214.7–17,298.0] | 25,555.4 | ||
| Success rate (%) | 99.1 | 99.0 | 94.7 | 100.0 | 100.0 | 100.0 | 0.270*** | |
Normally distributed continuous variables: mean
Patients undergoing UAE, more specifically UAE+C and UAE+H/S+S, exhibited a
greater reduction in post-operative serum
There was no significant difference in treatment success rates among the groups
(p
This study employed binary logistic regression to assess the relationship
between GA, pre-treatment serum
| UAE | D&C | |||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| GA (days) | 1.029 | 0.987–1.073 | 0.173 | 1.011 | 0.979–1.044 | 0.494 |
| Pre-treatment serum |
1.000 | 1.000–1.000 | 0.164 | 1.000 | 1.000–1.000 | 0.773 |
| GSD (cm) | 1.651 | 1.041–2.616 | 0.033 | 1.142 | 0.839–1.556 | 0.398 |
| EL (cm) | 1.871 | 0.861–4.065 | 0.114 | 0.990 | 0.556–1.760 | 0.972 |
| Myometrial thickness (mm) | 0.295 | 0.175–0.499 | 0.412 | 0.291–0.581 | ||
GA, gestational age; UAE, uterine artery embolization; D&C, dilation and curettage; OR, odds ratio;
95% CI, 95% confidence interval;
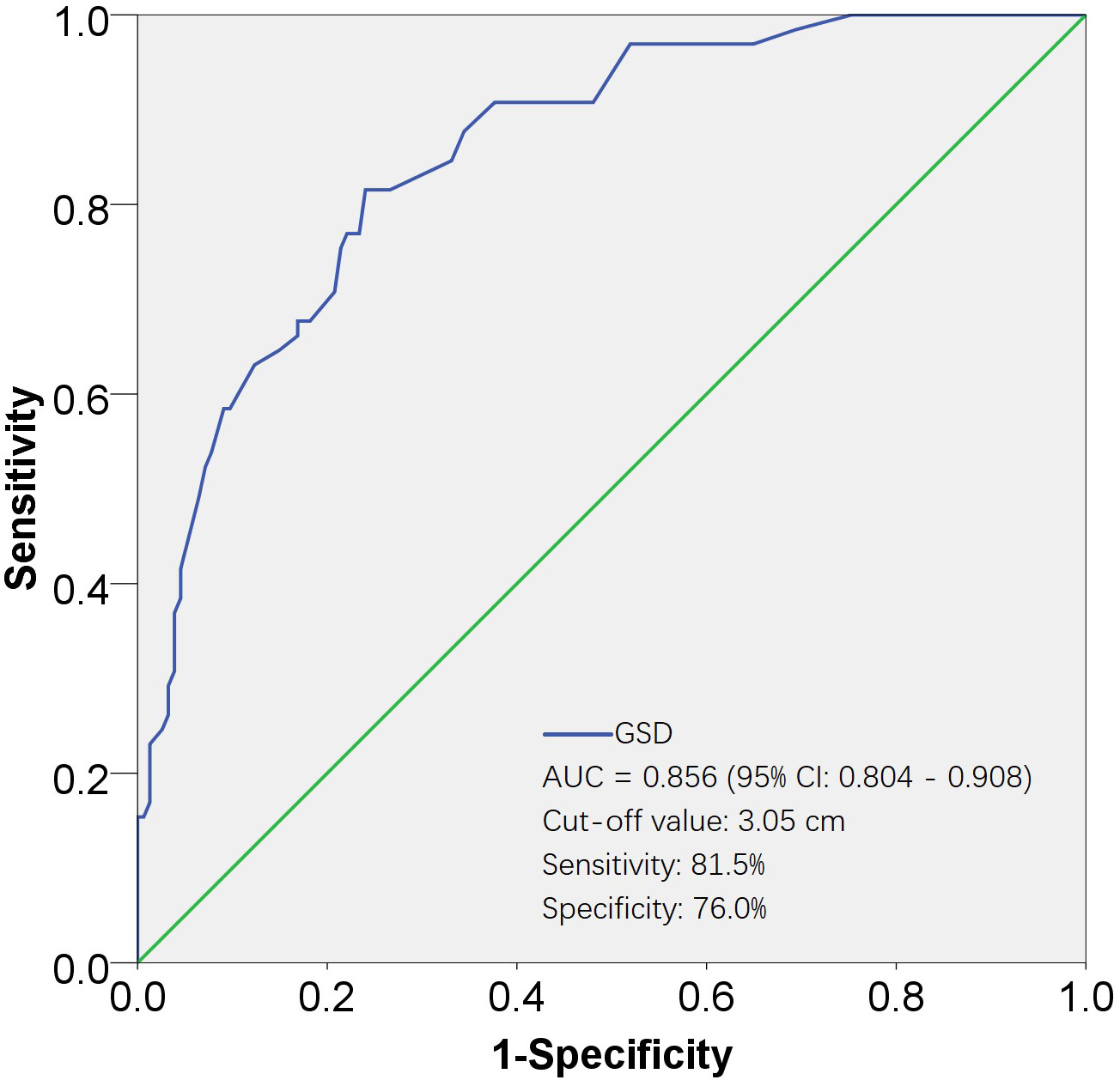
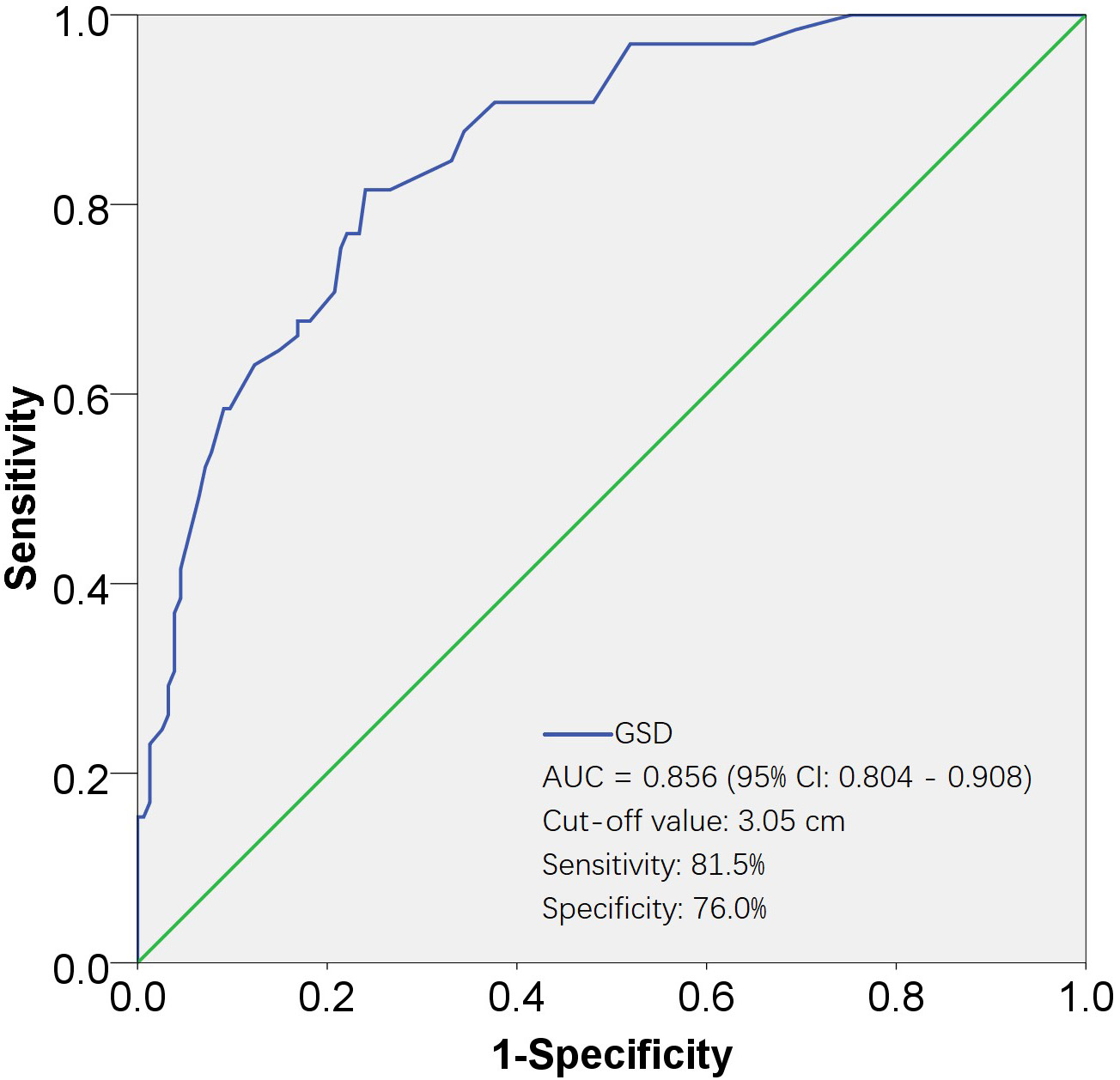 Fig. 2.
Fig. 2.ROC curves for evaluating the effectiveness of GSD in predicting UAE. The AUC for the GSD was 0.856, with a standard error of 0.026, and its 95% CI ranged from 0.804 to 0.908. The cut-off value was 3.05 cm, corresponding to a sensitivity of 81.5% and a specificity of 76.0%. AUC, area under the ROC curve; 95% CI, 95% confidence interval; ROC, receiver operating characteristic; GSD, gestational sac diameter; UAE, uterine artery embolization.
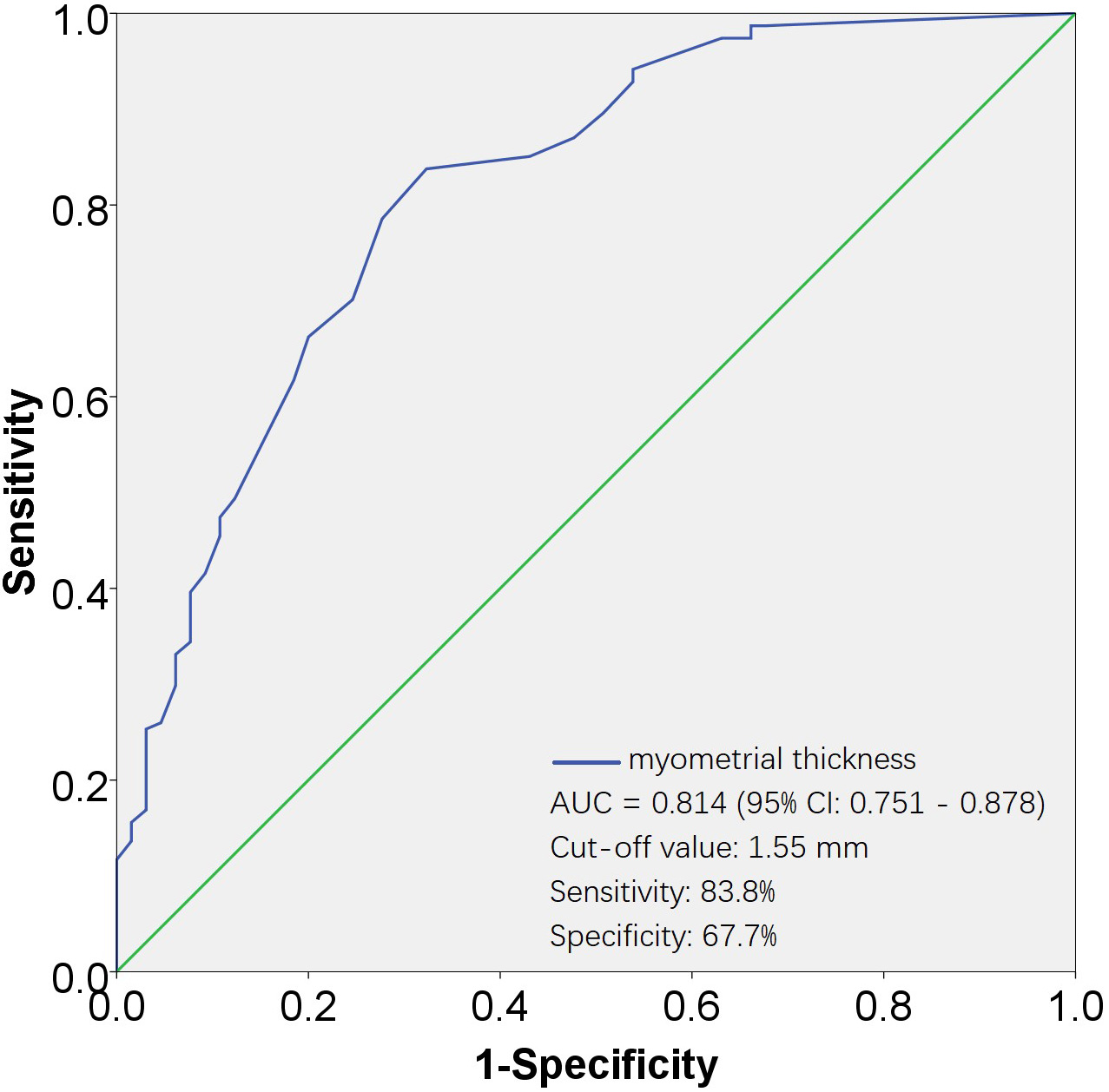
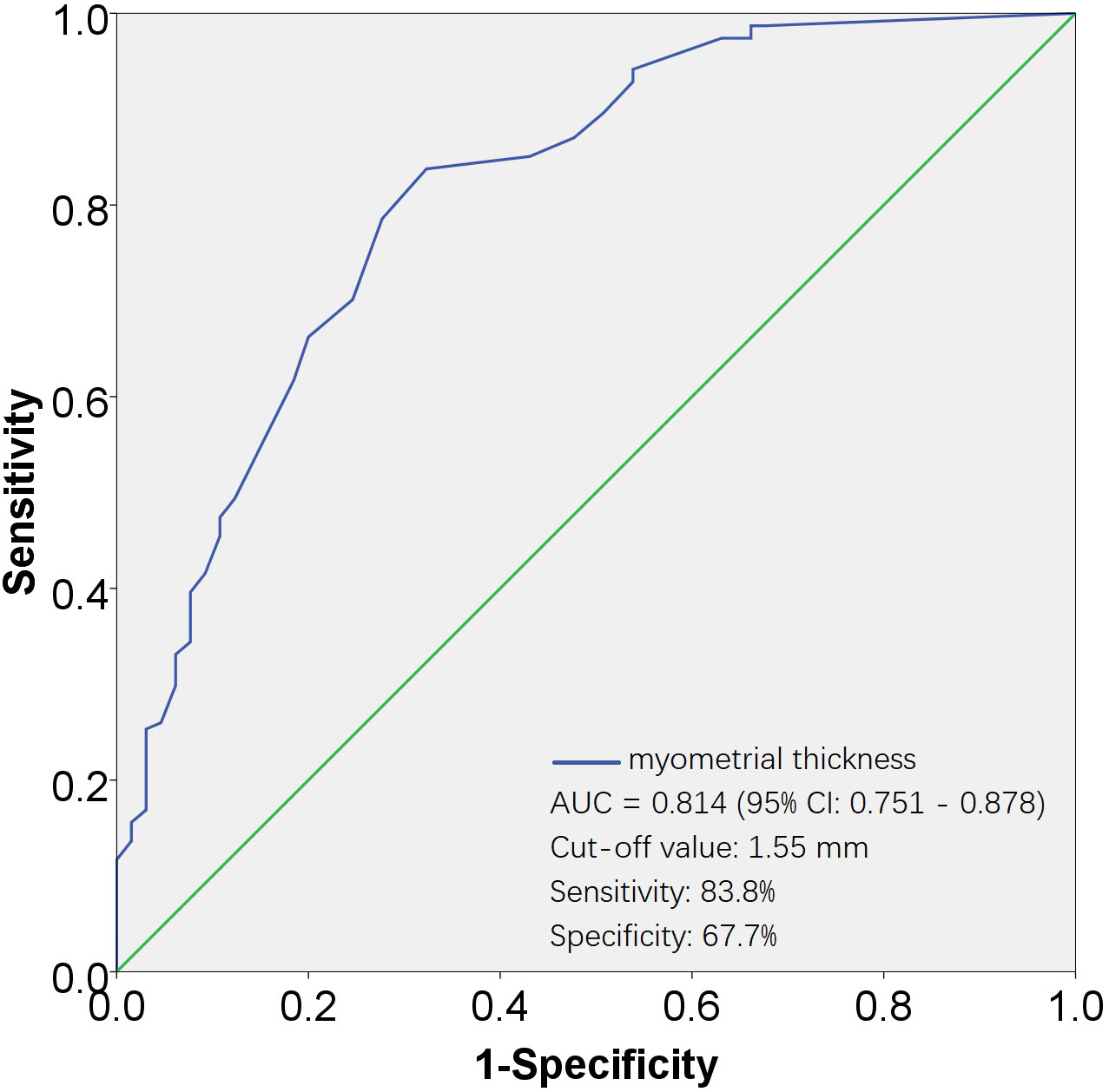 Fig. 3.
Fig. 3.ROC curves for evaluating the effectiveness of myometrial thickness in predicting none-UAE. The AUC for the myometrial thickness was 0.814, with a standard error of 0.032, and its 95% CI ranged from 0.751 to 0.878. The cut-off value was 1.55 mm, corresponding to a sensitivity of 83.8% and a specificity of 67.7%. AUC, area under the ROC curve; 95% CI, 95% confidence interval; ROC, receiver operating characteristic; UAE, uterine artery embolization.
The Pearson Chi-square test and Fisher’s exact test were used to assess the
association of fetal heartbeat and CSP subtype with UAE. Both variables were
significantly related to UAE, with p
| UAE | Surgical Procedures | ||||||
| UAE | None-UAE | p-value | D&C | H/S+C | p-value | ||
| Fetal heartbeat | Y | 40 | 25 | 51 | 42 | 0.898 | |
| N | 53 | 101 | 68 | 58 | |||
| Subtype n | |||||||
| Type I | 3 | 40 | 38 | 5 | |||
| Type II | 40 | 97 | 71 | 66 | |||
| Type III | 22 | 17 | 10 | 29 | |||
CSP, cesarean scar pregnancy; UAE, uterine artery embolization; D&C, dilation and curettage; H/S+C, hysteroscopy plus curettage.
The relationship of GA, pre-treatment serum
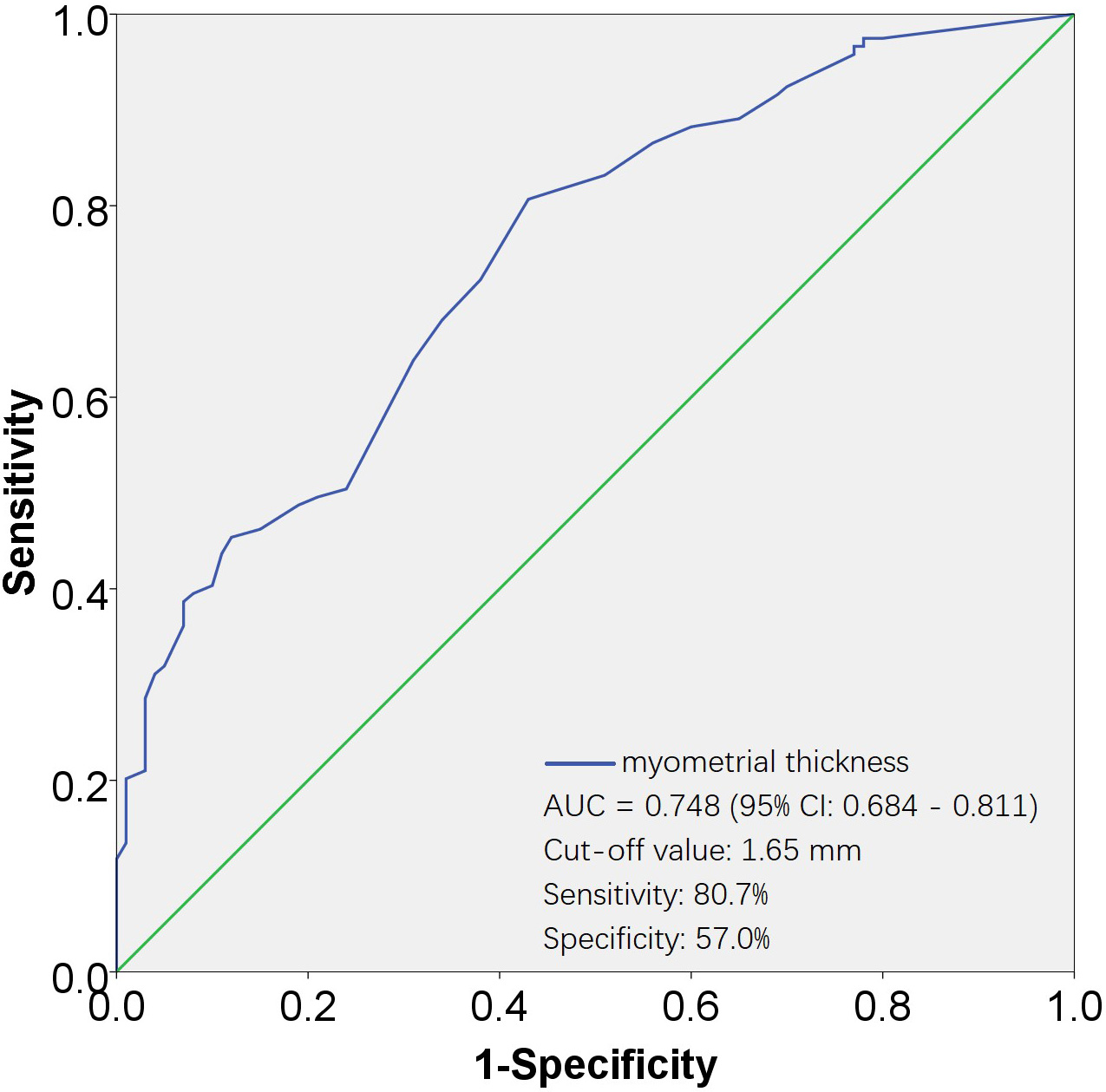
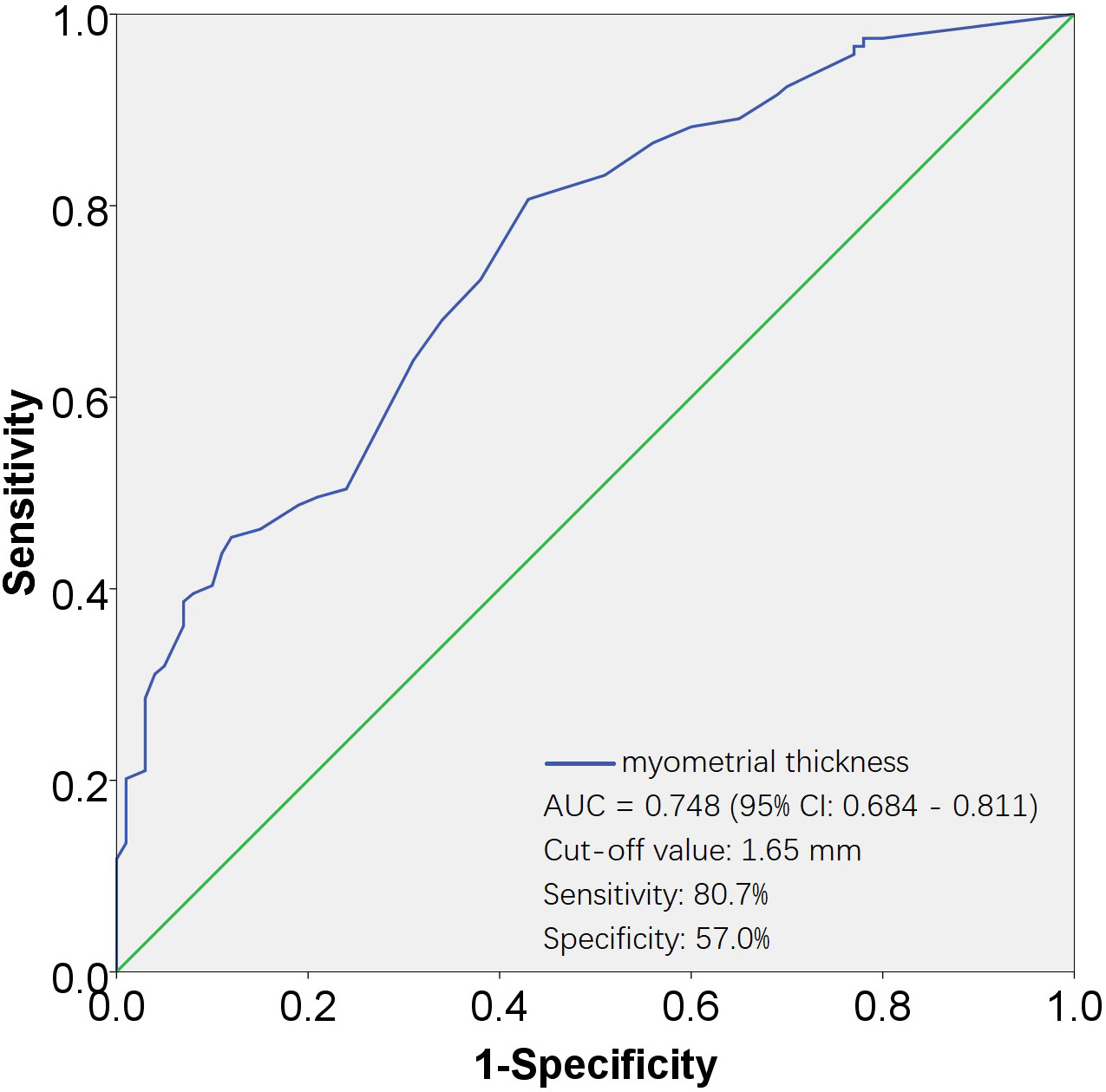 Fig. 4.
Fig. 4.ROC curves for evaluating the effectiveness of myometrial thickness in predicting D&C. The AUC for the myometrial thickness was 0.748, with a standard error of 0.032, and its 95% CI ranged from 0.684 to 0.811. The cut-off value was 1.65 mm, corresponding to a sensitivity of 80.7% and a specificity of 57.0%. AUC, area under the ROC curve; 95% CI, 95% confidence interval; ROC, receiver operating characteristic; D&C, dilation and curettage.
The association of fetal heartbeat and CSP subtype with the surgical approach
was evaluated using the Pearson Chi-Square test and Fisher’s exact test. Among
the two variables included, fetal heartbeat was not associated (p =
0.898), whereas CSP subtype was significantly associated with the surgical
approach (p
As the rate of CS increases and ultrasound diagnostic techniques advance [10], CSP has become increasingly common. To date, there is no unified standard for the treatment of CSP, both domestically and internationally. Treatment plans are primarily dependent on the expertise and judgment of specialist physicians [4], whereby the professional proficiency and depth of disease understanding of the attending physician significantly influence the treatment outcomes.
The primary treatment methods for CSP include local and/or systemic administration of MTX [11], UAE combined with MTX infusion chemotherapy, D&C, hysteroscopic lesion removal, as well as laparoscopic, abdominal, or transvaginal lesion removal [4]. Each of these diagnostic and therapeutic approaches present its own set of advantages and disadvantages.
The efficacy of MTX treatment is limited and depends upon the patient’s
hemodynamic stability. Studies [10, 12, 13] suggest that initiating treatment
early and encountering lower initial serum
UAE stands out as the most effective and rapid treatment for controlling
bleeding [18]. Nevertheless, it is associated with high costs and post-operative
complications [23], including spasmodic pain, fever (post-embolization syndrome),
ovarian failure, uterine-rectal fistulas, uterine-bladder fistulas, and uterine
necrosis. These complications have been reported to potentially affect the
outcomes of subsequent pregnancies [18, 24]. Previous studies have indicated that
adjunctive UAE may not be necessary for all CSP patients [25, 26], and therefore,
it should be carefully considered. A meta-analysis of 3101 patients with CSP
found that larger GSD, advanced GA, higher serum
In our study, there was no significant difference in the success rates between D&C and H/S+C. D&C stands as the simplest and least invasive surgical method. However, its non-selective and blind uterine evacuation may potentially result in uncontrolled massive bleeding or uterine rupture, thereby posing a risk of uterine resection [29]. Various studies recommend varied criteria for determining the suitability of D&C, and as of yet, there is no unified standard [26, 30, 31]. In contrast to D&C, hysteroscopy provides direct visualization of the uterine cavity, enabling a clear diagnosis and thorough removal of pregnancy tissue from the cesarean scar site [3, 32, 33].
Our study found that when myometrial thickness was less than 1.65 mm, H/S+C was
more likely to be chosen. This finding differs from previous studies [34], which
considered hysteroscopy a better choice for women with a myometrial thickness
Compared to D&C, hysteroscopy reduces the chances of initial treatment failure. However, hysteroscopy significantly increases the cost of treatment, and thus is not recommended for all types of CSP patients. Consistent with this study, another retrospective study conducted by our medical institution also showed that there is no difference in the failure rate of CSP treatment between ultrasound-guided D&C and hysteroscopy, regardless of whether UAE is performed [26].
In our study, a type III CSP patient underwent L/S+H/S, a comprehensive approach
that excises the CSP lesion and simultaneously repairs the original uterine scar
defect. This method is considered the preferred and optimal approach for cases of
conservative treatment failure, uterine rupture, or suspected uterine rupture
[35]. While UAE combined with surgical excision exhibits more favorable outcomes
compared to other treatments regarding the duration of vaginal bleeding, abnormal
serum
The strengths of this study primarily lie in its large sample size and rigorous research design, which encompassed well-defined inclusion and exclusion criteria, detailed treatment procedures, and comprehensive follow-up. However, due to the retrospective nature of the study, the treatment approach was non-randomized and primarily based on expert opinion. Therefore, to provide a nuanced analysis, patients were divided into subgroups, allowing for a separate examination of the risk factors associated with each treatment strategy. The limitations of the study stem from its retrospective cohort nature, wherein the allocation of patients was subject to many constraints, precluding strict randomization. Although initial risk factors might influence the decision on treatment strategy, the final treatment plan was chosen based on expert opinion. Moreover, the study focused solely on severe complications, leading to the omission of recording some relatively minor post-operative complications. Lastly, as a gynecological teaching and treatment center, the surgeons’ operative experience and diagnostic expertise were superior, which likely contributed to better diagnostic and surgical outcomes. However, this study’s failure to consider the surgeons’ experience as a potential risk factor represents a missed opportunity to explore its impact on outcomes. Therefore, future research will be designed with more rigorous methodologies to reduce allocation bias.
Overall, this study demonstrated that there is no significant difference in the success rate for treating CSP between D&C and H/S+C alone, or in combination with UAE. However, the incorporation of UAE or H/S+C into the treatment regimen significantly increases both the cost of treatment and the duration of hospital stay. Factors such as a larger GSD, reduced myometrial thickness, the presence of a fetal heartbeat, and a higher CSP subtype were identified as risk factors for choosing UAE, while a reduced myometrial thickness and a higher CSP subtype were identified as risk factors for opting for H/S+C. For different patients, it is essential to carefully select the treatment option based on the relevant risk factors.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request with the permission of the Ethics Committee of the Women’s Hospital, School of Medicine, Zhejiang University.
XN and YZ conceived and designed the study. SN, JY and SL collected the data. XN and JY analyzed the data. XN and SN wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors have read and approved the final manuscript.
Given Considering the nature of the retrospective approach and the anonymization of patient data, approval (IRB-20220061-R) for the study protocol has been granted by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University. Consequently, the necessity of obtaining informed consent from individual patients has been waived. This exemption is due to the low risk posed to participants by the retrospective analysis of anonymized data, which ensures that patient confidentiality and privacy are maintained.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This study was funded by the General Research Project of Department of Education of Zhejiang Province, P.R. China (Grant No. Y202146817).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.