1 Department of Critical Care Medicine, The Third Affiliated Hospital of Guangzhou Medical University, 510150 Guangzhou, Guangdong, China
2 Guangdong Provincial Key Laboratory of Major Obstetric Diseases, 510150 Guangzhou, Guangdong, China
3 Guangdong Provincial Clinical Research Center for Obstetrics and Gynecology, 510150 Guangzhou, Guangdong, China
Abstract
Objective: This review provides a comprehensive intensive care unit (ICU) perspective on amniotic fluid embolism (AFE), encompassing its epidemiology, pathophysiology, diagnosis, and management. Mechanism: AFE is an abrupt and perilous condition. The enhancement of diagnostic criteria, starting from the understanding of pathologic physiology, can facilitate the development of more specialized disease management approaches and targeted interventions. Findings in Brief: Significant research advancements have improved the timeliness and accuracy of clinical diagnosis and treatment for AFE, leading to the development of several effective rescue procedures. Progress is expected to be made in various aspects including a comprehensive exploration of pathophysiological mechanisms, identification and utilization of biomarkers, innovation in treatment methods, enhancement of personnel training and collaboration within treatment teams, as well as the application of big data technology. Conclusions: The recent research has greatly contributed to substantial progress in the clinical diagnosis and treatment for AFE. However, further research holds potential to provide even greater benefits for patients.
Keywords
- amniotic fluid embolism
- ICU
- diagnosis
- management
Amniotic fluid embolism (AFE) is a series of severe birth complications, including acute pulmonary embolism, anaphylactic shock, disseminated intravascular coagulation (DIC), renal failure, and other pathophysiologic changes caused by the entry of amniotic fluid into the maternal bloodstream during delivery [1]. AFE is characterized by its sudden onset, high risk nature, and unpredictable course, which can result in serious adverse outcomes such as maternal and fetal disability or even death. It is crucial to accurately identify and effectively manage cases of AFE. The aim of this study is to provide a comprehensive review of the pathophysiology, diagnosis, and multidisciplinary management of amniotic fluid embolism from the intensive care unit (ICU) perspective, aiming to enhance our understanding and optimize the management strategies for this condition.
The incidence of AFE varies slightly in different countries and regions. According to a European study, the reported incidence ranges from 1/5500–12,500 pregnancies [2], while a Chinese study reports a fatal incidence rate of 0.99 per 100,000 pregnancies [3]. Among pregnant women experiencing cardiac arrest, the incidence of AFE is approximately 13% [4]. Despite its low occurrence rate, AFE can lead to serious adverse outcomes such as maternal and infant death. Studies have shown that the mortality rate or permanent central nervous system damage caused by AFE can range from 30% to 41% [2], with some reports suggesting rates as high as 33%–48% [4].
The pathogenesis of AFE remains incompletely understood; however, further research has gradually revealed the physiologic and pathologic changes that occur during its onset. The pathophysiologic manifestations of AFE include inflammatory injury, anaphylaxis, pulmonary hypertension, and DIC. However, it is incorrect to use the term “amniotic fluid embolization” as it does not adequately explain the disease’s pathophysiology in clinical observations, animal studies or other experiments [5]. In fact, a study published in 1950 suggested that the presence of fetal tissue alone in the maternal circulation may not be sufficient to cause AFE; instead, individual responses involving physiologic and pathologic changes are key factors [6].
Building on previous knowledge, Clark et al. [7] noted that AFE shares
similar clinical manifestations with septic shock and anaphylactic shock.
Subsequently, Romero et al. [8] discovered elevated levels of
TNF-
Some researchers have argued that the explanation of AFE solely based on the release of inflammatory mediators is still insufficient. It is also considered a physiologic and pathologic manifestation of AFE when amniotic fluid enters the circulation, triggering the activation of the coagulation cascade [11]. Some researchers have utilized thromboelastography or thromboelastometry to investigate the transference of amniotic fluid contamination into maternal blood [12, 13, 14]. These studies observed that the addition of amniotic fluid to the blood resulted in a shortened clotting time and enhanced platelet function, thereby accelerating coagulation without any evidence of fibrinolysis, thereby accelerating thrombosis without any evidence of fibrinolysis. Additionally, it has been reported that the addition of amniotic fluid to maternal plasma can impede the anticoagulant pathway of activated protein C [15]. However, conflicting findings exist as Fudaba et al. [16], utilizing thromboelastography to monitor coagulation function in AFE patients, revealed that primary hyperfibrinolysis may precede the occurrence of coagulation dysfunction during the pathologic process of AFE. There is a discrepancy between the results of the study which reproduced amniotic fluid contamination into whole blood and the coagulopathy in the amniotic fluid embolism case reports. Therefore, the cause and etiology of coagulopathy in amniotic fluid embolism remain to be clarified.
The presence of evident thrombosis in the pulmonary artery and uterine artery of AFE patients has been observed by researchers through anatomical examination [17]. Additionally, microembolism can trigger a significant release of arachidonic acid metabolites, leading to the development of pulmonary vasospasm. These aforementioned pathophysiologic changes result in intrapulmonary shunting, bronchoconstriction, pulmonary hypertension, and ultimately severe hypoxia [17, 18].
Mast cell activation serves as a significant mechanism in AFE. Benson et al. [5] observed mast cell degranulation and a decrease in complement C3 and C4 levels within the lungs of patients with fatal AFE. Other studies have identified mast cell and complement activation in the uterine tissue of patients with AFE and have demonstrated that the pathologic changes associated with postpartum acute myometritis (PAM) are linked to severe postpartum hemorrhage caused by uterine atony [19, 20, 21]. Shen et al. [22] reported an upregulation of bradykinin B1 receptor expression in PAM uterine tissues, which may be associated with uterine edema. Tamura et al. [23, 24] found that the concentration of C1 esterase inhibitors was reduced in AFE patients and inhibited the activity of factors XIIa, XIa and kallikrein, and appeared to play an important role in the pathophysiology of AFE patients.
The onset of AFE is rapid, and it presents with complex clinical manifestations. Approximately 70% of cases occur during labor, 11% after vaginal delivery, and 19% during or after cesarean section. Typically, it manifests during labor or immediately following delivery, mostly occurring within 2 hours before the fetus is delivered and within 30 minutes after the placenta is delivered [10, 25]. A small number of cases may arise during second-trimester labor induction, amniocentesis procedures, or as a result of trauma [26].
Classic AFE is characterized by a sudden onset of hypoxemia, hypotension, and coagulopathy (Table 1). The clinical manifestations include sudden onset of respiratory and circulatory failure, with the possibility of respiratory and cardiac arrest occurring following episodes of screaming, yawning, or convulsions. In severe cases, death may occur within minutes [7]. Research has indicated that right ventricular failure is attributed to pulmonary hypertension and pulmonary vasospasm [27, 28, 29], while left ventricular failure results from myocardial ischemic injury or cardiac depression secondary to circulating inflammatory mediators [10]. Initially, respiratory failure may be caused by pulmonary vasoconstriction and pulmonary edema resulting from acute left heart failure; however, subsequent impairment of respiratory function is attributed to capillary leakage due to immune-mediated processes [30].
| Clinical manifestations of Amniotic Fluid Embolism | |
| Circulatory system | increased heart rate; decrease in blood pressure; shock; right heart failure; left heart failure; cardiac arrest |
| Respiratory system | dyspnea; wet rales at the basal regions of both lungs; decreased blood oxygen; ARDS; respiratory failure |
| Coagulation system | bleeding; DIC |
| Nervous system | change of consciousness; epilepsy; coma |
| Urinary system | oliguria; acute renal failure |
| Obstetric conditions | uterine atony; fetal distress |
ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation.
The presence of coagulopathy in AFE is characterized by a systemic bleeding tendency, including uterine bleeding, incision bleeding, mucocutaneous bleeding, needle piercing bleeding, hematuria, and gastrointestinal bleeding. The occurrence of coagulopathy may arise following cardiopulmonary failure, and in certain cases of atypical AFE, it can manifest as the initial symptom. Consumption coagulopathy and hyperfibrinolysis contribute to the development of coagulopathy in AFE as evidenced by elevated prothrombin time and activated partial thromboplastin time and decreased fibrinogen levels [31].
Laboratory tests alone cannot diagnose AFE in cases without clinical features [25, 32]. Auxiliary examinations are valuable for diagnosis and disease monitoring. Recent studies have emphasized the crucial role of auxiliary examinations in diagnosing and treating AFE. Some studies suggest that early monitoring of the hemoglobin/fibrinogen ratio can reduce or prevent worsening of coagulation dysfunction in patients with AFE [33]. Matsunaga et al. [34] found that fibrinogen exhibited the strongest correlation among test findings at onset. The blood loss/fibrinogen ratio may help differentiate postpartum hemorrhage from DIC-type AFE and diagnose clinical AFE, facilitating optimal replacement of coagulation factors during the early stages. Legrand et al. [35] proposed insulin-like growth factor binding protein-1 (IGFBP-1) as a diagnostic marker for AFE, but experienced subsequent scrutiny from other researchers [36, 37]. Echocardiography has gained increasing importance as an imaging tool for diagnosing AFE and assessing therapeutic efficacy; it may even be considered as one of the diagnostic criteria [38, 39, 40, 41, 42, 43].
The diagnosis of AFE should be based on clinical manifestations and predisposing factors while excluding other potential causes. Currently there are no internationally unified diagnostic criteria or laboratory indicators for AFE diagnosis. The recommended diagnostic criteria are outlined in Table 2 (Ref. [44]); all five criteria must be met for the diagnosis of AFE.
| Diagnostic criteria for amniotic fluid embolism [44] | |
| 1 | Sudden onset of cardiorespiratory arrest or hypotension (systolic blood pressure |
| 2 | Respiratory compromise-dyspnea, cyanosis, peripheral capillary saturation |
| 3 | Documentation of DIC using the scoring system of the Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Hemostasis (ISTH), modified for pregnancy |
| 4 | Clinical onset during labor, cesarean delivery, or dilation and evacuation or within 30 min of delivery of the placenta |
| 5 | No fever (38.0 °C) and absence of any other significant confounding condition or explanation for the signs and symptoms observed |
Note: All 5 items are mandatory for diagnosis of AFE. AFE, amniotic fluid embolism.
Since the introduction of the diagnostic criteria, several studies have conducted retrospective analyses on previous cases and excluded a subset of patients who were previously classified as AFE. These findings suggest that the new diagnostic criteria can provide a more accurate diagnosis for AFE [1]. Additionally, some studies have indicated that the new diagnostic criteria offer valuable assistance in achieving improved accuracy in diagnosis [45]. However, it has been suggested that the concurrence rate of the new diagnostic criteria among patients with strong suspicion of AFE is less than fifty percent, thereby proposing further enhancements to these diagnostic criteria [46].
Once AFE is suspected, immediate rescue measures should be initiated and a multidisciplinary team should be called for assistance. The involvement of various disciplines such as obstetrics, anesthesiology, respiratory medicine, and critical care medicine is crucial in accordance with the guidelines provided by the Society for Maternal-Fetal Medicine and the American Heart Association [25, 47, 48]. Numerous successful cases have demonstrated the effectiveness of this approach [49, 50, 51]. Throughout the rescue process, comprehensive monitoring is essential to maintain vital signs and preserve organ function. Treatment for AFE primarily focuses on life support, symptomatic management, and protection of organ function. Respiratory and circulatory support as well as correction of DIC play a significant role.
Ensuring immediate airway patency, providing adequate oxygenation, and promptly establishing optimal ventilation are crucial for successful management. This may involve administering oxygen via a mask, utilizing non-invasive ventilation or endotracheal intubation to support respiration. In cases where necessary, tracheal intubation, positive pressure high concentration oxygen therapy, or even tracheotomy should be considered. Maintaining oxygen saturation above 90% [52] is essential to alleviate alveolar capillary hypoxia, prevent/reduce pulmonary edema, and improve tissue perfusion in vital organs such as the heart, brain, and kidneys. Following cardiopulmonary resuscitation (CPR), advanced life support, hyperbaric oxygen therapy, or mechanical ventilation can be implemented.
Since AFE often presents as cardiac arrest, it is crucial to promptly initiate high-quality CPR. In the initial stage of cardiac arrest resuscitation, a definitive diagnosis of AFE is not necessary. At this critical moment, the primary focus should be on delivering high-quality CPR. For women who have not yet given birth, maintaining a leftward tilt position is recommended. This can be achieved by placing the patient in a supine position or applying uterine left traction to prevent compression of the inferior vena cava caused by the weight-bearing uterus.
Because shock is one of the most crucial clinical manifestations of AFE, it is
imperative to maintain hemodynamic stability. First, crystalloid-based fluid
resuscitation, often with Ringer’s solution, should be administered. During
circulatory support treatment, caution must be exercised to restrict fluid intake
in order to prevent further right ventricular dilatation [52], which can lead to
heart failure and pulmonary edema. Pulmonary edema also contributes to severe
infection and sepsis during later stages of treatment [53]. Second, vasoactive
agents and inotropic agents are necessary for maintaining hemodynamic stability.
In cases of hypotension, excessive fluid resuscitation should be avoided as it
can exacerbate the condition; instead drugs such as norepinephrine or vasopressin
should be used to maintain blood pressure. Mean arterial pressure during the
initial resuscitation phase should be maintained at
Pulmonary artery spasm and embolism, along with increased pulmonary vascular resistance, can result in acute pulmonary hypertension, severe dyspnea, cyanosis, and hypoxia. Severe hypoxia can rapidly lead to multi-organ failure including heart, brain, and kidney hypoxia. The initial stage of AFE primarily presents as right ventricular failure. Cardiac ultrasound is recommended for assessment at the earliest opportunity. Transthoracic echocardiography is suggested as a simple and reliable method to identify right ventricular failure [56, 57, 58]. Specific relaxation of pulmonary vascular smooth muscle can be achieved by using drugs such as prostacyclin, sildenafil, nitric oxide, and endothelin receptor antagonists [59]. Additionally, drugs like papaverine hydrochloride, atropine, aminophylline, and phentolamine may also be considered.
The use of glucocorticoids in the management of AFE remains a subject of controversy. Based on clinical experience, early administration of high-dose glucocorticoids should be considered as a beneficial intervention [32, 60]. Hydrocortisone, methylprednisolone, or dexamethasone are all viable options to consider.
Invasive hemodynamic support may prove advantageous for pregnant women with refractory shock unresponsive to vasoactive medications. Over the past few decades, extracorporeal membrane oxygenation (ECMO) technology has undergone significant advancements, rendering it a more accessible and safer therapeutic approach; however, its availability is limited across healthcare settings. Nevertheless, several case reports have demonstrated the efficacy of ECMO in managing AFE in pregnant women even when complicated by bleeding and coagulation disorders; thus making it the sole means to provide hemodynamic support [61, 62, 63].
Coagulopathy can occur following AFE complicated by cardiovascular system abnormalities or it may present as the initial manifestation. Early assessment of coagulation status is recommended. The coagulopathy associated with AFE may be attributed to activation of the exogenous pathway, specifically the factor VII pathway, induced by tissue factor [25]. Excessive administration of crystalloids or colloids is not advised due to its potential to further dilate the right ventricle and causing dilutional coagulopathy. Prompt supplementation of red blood cells and coagulation factors (i.e., fresh frozen plasma, cryoprecipitate, fibrinogen, platelets) is recommended, particularly emphasizing fibrinogen supplementation. Fibrinogen plays a crucial role in hemostasis, as it is the earliest clotting factor to decrease during obstetric hemorrhage. Fibrinogen levels can serve as a prognostic indicator for the severity of obstetric hemorrhage, with significantly reduced levels often observed in cases of AFE [64, 65]. A study on obstetric hemorrhage identified fibrinogen levels below 200 mg/dL as a positive predictor for severe bleeding [66], thus suggesting that early administration of fibrinogen supplementation should be considered when levels are below 200 mg/dL. Concurrently, anti-fibrinolytic therapy should be implemented. Case reports have documented the use of anti-fibrinolytic drugs such as tranexamic acid in managing secondary bleeding during AFE [31], and evidence supports their efficacy in non-AFE obstetric and traumatic bleeding scenarios [67]. Nevertheless, the utilization of recombinant factor VIIa remains controversial. Recent systematic review findings regarding its application in AFE patients indicate unfavorable outcomes and increased mortality rates; hence routine usage is not currently recommended [68]. The role of heparin in treating DIC caused by AFE remains contentious within clinical practice. Due to the rapid progression of AFE, accurately determining the hypercoagulable stage at which heparin therapy would be beneficial becomes challenging; therefore, routine administration is not advised due to potential harm outweighing benefits [10, 25].
The application of thromboelastography enables a more comprehensive understanding of the specific etiology underlying abnormal coagulation and provides guidance for the management of coagulation dysfunction. As necessary, blood products can be administered or anti-fibrinolytic therapy can be implemented, thereby reducing fluid requirements and mitigating the risk of further right ventricular dilatation [16, 61, 69].
If AFE occurs prior to delivery, prompt termination of the pregnancy should be performed by either vaginal delivery or cesarean section. In cases of maternal cardiac arrest beyond 23 weeks gestation, immediate CPR and preparation for emergency cesarean section are necessary. If spontaneous heart rate does not return after 4 minutes of maternal CPR, emergency cesarean section may be considered [25], as it can potentially save both the fetus’s life and aid in maternal resuscitation by relieving pressure on the inferior vena cava [48]. However, when AFE-induced maternal cardiac arrest occurs during the perinatal period leading to maternal death, making a decision regarding cesarean section becomes challenging. The specific circumstances at the rescue scene must guide this decision-making process since there is no standardized treatment protocol.
AFE is frequently associated with uterine atony and necessitates aggressive treatment with uterotonic agents, such as oxytocin, ergonovine, and prostaglandins [70, 71]. In the case of vaginal delivery, careful attention should be given to cervical, vaginal, and other birth canal lacerations. Hysterectomy is not required for managing AFE and prophylactic hysterectomy should be avoided. However, if postpartum hemorrhage becomes uncontrollable and poses a threat to the mother’s life, prompt hysterectomy becomes imperative [32, 70].
In recent years, significant research advancements have led to more timely and accurate clinical diagnosis and treatment of AFE, resulting in the development of several effective rescue procedures (Fig. 1). However, despite notable progress, AFE continues to pose a substantial threat to maternal survival. Further progress is anticipated in the research and management of AFE in the following areas: (1) Comprehensive investigation into pathophysiologic mechanisms by exploring coagulation, immune response, and inflammatory pathways. (2) Biomarker discovery with continued exploration of non-invasive diagnostic methods to aid in identifying highly sensitive and specific biomarkers for accurate detection of AFE. (3) Research and development of novel therapeutic approaches based on the pathophysiologic processes and pathogenesis, with the anticipation for the development of new medications targeting AFE. (4) Enhancing personnel training and team building with the likelihood that this collaborative model will reduce mortality associated with AFE and improve patient outcomes. (5) Application of big data technology in the study of AFE with the anticipation that the prediction accuracy and treatment efficacy for AFE can be enhanced. Encompassing all of these suggestions will bring benefits to patients and offer new opportunities for research and management in this field.
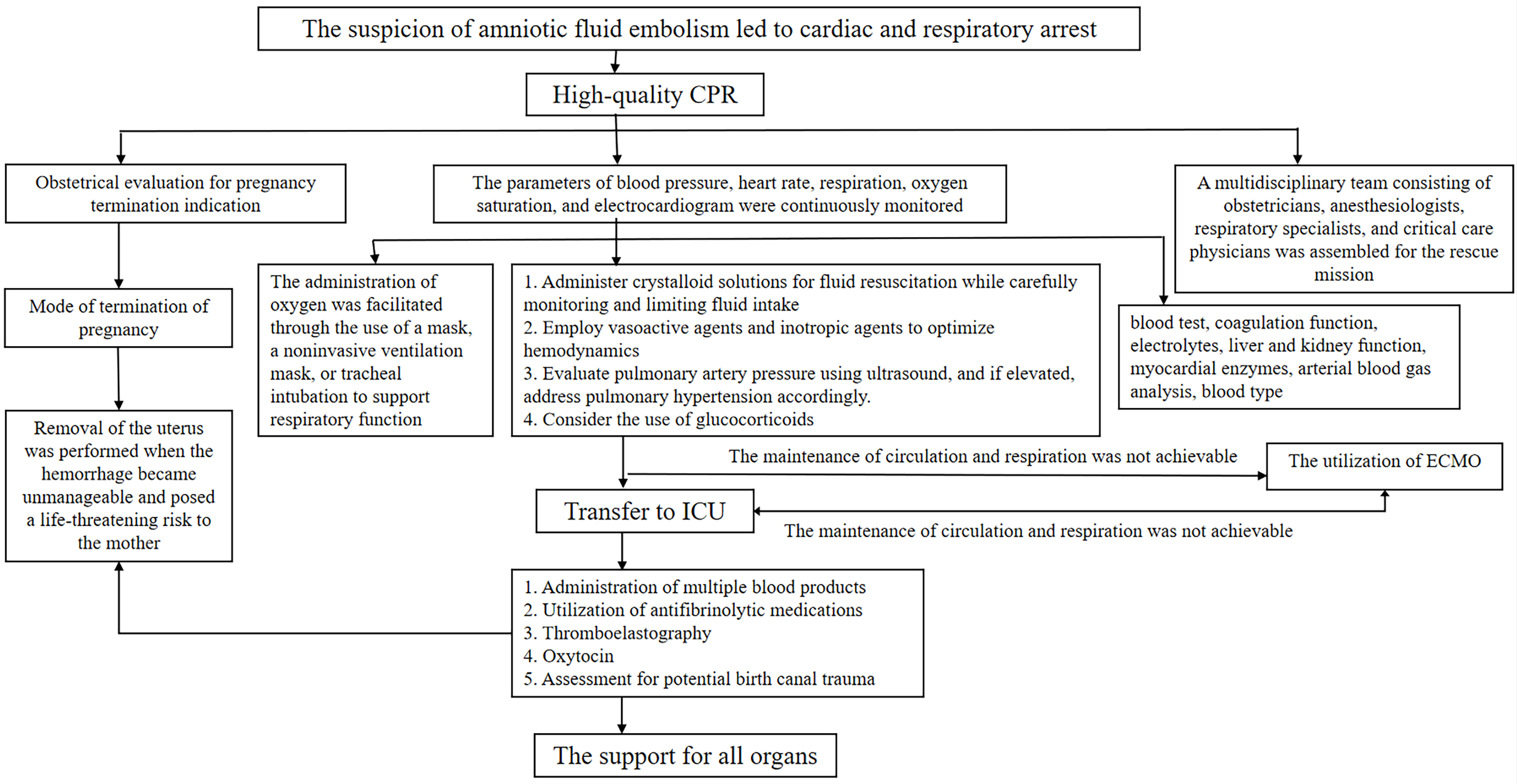 Fig. 1.
Fig. 1.Flow chart of amniotic fluid embolism rescue. CPR, cardiopulmonary resuscitation; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
AFE remains a diagnosis of exclusion based on clinical manifestations; however, an increasing number of studies suggest that auxiliary examinations play a pivotal role in the accurate diagnosis and treatment of AFE. In cases where AFE is highly suspected, early intervention is imperative. Ensuring successful rescue from AFE relies heavily on precise first aid drills. The primary approach to treatment involves supportive and symptomatic measures, encompassing respiratory support, circulatory support through appropriate fluid administration, vasoactive drugs, positive inotropic agents, pulmonary vasodilators, glucocorticoids, ECMO, active management of coagulopathy, timely delivery or hysterectomy when necessary, as well as organ function support and protection. Rapid and comprehensive monitoring serves as the cornerstone for effective treatment. Moving forward, the research and management of AFE are expected to make progress in pathophysiologic mechanisms, biomarkers, new treatment methods, the development of treatment collaboration teams, and the application of big data technology.
MJ: Contributions to the conception and design of the work, retrieval, review, and analysis of literature, specifically writing the initial draft. JY: Contributions to retrieval, review, and analysis of literature, specifically writing the initial draft. BL, YL and YY: Contributions to retrieval, review, and analysis of literature. YW: Contributions to the conception and design of the work, responsible for protocol development, manuscript editing, and given final approval of the version to be published. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Clinical characteristic technology project of Guangzhou region (No. 2023C-TS13).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

