1 School of Basic Medicine, Wannan Medical College, 241002 Wuhu, Anhui, China
2 Department of Obstetrics and Gynecology, First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), 241000 Wuhu, Anhui, China
3 Department of Ultrasound, First Affiliated Hospital of Wannan Medical College, 241000 Wuhu, Anhui, China
4 Pharmacy School of Wannan Medical College, 241002 Wuhu, Anhui, China
†These authors contributed equally.
Abstract
Background: Infiltration of immune cells associated with tumor clinical results affects different cancers. However, the composition and the clinical significance of tumor-infiltrated immune cells in epithelial ovarian cancer has not been completely investigated. Methods: The metagene deconvolution algorithm (Cell type Identification by Estimating Relative Subsets of known RNA Transcripts (CIBERSORT)) was used to analyze gene expression profiles in public databases (GEO and TCGA) to infer the composition of 22 immune cell subgroups in ovarian tumors. The proportions obtained in this study were used to investigate the association between each cell type and the clinical outcomes for ovarian cancer diagnosis and prognosis. Quantitative real-time polymerase chain reaction (qRT-PCR) was used for detecting the expression levels of related genes. Results: The profiles of immune cells infiltration were altered in malignant ovarian neoplastic tissue. Ovarian cancer tissues contained higher proportion of T follicular helper cells (Tfh) and macrophages (M0 and M1) rather than the normal ovarian tissue. Meanwhile, lower proportion of monocytes and neutrophils was also observed in ovarian cancer tissues compared with normal tissues. The qRT-PCR test confirmed the conclusion that the contents of CD80 (M1 cells) and CD4+ (Tfh cells) were high in the interstitium of ovarian cancer tissue, while the contents of CD21 (B cells) and CD66b (neutrophil) were low. Interestingly, immune cell infiltration was observed to be correlated with the change in clinical outcome. The activated mast cell subpopulation was associated with poor prognosis, while the subpopulation of resting dendritic cells was correlated with pathological grade of the ovarian cancer. Conclusions: Our bioanalysis revealed that the composition of tumor-infiltrating immune cells was closely related to the clinical outcome of ovarian cancer, which was also validated in clinical samples. These results provide a new strategic basis for the prognosis and treatment of ovarian cancer.
Keywords
- tumor-infiltrated immune cells
- ovarian cancer
- CIBERSORT
- qRT-PCR
- clinical outcome
Ovarian carcinoma is the third most common gynecological malignancy in the world [1, 2], posing a substantial threat to women’s health due to its high incidence. According to organizational classification, ovarian cancer can be categorized into epithelial cell (90%) and non-epithelial origin (10%). The latter encompasses germ cell tumors, sex cord stromal tumors, and exceptionally rare tumors like small cell carcinoma [3]. Distinct clinical behaviors and treatment approaches exist for various ovarian cancer types; germ cell tumors exhibit earlier onset, rapid growth, predominantly unilateral localization (95% of cases), and a generally favorable prognosis compared to epithelial ovarian cancers (EOCs) [4]. Notably, a subset of EOC patients harbors germline mutations in the BRCA1/2 genes, prompting the clinical application of poly (ADP-ribose) polymerase (PARP) inhibitors employing synthetic lethality as targeted therapy in this specific situation [5].
According to recent global cancer open-source data in GLOBOCAN 2020 and the United Nations [6], among women worldwide, ovarian cancer is the most common malignancy. The increased utilization of ultrasound has facilitated the detection of asymptomatic ovarian masses during pregnancy [7]. Approximately 5% of ovarian tumors identified during pregnancy are proven malignant. Surgical intervention is recommended for ovarian tumors less than 6 cm in diameter or cases with clinical symptoms [8].
Despite 314,000 new cases annually, effective tools for early ovarian cancer screening in the general population are currently lacking. Ovarian cancer’s inconspicuous symptoms in its early stages, coupled with the low pelvic location of the ovaries, contribute to delayed detection, resulting in advanced-stage diagnoses [9]. Consequently, the challenge of identifying ovarian carcinoma in its early stages persists, leading to escalating treatment costs and patient morbidity. The financial burden of ovarian carcinoma treatment remains the highest among all cancer types, with an initial average cost of approximately $80,000 in the first year and a subsequent increase to $100,000 in the final year [10, 11].
Meanwhile, ovarian carcinoma was also defined as the second most common female reproductive system tumor in terms of mortality (207,000 deaths annually). Ovarian cancer is insidious and progresses rapidly. It is prone to invasion and metastasis in advanced stages, which results in poor prognosis and high patient mortality [12, 13]. Shih et al. [14], based on clinicopathological and molecular studies, proposed a progression model for ovarian cancer, dividing epithelial ovarian tumors into two main groups: type I and type II tumors. Type I tumors are considered relatively inert and genetically stable, often originating from identifiable precursor lesions such as endometriosis or borderline tumors with low malignant potential. Conversely, type II tumors are deemed biologically aggressive, characterized by a propensity for early metastasis from a small primary lesion. High-grade serous ovarian cancer, the most prevalent type, constitutes approximately 75% of epithelial ovarian cancers, follows the type II pathway, and is marked by p53 and Breast Cancer Susceptibility Gene (BRCA) mutations. Ovarian malignant cells possess high invasion and metastatic capacity. However, tumor cells and infiltrating immune cells, the main components of the tumor, determine the malignant phenotype of the tumor [15, 16].
Tumor-infiltrated immune cells (TIICs), whose composition and function subtly vary depending on the host’s immune status, have been correlated with the clinical outcomes of different cancers [17, 18, 19, 20]. Mechanistic studies have reported that, the changes of the composition of TIICs and malignant cells in the tumor stroma may affect the immune function and play an important role in promoting or inhibiting tumor progression. Recent studies demonstrated that the composition of TIICs was related to the prognosis of breast and colorectal cancer [21, 22]. Unfortunately, the landscape and clinical implications of TIICs in the ovarian tumor microenvironment remain unclear due to technical limitation [23]. The development of the mutagen method Cell type Identification by Estimating Relative Subsets of known RNA Transcripts (CIBERSORT) has received extensive attention. Relying on the deconvolution algorithm based on gene expression, the relative difference of expression between a group of genes and all other genes in one sample can be examined by using CIBERSORTAs (Stanford University, Stanford, CA, USA) [22, 24]. Using this approach can precisely predict the landscape of tumor- infiltrated immune cells.
Due to these advantages of CIBERSORT, its superiority in cell heterogeneity studies has drawn wide attention. In this study, we quantified 22 TIICs subpopulations using CIBERSORT for the first time to elucidate the relationship between TIICs and molecular subpopulations, diagnosis, and survival of ovarian cancer patients. The content distribution of immune cells in ovarian cancer tissues was preliminarily verified by quantitative real-time polymerase chain reaction (qRT-PCR). This study provides some significant insights into the intricacy correlation between the TIICs heterogeneity and ovarian cancer progression.
All the data used in this study was extracted from public databases. We focused on the different gene expression matrices between epithelial ovarian carcinoma and normal ovarian epithelial tissues. We downloaded publicly available gene expression datasets from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). Datasets with recurrent ovarian cancer or chemotherapy utilization were excluded. Analyzing the sample annotation information of the three chips, the tissue samples we collected were high-grade serous ovarian adenocarcinoma and normal ovarian epithelial tissue. Gene expression data and corresponding prognostic information for epithelial ovarian cancer were obtained from TCGA database (https://cancergenome.nih.gov/). The last data update was reported on October 4, 2022.
Downloaded gene expression data was normalized and filtered. Then the CIBERSORT algorithm was utilized to infer the abundance of 22 types of invasive immune cells in ovarian cancer and normal ovarian tissues, including B cells, eosinophils, macrophages, dendritic cells, mast cells, monocytes, neutrophils, natural killer cells (NK), plasma cells, T cells and their different subtypes.
The expression profile of the “signature matrix” constituting 547 reference
genes was used by CIBERSORT. These profiles were used for describing the ratio of
diverse cell classifications in a tumor sample. A p-value was derived by
CIBERSORT for the deconvolution for each sample that used Monte Carlo sampling,
which provides a confidence evaluation for the results. We sorted the data
obtained and excluded the data with p
A total of 10 ovarian cancer specimens and 10 ovarian cancer adjacent specimens were collected from patients from 2023 to 2024 who were diagnosed with ovarian cancer in the Yijishan Hospital of Wannan Medical College (Wuhu, Anhui, China). Other malignant tumors were ruled out in the individuals who were included. Following surgical excision, the tissue was promptly frozen in liquid nitrogen for future study. Each patient who was enrolled completed an informed consent. The Yijishan Hospital of Wannan Medical College Ethics Committee approved this study. The ethical approval number is 2021(37).
Total RNA was extracted using TRIzol reagent (15596026CN, Invitrogen, Waltham, MA, USA) to ovarian cancer tissues and adjacent tissues. Microphotometer (MSP300R, Roche, Basel, Switzerland) was used to measure the quality of total RNA. The complementary DNA (cDNA) was obtained using Quantscript RT Kit (KR103-04, Tiangen, Beijing, China) applied to total RNA. SYBR Green mRcute mRNA qPCR Kit (FP411-02, Tiangen, Beijing, China) was used to conduct quantitative real-time polymerase chain reaction (qRT-PCR) on a Lightcycler 480 system (Roche, Basel, Switzerland). The internal parameters that were detected included CD80, CD4+, CD21 and CD66b with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All experiments were repeated 3 times. The primer sequences are shown in Table 1.
| The primer | Sequences |
| CD80 | F-5 |
| R-5 | |
| CD4+ | F-5 |
| R-5 | |
| CD21 | F-5 |
| R-5 | |
| CD66b | F-5 |
| R-5 | |
| GAPDH | F-5 |
| R-5 |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cases with a CIBERSORT p-value
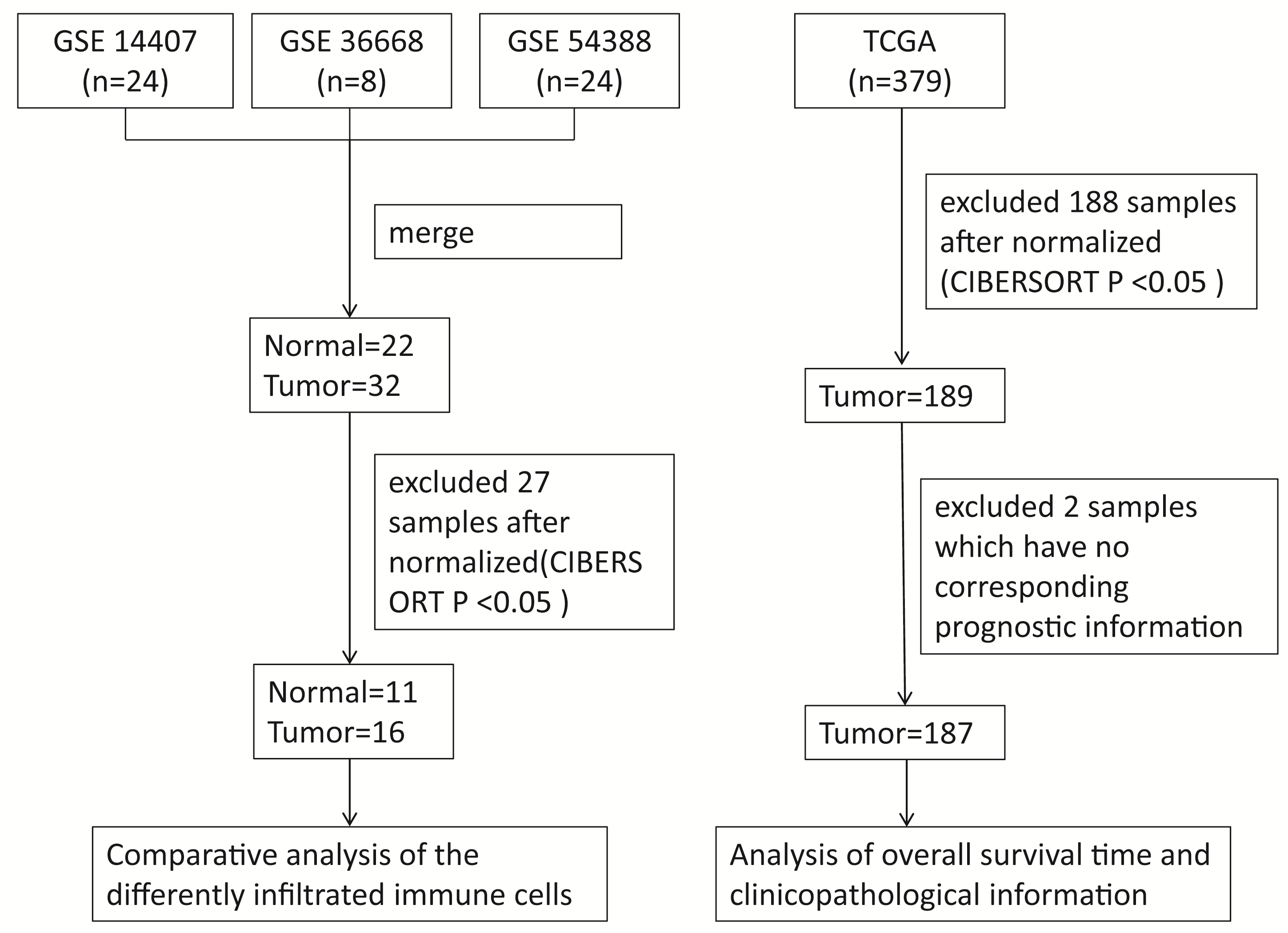
Gene expression microarray datasets of epithelial ovarian cancer and normal ovarian tissues, including GSE14407, GSE36668 and GSE54388, were downloaded from the GEO database. All the datasets used in this study were obtained by the GPL570 platform (https://www.ncbi.nlm.nih.gov/gds/?term=GPL570). We merged all datasets into one dataset that contained 22 normal and 32 tumor samples. A total of 27 samples were excluded after normalization and filtering by the R software, including 11 normal samples and 16 tumor samples. Similarly, the gene expression data and corresponding prognostic information of 187 valid samples from 379 tumor samples, downloaded from the TCGA database were selected for the further analysis after normalization and filtering. The flow chart of the study design is shown in Fig. 1.
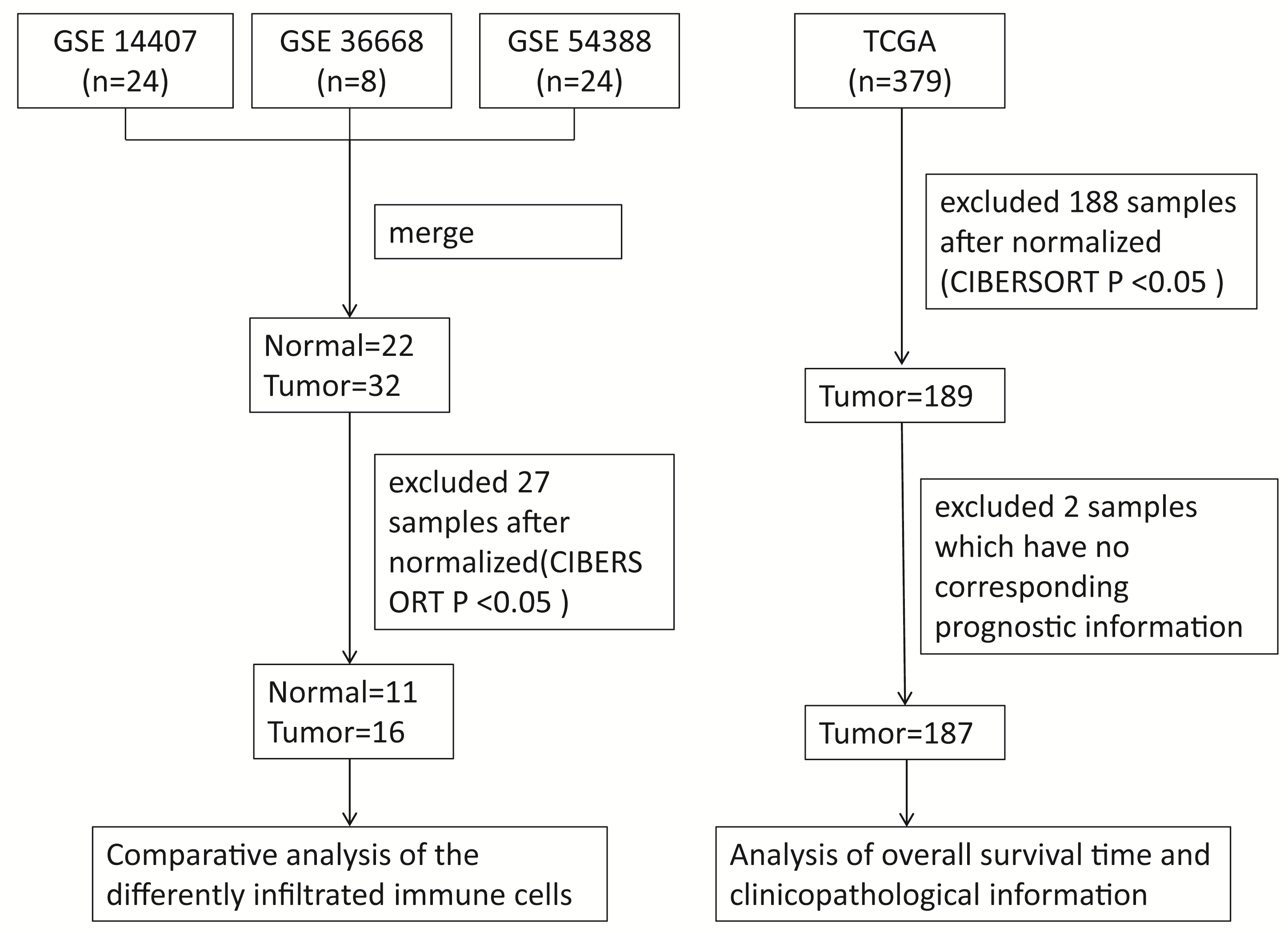 Fig. 1.
Fig. 1.Representative the flowchart of the study design and samples at each stage of analysis. CIBERSORT, Cell type Identification by Estimating Relative Subsets of known RNA Transcripts.
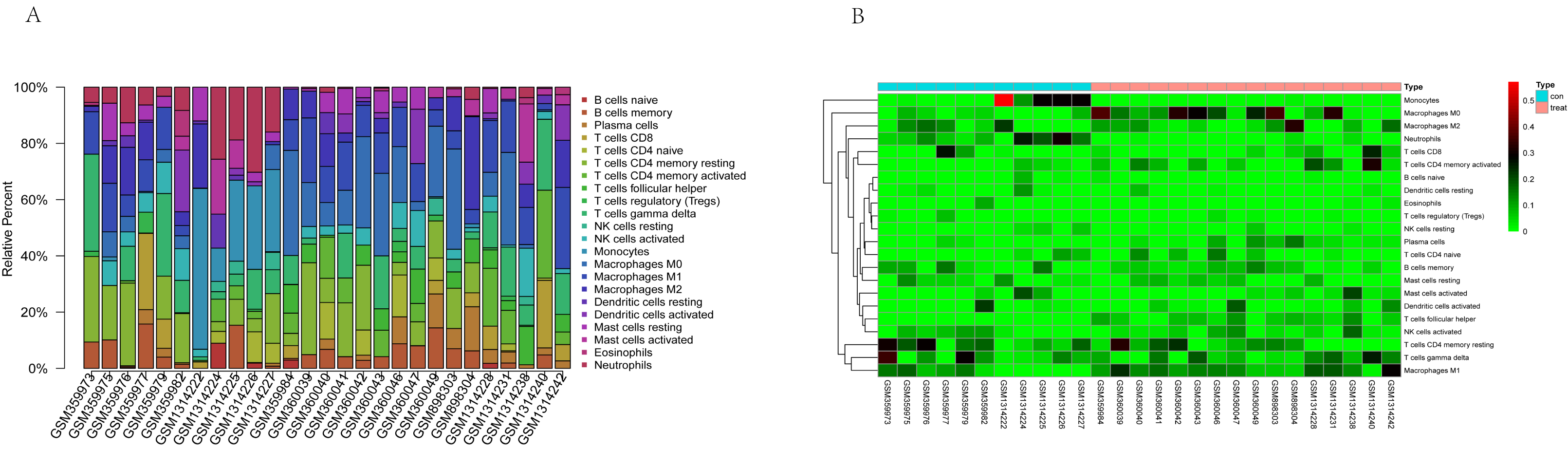
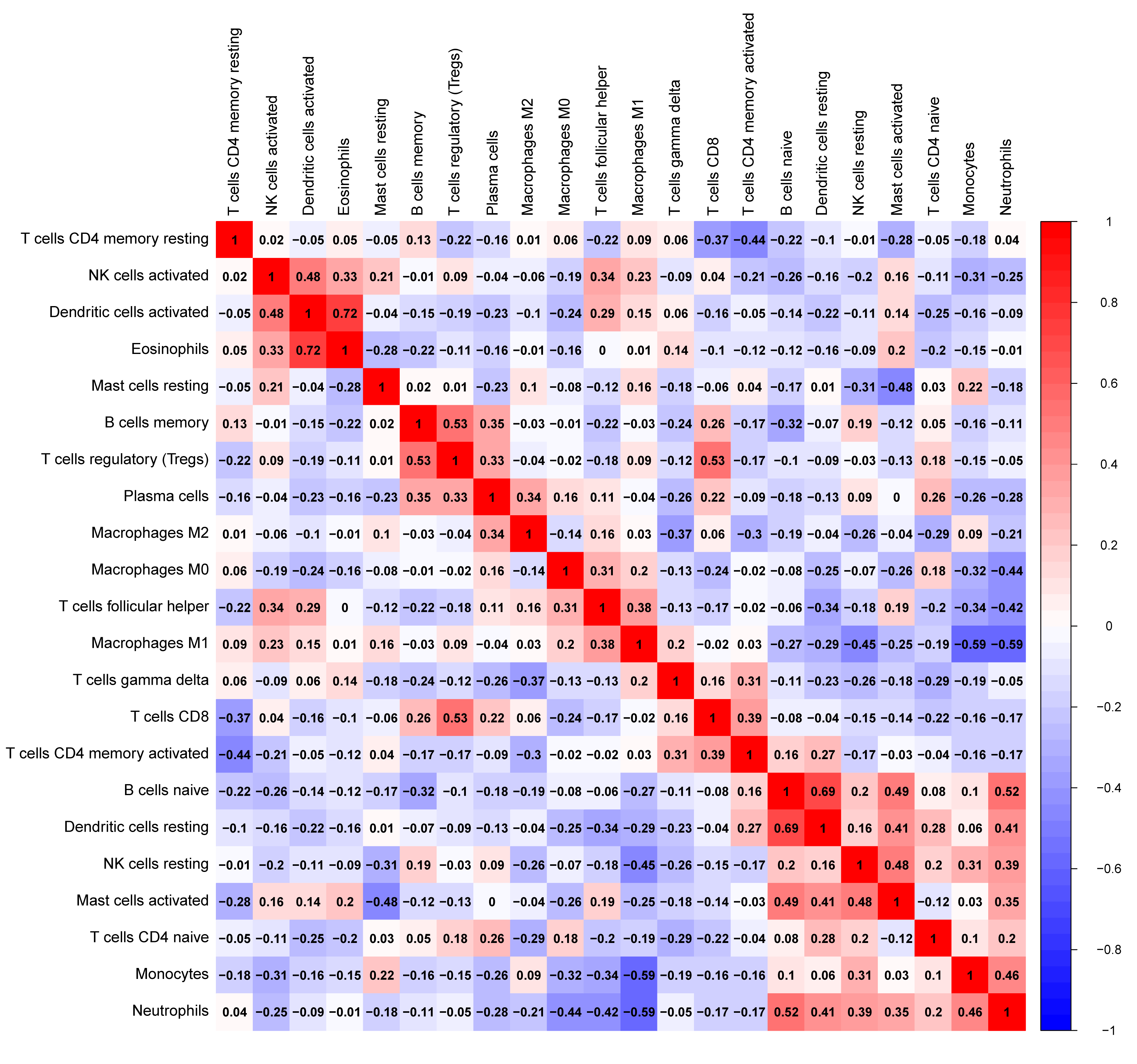
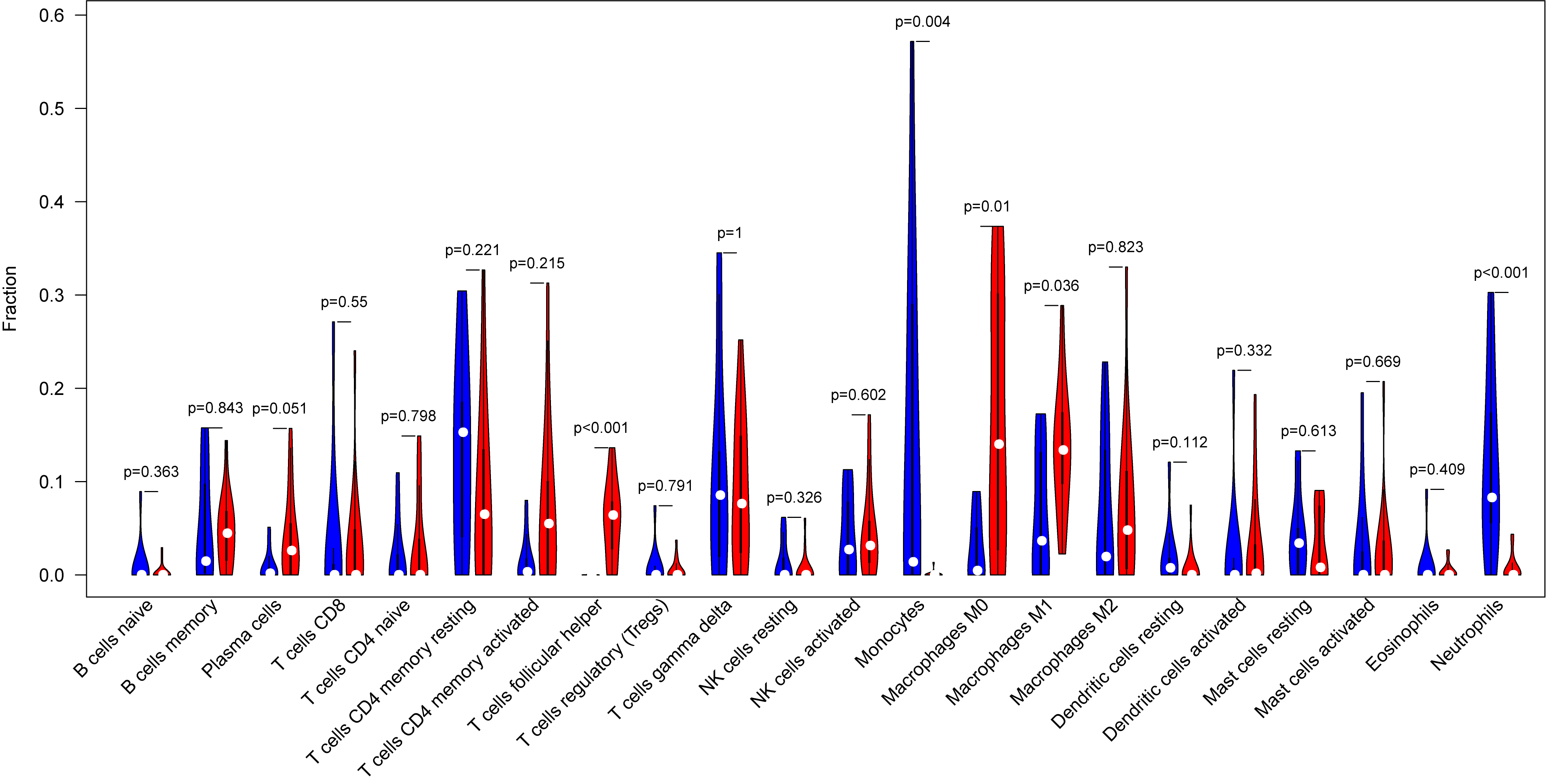
The tumor-infiltrated immune cells, especially those with low abundance, have not been fully defined in ovarian neoplasm because of the lack of detection methods. Taking advantages of the newly reported CIBERSORT algorithm, our team researched the infiltration of 22 immune cell subgroups in ovarian cancer tissue, and found that its composition was different from normal ovarian tissue. The results obtained from 11 normal tissue samples and 32 ovarian cancer samples were summarized using R software. As shown in the bar plots and heatmap, the proportions of TIICs in ovarian cancer vary significantly between benign and malignant neoplasms (Fig. 2). In addition, the proportions of different TIICs subgroups were also relevant (Fig. 3). The expression profile of resting dendritic cells was positively correlated with naive B cells (0.69), while the expression profile of activated dendritic cells was positively correlated with eosinophils (0.72). Compared to the normal ovarian tissue, higher content of M0 and M1 macrophages, T follicular helper cells (Tfh), and lower content of monocytes and neutrophils were observed in ovarian cancer tissue (Fig. 4). These results suggested that abnormal immune cell infiltration was related to the occurrence and development of the ovarian neoplasm, which provides better understanding for the biological behavior of ovarian cancer.
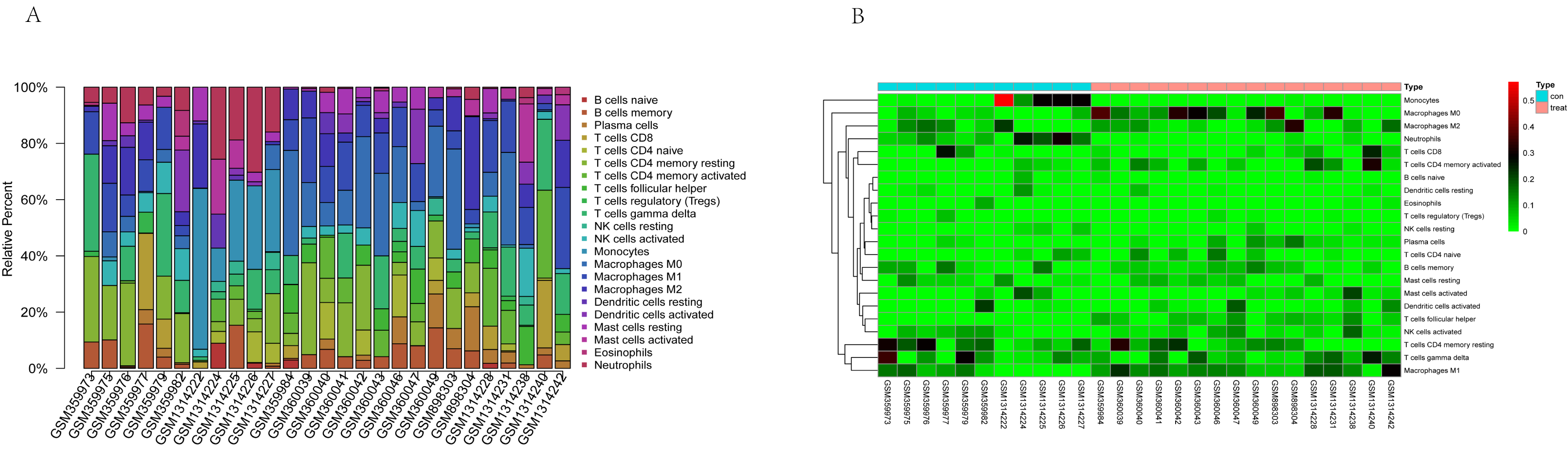 Fig. 2.
Fig. 2.Representative bar plot (A) and heatmap (B) of tumor-infiltrated immune cells in ovarian cancer and normal tissue. Bar plots and heatmap were drawn in R environment, with 11 normal tissue samples and 32 ovarian cancer samples. The proportions of tumor-infiltrated immune cells (TIICs) in ovarian cancer vary significantly between benign and malignant neoplasia.
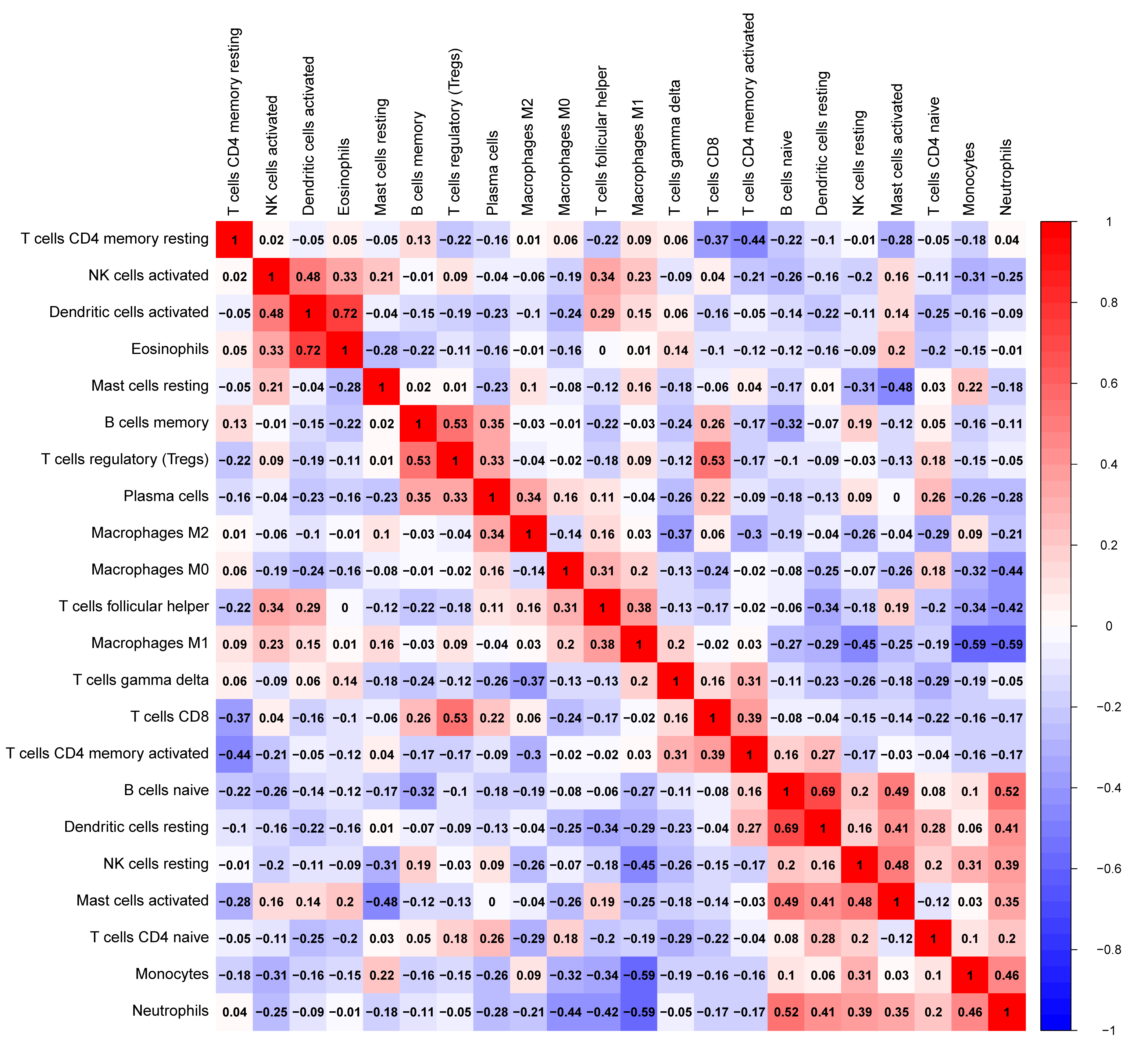 Fig. 3.
Fig. 3.The correlation heatmap of expression of 22 tumor-infiltrating immune cells in ovarian cancer and normal tissue. Resting dendritic cells were positively correlated with naive B cells (0.69); activated dendritic cells were positively correlated with eosinophils (0.72).
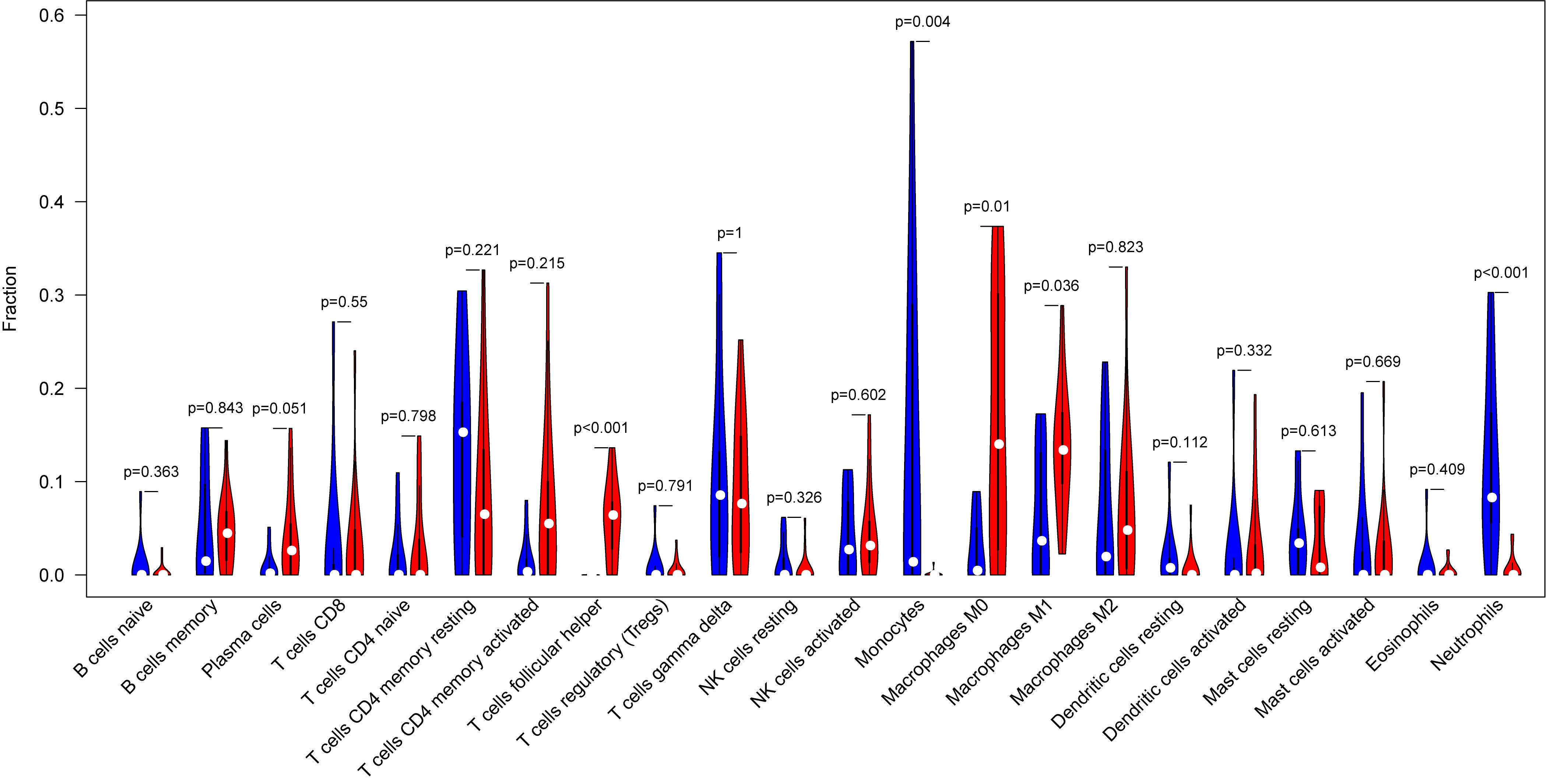 Fig. 4.
Fig. 4.Representative the vioplot of tumor-infiltrated immune cells in ovarian cancer tissue. Compared to normal ovarian tissue, ovarian cancer tissue generally contained higher proportion for T follicular helper cells (Tfh), M0 and M1 macrophages, and lower proportions of monocytes and neutrophils.
After excluding the samples with missing information, the data of the survival
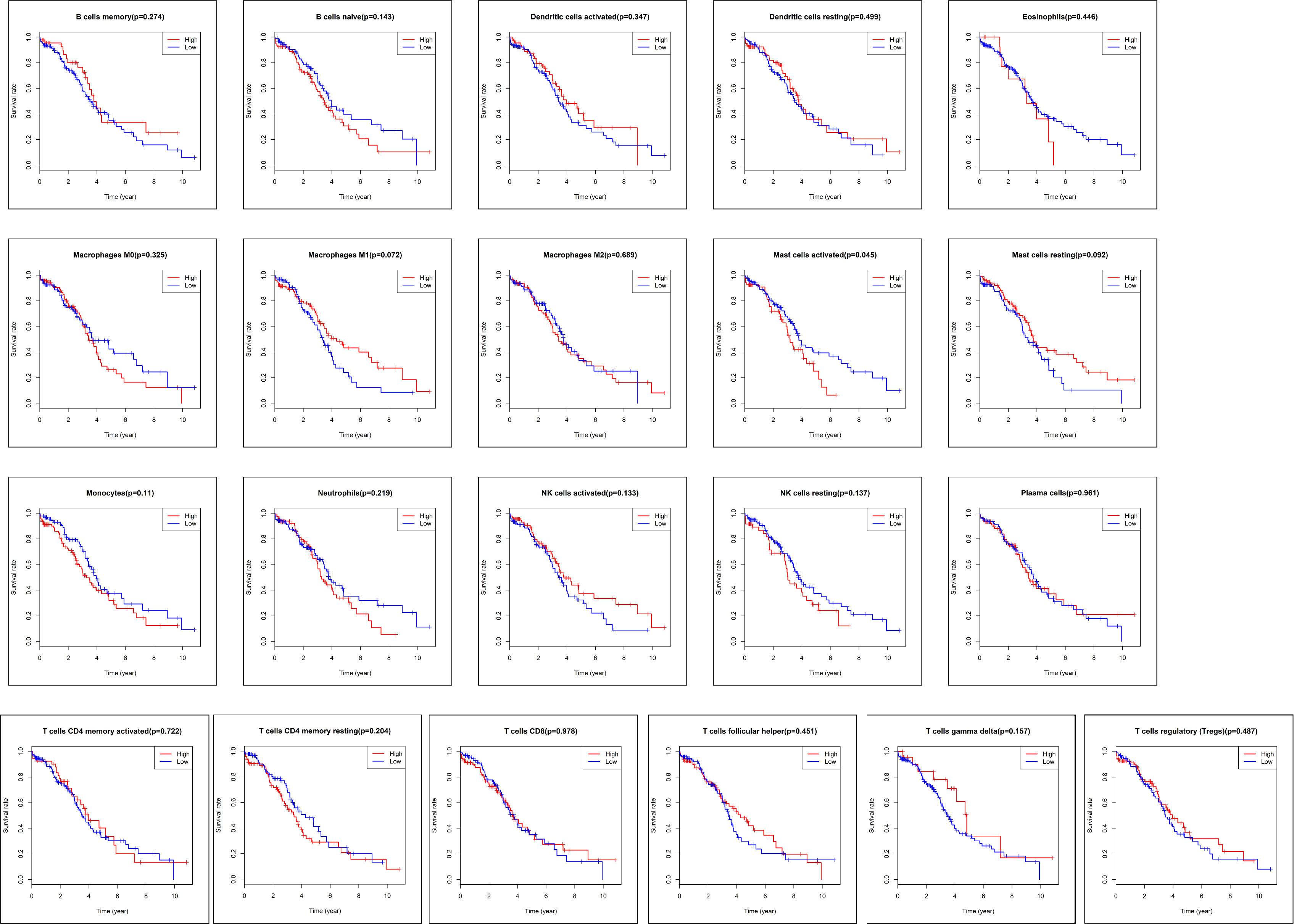
time and survival status for 187 samples were downloaded from TCGA database (Fig. 1). Kaplan-Meier analysis (Fig. 5) and log-rank test demonstrated that only the
subpopulations of activated mast cells was associated with poor prognosis in
ovarian cancer patients (p
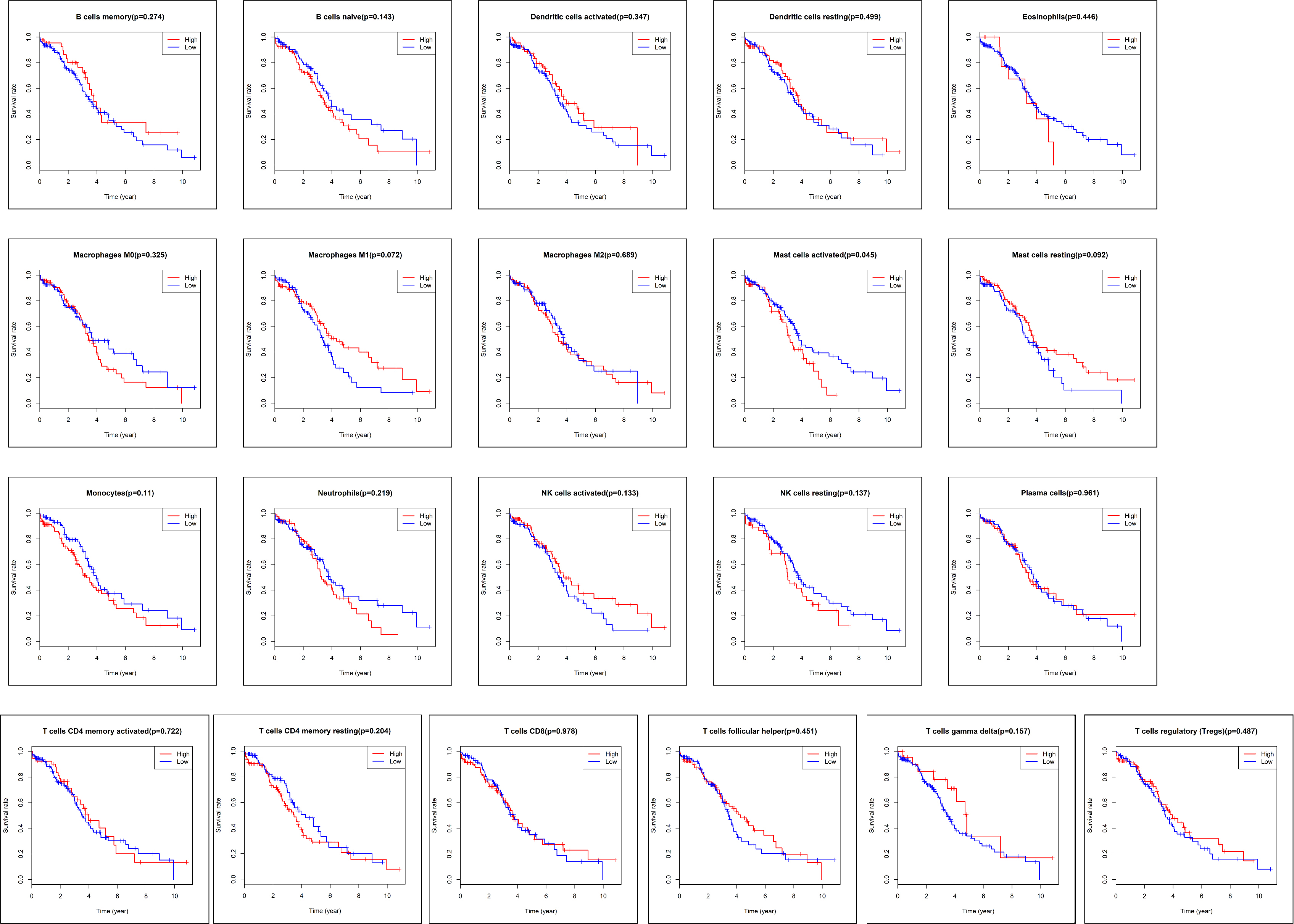 Fig. 5.
Fig. 5.The Kaplan-Meier survival curve of tumor-infiltrated immune cells in ovarian cancer. In 22 subpopulations of tumor-infiltrated immune cells, only activated mast cell subpopulations were associated with poor prognosis in patients with ovarian cancer (p = 0.05).
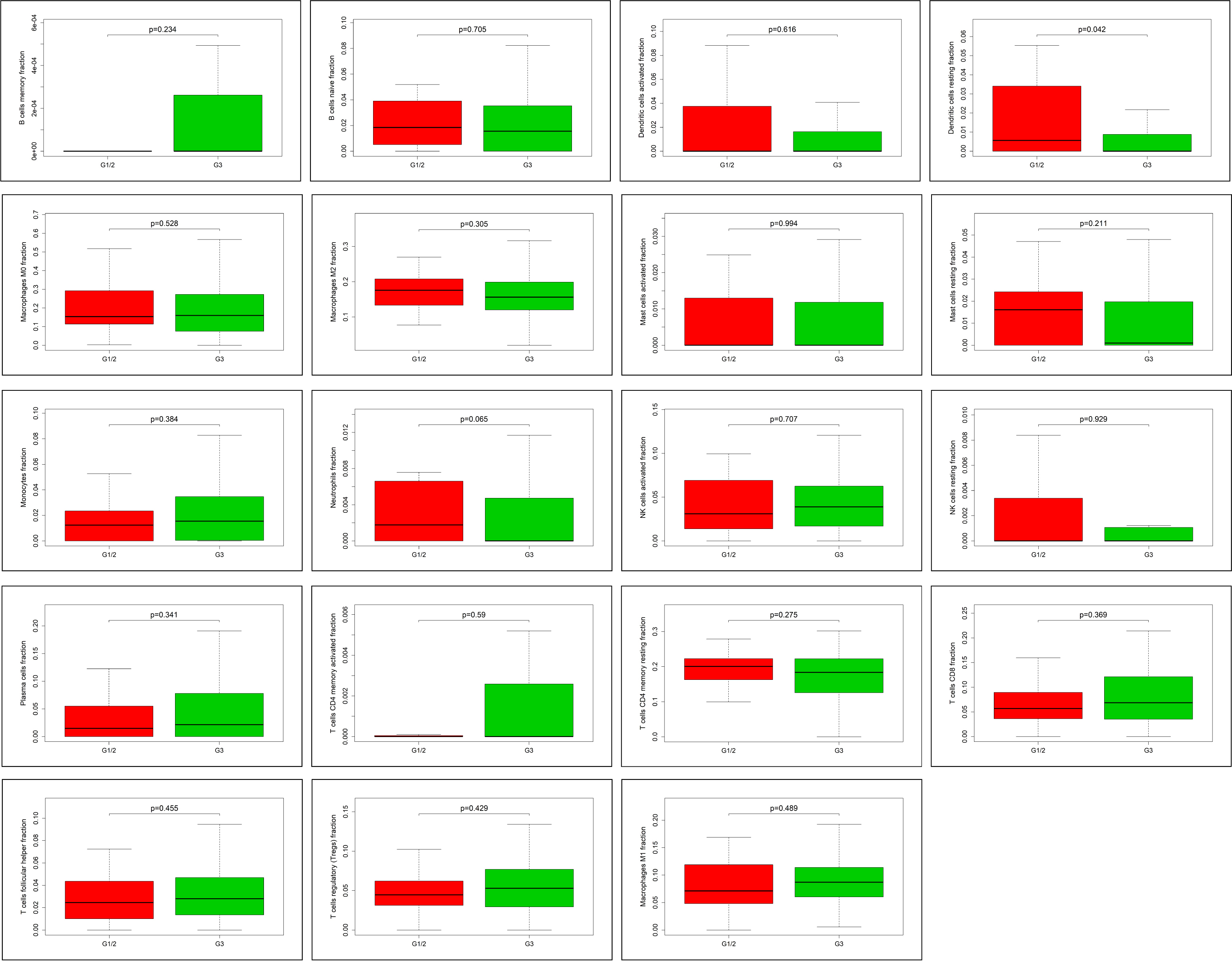
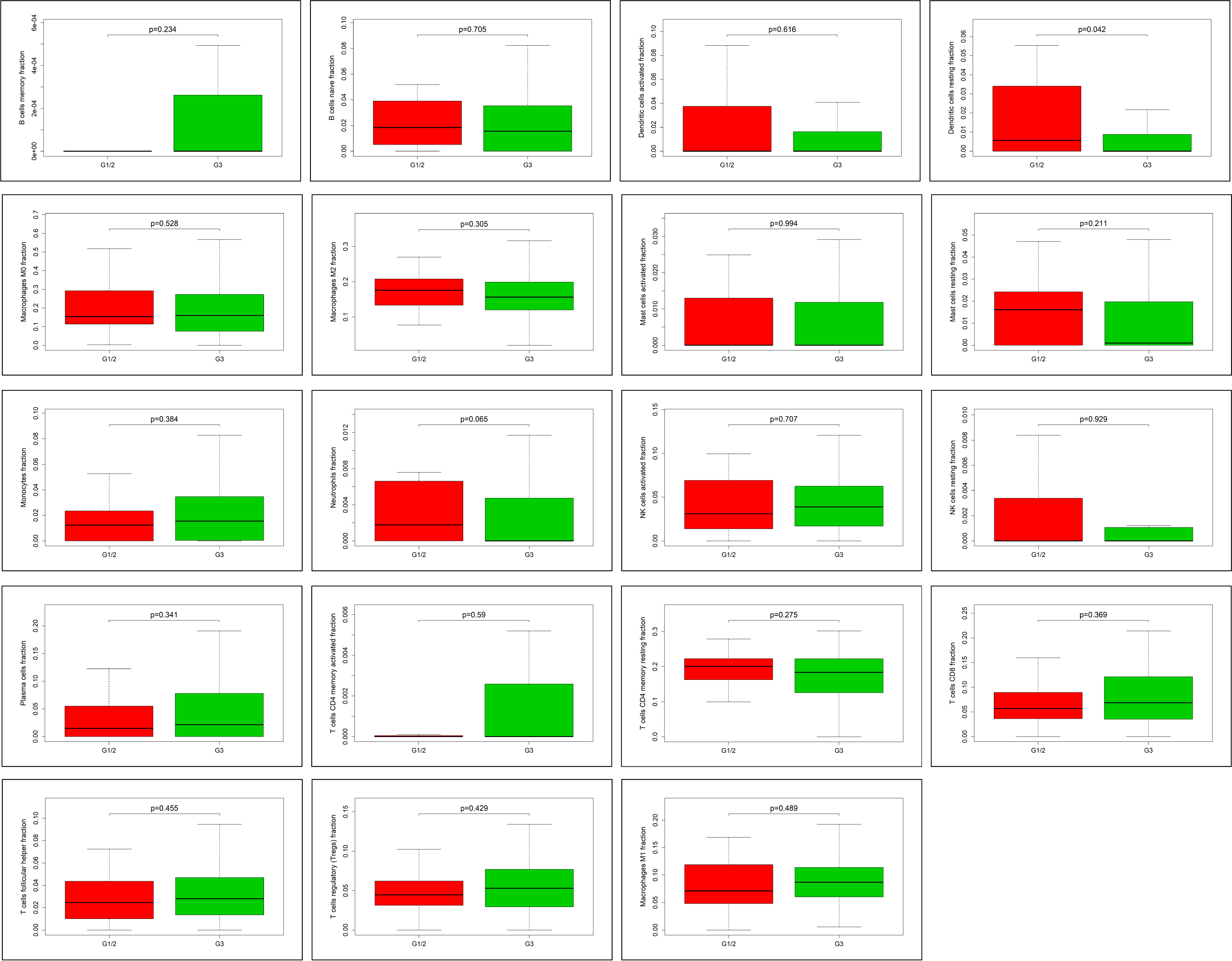 Fig. 6.
Fig. 6.The correlation between tumor-infiltrated immune cells and
clinicopathological information. In 22 subpopulations of tumor-infiltrated
immune cells, only resting dendritic cells are associated with pathological grade
of ovarian cancer, and highly expressed in G1/2 (p
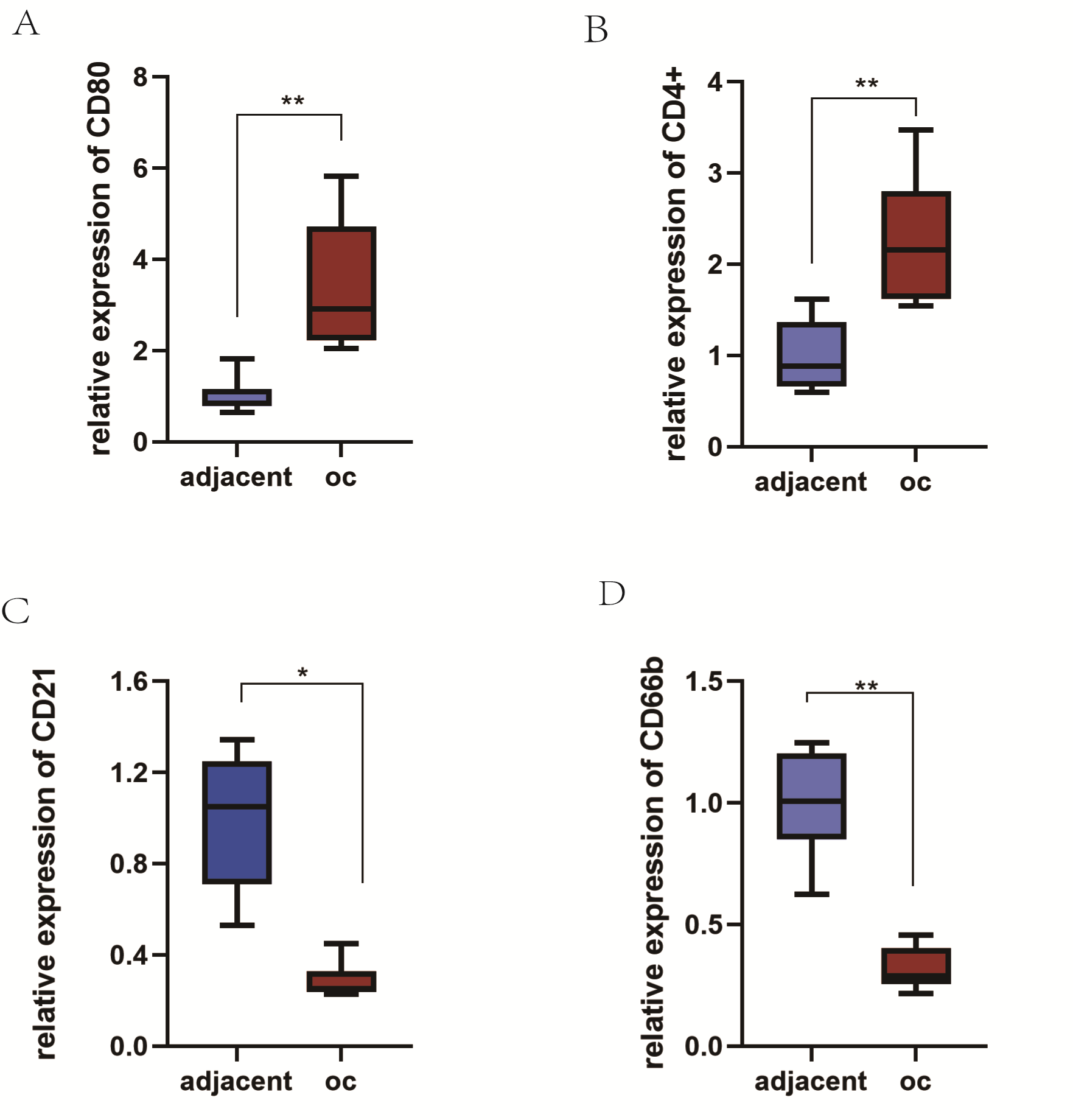
To further confirm the expression of TIICs between different tissues, we
collected 10 ovarian cancer specimens and 10 para-cancerous tissues. qRT-PCR was
used to test the content of the signature protein on the surface of the immune
cells at the transcriptional level. The results suggested that the mRNA
expression levels of CD80 and CD4+ in ovarian cancer tissues were significantly
higher than those in adjacent non-tumor tissues, with the contents of M1 cells
and Tfh cells being higher in ovarian cancer tissues (p
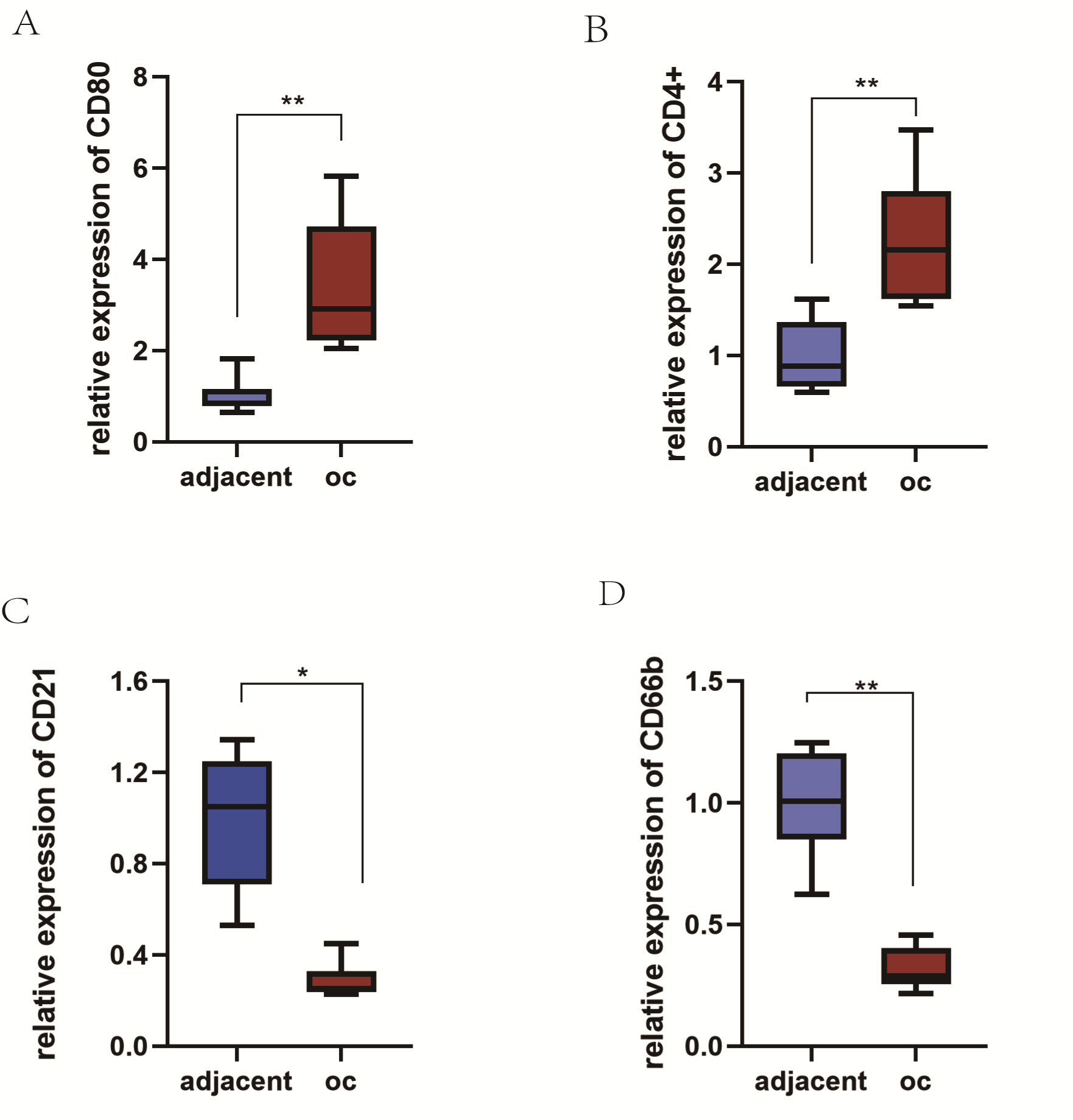 Fig. 7.
Fig. 7.The expression of tumor-infiltrating immune cells surface
markers in ovarian cancer and paracancer tissues was verified. The contents of
MI cells (A) and Tfh (B) were high in ovarian cancer, while the contents of B
cells (C) and neutrophils (D) were low (p
In addition to malignant tumor cells, immune cells, endothelial cells, fibroblasts, and a large arsenal of bioactive molecules are considered as the major components of solid tumors [23, 25, 26]. Ovarian cancer is the world’s deadliest gynecologic malignancy, with the majority of women with epithelial ovarian cancer in advanced stages [1, 27]. Despite the use of surgery and platinum-based chemotherapy, ovarian cancer continues to spread with frequent relapses and poor prognosis [28]. BRCA1/2 germline mutations, constituting the most potent known genetic risk factor for epithelial ovarian cancer, are found in 14% of diagnosed women. Given that BRCA1/2 carriers respond more favorably to platinum-based chemotherapy, their status serves as a valuable prognostic indicator. The timely detection and targeted treatment of BRCA1/2 status, although often diagnosed at advanced stages, can enhance survival prospects [29].
Consequently, an urgent imperative exists to identify novel targeted treatments for ovarian cancer, aligning with distinct mechanisms of tumorigenesis. Emerging research indicates frequent upregulation of the phosphatidyl-inositol 3 kinase activity (PI3K) pathway in epithelial ovarian cancer, contributing significantly to chemotherapy resistance and genomic stability preservation, crucial in DNA replication and cell cycle regulation [30, 31]. Thus, the application of PI3K inhibitors may impede ovarian cancer progression. The underlying principle involves PI3K inhibition leading to genomic instability and mitotic catastrophes by diminishing the activity of spindle assembly checkpoint protein Aurora kinase B, consequently elevating the occurrence of prolog chromosomes [32].
Intricate biological processes between immune cells and malignant cells in the tumor stroma affect the balance of the tumor microenvironment and tumor progression [33]. The tumor microenvironment plays a significant role in the development of ovarian cancer, particularly genomic research, transcriptome and proteome.
Through proteomic analysis, a deeper understanding of the regulatory mechanisms, interactions, and pharmacological mechanisms of signaling pathways in ovarian cancer is achieved, aiding in the discovery of potential biomarkers, disease mechanisms, and targeted therapies. PARP inhibitors, tailored to the proteomic characteristics of ovarian cancer, disrupt the double-strand DNA repair mechanism, induce synthetic lethality in cells, particularly in BRCA1/2-deficient tumor cells, leading to accumulation of double-strand DNA damage and apoptosis, thereby achieving therapeutic effects. This approach also reduces resistance to other chemotherapy drugs and improves patient prognosis [34, 35].
Tumor microenvironment (TME) may be an underlying therapeutic target in ovarian neoplasm [36, 37, 38]. Studies [24, 25] have confirmed that, as an important determinant of immunotherapy response, TIICs can influence the prognosis of lung cancer. Previous studies [39, 40] have shown that some genes, such as HLA-G, COX, may have an effect on the immune condition of ovarian neoplasm. HLA-G, a non-classical MHC class I molecule, plays a key role in immune regulation. In ovarian cancer immunotherapy, HLA-G likely contributes to immune evasion and suppression mechanisms. HLA-G overexpression inhibits NK cell and T cell activity, impairs antigen-presenting cell function, suppresses antigen-specific T cell activation, and promotes the generation of immunosuppressive cells, aiding tumor immune evasion and growth [41]. Additionally, HLA-G binding to receptors like immunoglobulin-like transcript2 (ILT2) and immunoglobulin-like transcript4 (ILT4) suppresses immune cell activity, reducing NK cell and T cell cytotoxicity against tumor cells, impairing antigen-presenting cell function, and lowering pro-inflammatory cytokine secretion, favoring tumor survival and progression. COX inhibition enhances ovarian cancer immunotherapy by suppressing inflammation, modulating immune cell activity, and influencing the tumor microenvironment [42, 43].
COX-produced metabolite prostaglandin E2 (PGE2) is an immunosuppressive factor, inhibiting antigen-presenting cell function, reducing T cell activation, proliferation, and suppressing NK cell and dendritic cell activity. Therefore, COX inhibition may enhance immunotherapy efficacy by reducing PGE2 levels [44, 45, 46].
A recent study found that in the new treatment of high-grade serous ovarian cancer (HGSOC), inflammatory microenvironment and immune cell rejection co-exist, revealing that there are universal existences in immune cells infiltration [23]. In this current study, the gene expression data were based on TCGA and GEO with a large number of deconvolutions. We investigated the association between different TIICs with the clinical outcome and then analyzed the landscape and prognostic value of TIICs in ovarian cancer by using the metagene deconvolution algorithm CIBERSORT.
Different from normal ovarian epithelial tissues, ovarian carcinoma tissues generally contained higher proportions of macrophages (M0 and M1) and T follicular helper cells (Tfh), whereas monocytes and neutrophils were relatively lower. Macrophages can be divided into M1 and M2 subtypes according to their immune function [47]. The M1 macrophages were considered as anti-tumoral macrophages, while M2 macrophages were considered as the pro-tumoral cells and are associated with distinct immunoregulatory functions [48, 49]. A study has confirmed that M1 macrophages secrete pro-inflammatory cytokines such as interferon-c (IFN-c), interleukin-16 (IL-16) and interleukin-12 (IL-12), which activate inflammatory responses, and induce tumor cells death [50]. The main reason for the rigidity and degradation of extracellular matrix (ECM) is cancer-associated fibroblasts (CAF), which interact with almost all cells in TME to regulate ECM components for carcinogenesis. The interaction of M2 cells and CAF is the key to ECM stiffness and degradation [51]. In general, tumor-associated macrophages resemble M2-polarized macrophages, which are key regulators of the immune-suppressive microenvironment [52].
However, certain studies have argued that M1 and M2 macrophages do not represent different cell types, and that the difference is that they represent a series of functional state of extreme [53, 54]. Tumors may contain various types of macrophages, and we found high expression of M1 macrophages in ovarian cancer tissues, which is likely to reflect the level of functionality. Similarly, Tfh cells were highly expressed in ovarian cancer tissues in our study. Tfh cells have a great effect on promoting B-cell differentiation and guide the antibody response in humoral immunity and immune-associated inflammatory diseases, such as infections, autoimmune diseases and cancers [55]. It is Tfh cells that play a decisive role in the selective evolution from the antigen-specific B cells and high affinity plasma cells to memory B cells [56].
Infiltration of Tfh cells was observed in breast tumors, especially in response to chemotherapy, suggesting the significant role of Tfh cells in the umor immune process [22, 57]. Most studies have confirmed that monocytes and neutrophils in tumor tissues can promote the sensitivity of malignant tumors to treatment [58, 59]. A previous study revealed that healthy women have significantly higher phagocytosis index of monocytes and neutrophils than ovarian cancer patients. The number of monocytes and neutrophils, and their phagocytic activity increased after ovarian cancer surgery. Ovarian cancer cells may secret a kind of cytokine that inhibits monocytes and neutrophils, and this cytokine is reduced after tumor removal [60]. This is consistent with the results of quiet infiltration of monocytes and centrosomes in ovarian cancer tissues in this study. Neutrophils are crucial in fighting infection and activating and regulating immunity. Tumor-associated neutrophils have become an important part of the tumor microenvironment. Studies have shown that neutrophils also cause tissues damage in various autoimmune and inflammatory diseases, and play an important role in cancer progression [61, 62, 63].
There are different TIICs between epithelial ovarian cancer and normal ovarian tissues, such as M0 and M1 macrophages, Tfh cells, monocytes and neutrophils, which can play a major role in the progression of ovarian cancer. With regards to the relationship between immune cells and ovarian cancer prognosis, we found that the increase of mast cells in tumor tissue was related to poor overall survival (OS). Mast cells can be activated and become pro-tumorigenic cells after direct contact with cancer cells [64]. Study has reported that activated mast cells could regulate hypothermia by affecting the function of temperature-regulating neuronsin allergic reactions [65]. This could be a reason why the infiltration of activated mast cells could lead to the decrease of OS in patients with ovarian cancer. In addition, a correlation between resting dendritic cells and ovarian cancer grade was also found in our study. Increased infiltration of resting dendritic cells could lead to better clinical outcomes, which helps achieve better diagnosis and prognosis of ovarian cancer.
Immune cell infiltration is heterogeneous, and previous studies were mostly limited to detecting immune responses as a result of technical limitations. Immunohistochemical based methods were used to analyze tumor immune cell infiltration, depending on individual surface markers to identify subpopulations of TIICs. However, this approach is not conducive in distinguishing closely related cell types. What’s more, many markers are expressed in different cell types, so it may lead to be confusing results. Therefore, inconsistent results are often observed in different clinical studies. It is necessary to develop reliable and accurate methods to verify the TIICs in the tumor-related microenvironment in future research. The sample size in this study may constrain the analysis of ovarian cancer progression in relation to other immune cell functions.
There exist some deficiencies in the study. Since the data samples are finite, this research could not cover all conditions. At present, analyses were conducted on three data sets, including GSE14407, GSE36668 as well as GSE54388, and 379 raw data of the TCGA data set. Ovarian cancer includes many pathological types, such as serous and mucinous carcinoma. The pathological grade can be divided into high-grade and low-grade cancers, and there are differences in malignant behavior. However, the data set is limited to the bioinformatics level, and the research does not distinguish between high-level and low-level. Therefore, the preliminary conclusions of this study need to be verified by a large number of clinical samples at the level of future experiments.
Our study reveals that the composition of tumor-infiltrating immune cells is altered in ovarian cancer and correlates with clinical outcomes. With the future validation of clinical samples, the results may provide a new strategic basis for the prognosis and treatment of ovarian cancer.
The data underlying this article are available in GEO databases, including GSE14407, GSE36668 and GSE54388 (https://www.ncbi.nlm.nih.gov/geo/). We obtained gene expression data and prognostic information of epithelial ovarian cancer from TCGA database (https://cancergenome.nih.gov/). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
These should be presented as follows: CD, GN and JD designed the research study. LW and CD performed the research. CS designed primer sequence and drew figures. GN reviewed entire manuscript. QM and LX analyzed the data. CD, QM and JD wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Wannan Medical College, Yijishan Hospital (approval number: 2021(37)).
We extend our sincere appreciation to all individuals who contributed to this manuscript. We thank the peer reviewers for their valuable insights and suggestions.
This research was funded by The National Natural Science Foundation of China (No.82201820), Young and middle-aged research projects of Wannan Medical College (No.WK202215), Anhui Province College Students’ Innovation and Entrepreneurship Projects (No.S202310368108), Wuhu City Science and Technology Program Achievement Transformation Project (No.2022cg26) and Scientific Research Fund for Introduced Talents of the First Affiliated Hospital of Wannan Medical College (No.YR20220220).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.