1 Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430022 Wuhan, Hubei, China
2 Department of Surgery, Wuhan Red Cross Hospital, 430015 Wuhan, Hubei, China
Abstract
Anaphase-promoting complex/cyclosome (APC/C) is a multi-subunit E3 ubiquitin ligase that recruits substrates for ubiquitination and subsequent degradation. As one of the two co-activators of APC/C, cell division cycle protein 20 (CDC20) plays a crucial role in cell cycle regulation. The objective of our study was to explore the therapeutic potential of targeting APC/CCDC20 in endometrial carcinoma (EC).
We performed comprehensive bioinformatics analysis to screen novel targets for EC treatment. The expression of CDC20 in normal endometrial and EC tissues was analyzed by immunohistochemistry. We treated EC cells with varying concentrations of APC/C inhibitors and evaluated their effects on cell proliferation and apoptosis using Cell Counting Kit-8 (CCK-8) assay and flow cytometry analysis. We performed wound healing and transwell assays to evaluate the migration ability of EC cell lines.
CDC20 was identified as a potential therapeutic target for EC. We found that the expression level of CDC20 in EC tissue is significantly higher than in nonmalignant tissue. Treatment with pro-Tosyl-L-Arginine Methyl Ester (TAME) inhibited the proliferation of EC cells in a time- and dose-dependent pattern. High concentrations of pro-TAME induced apoptosis in EC cells. Furthermore, the inhibitory effects of pro-TAME on EC cells were amplified by the co-addition of Apcin at low concentrations. However, treatment with pro-TAME did not affect the migratory ability of EC cells.
Our findings suggest that the inhibition of APC/CCDC20 by pro-TAME, in combination with Apcin, may represent a promising approach for the treatment of EC that warrants further investigation.
Keywords
- endometrial carcinoma
- anaphase promoting complex/cyclosome
- CDC20
- pro-TAME
Ubiquitination is an important post-translational modification catalyzed by a
series of enzymes, including ubiquitin-activating enzymes (E1),
ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3) [1]. Ubiquitin is
a 76-residue regulatory protein and ubiquitously expressed in eukaryotic cells
[2]. During the ubiquitination process, ubiquitin is activated and transferred to
the target substrate, which results in protein degradation through the 26S
proteasome complex [3]. Moreover, ubiquitination participates in several
proteasome-independent biological processes, including the inflammatory response,
immune regulation, signal transduction, genome stability and selective autophagy
[4, 5, 6, 7, 8]. Accumulating evidence has revealed that ubiquitination is tightly involved
in cell cycle regulation and tumorigenesis [9]. Specifically, it has been noted
that ubiquitination of proteins involved in critical signaling pathways plays a
crucial role in the development of endometrial carcinoma (EC), including the
Transforming Growth Factor-
The anaphase-promoting complex/cyclosome (APC/C) is an evolutionarily conserved multi-subunit E3 ubiquitin ligase that catalyzes the generation of polyubiquitin chains on its substrates via two co-activators, CDC20, and Cdh1 [13]. APC/CCDC20 directs ubiquitin-dependent degradation of cell cycle regulators, such as cyclin A, cyclin B and securin [14], and plays a critical role in cell cycle control, especially during the transition from metaphase to anaphase in mitosis [15]. In case of aberrant mitotic events, such as incorrect spindle assembly, the spindle assembly checkpoint (SAC) inhibits the activity of APC/CCDC20, thereby inducing mitotic arrest [16]. APC/C dysfunction may lead to uncontrolled cell proliferation and tumorigenesis. Notably, CDC20 has been found to be overexpressed in various types of cancers and functions as an oncogene, while Cdh1 is mainly reported as a tumor suppressor [14, 15, 17]. Therefore, inhibition of APC/CCDC20 may represent an attractive strategy for cancer therapies.
Tosyl-L-Arginine Methyl Ester (TAME) is an APC/C inhibitor that prevents the loading of CDC20, thereby stabilizing APC/C substrates [18, 19]. A TAME prodrug (pro-TAME), which is cell-permeable while TAME is not, can be converted into the active form of TAME by intracellular esterases [18]. In addition, Apcin is another cell-permeable APC inhibitor and inhibits the binding of substrates to CDC20, thereby slowing down APC/C-dependent proteolysis [20, 21]. However, Apcin has been reported to be an inefficient inhibitor of APC/C because the substrates of APC/CCDC20 can outcompete Apcin for binding to CDC20 or be recruited to APC/C through other mechanisms [21].
In the present study, we aim to investigate whether APC/CCDC20 could serve as a target for the EC treatment. The results confirmed that pro-TAME inhibited proliferation and induced apoptosis in EC cells. Notably, the combination of pro-TAME and Apcin at minimal doses significantly suppressed EC cell proliferation.
Based on criteria such as sample size, data integrity, and unified platforms, we
searched the Gene Expression Omnibus (GEO) database
(https://www.ncbi.nlm.nih.gov/geo) and selected GSE17025 and GSE39099 for
bioinformatics analysis. Differentially expressed genes (DEGs) between EC and
normal endometrial tissues were screened using the GEO2R online program, with
screening criteria at
Human EC cell lines, AN3CA (catalog number: GDC0127) and KLE (catalog number: GDC0115), were purchased from the China Center for Type Culture Collection (Wuhan, Hubei, China). The cell lines used were tested for mycoplasma contamination and the results are negative (Supplementary Fig. 1) and verified by short tandem repeat (STR) verification. Cells were cultured in Minimum Essential Medium (MEM) or Dulbecco’s Modified Eagle Medium (DMEM)/F12 medium containing 10% (v/v) fetal bovine serum (all from Life Technologies, Grand Island, NY, USA) at 37 °C with 5% CO2. Pro-TAME was purchased from Boston Biochem, Inc. (Cambridge, MA, USA). Apcin was purchased from Sigma–Aldrich Co. LLC. (St. Louis, MD, USA).
EC and normal endometrium tissues were fixed in formaldehyde and dehydrated in alcohol before being embedded in paraffin. Sections (5 µm thickness) were stained with HE and subsequently observed under a light microscope (Olympus IX71, Olympus Corp., Tokyo, Japan).
After deparaffinization and hydration through an ethanol gradient, the sections were boiled in EDTA buffer (Sigma–Aldrich Co. LLC., St. Louis, MO, USA). Next, the sections were treated with 3% hydrogen peroxide (H2O2, catalog number: 73113762, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) to eliminate endogenous peroxidase activity. After blocking with 5% goat serum (catalog number: G1208, Servicebio, Wuhan, Hubei, China), the sections were incubated overnight at 4 °C with rabbit polyclonal antibody specific to CDC20 (1:200, catalog number: 10252-1-AP, Proteintech, Wuhan, Hubei, China). The sections were washed with phosphate-buffered saline (PBS) and subsequently incubated with a biotinylated secondary antibody. The immunohistochemical staining was visualized using a 3,3′-Diaminobenzidine (DAB) detection kit (DAKO, Glostrup, Denmark), and the sections were counterstained with hematoxylin. The sections were observed under a light microscope (Olympus IX71, Olympus Corp., Tokyo, Japan). The optical density values were measured using Image J software (Version1.51j8, National Institutes of Health, Bethesda, MD, USA) for quantitative analysis.
Cells were lysed on ice using radio-immunoprecipitation assay (RIPA) buffer (catalog Number: G2002, Servicebio, Wuhan, Hubei, China) supplemented with a protease inhibitor. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (catalog number: G2043, Servicebio, Wuhan, Hubei, China) and transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with 5% skim milk (catalog number: GC310001, Servicebio, Wuhan, Hubei, China), the membranes were subsequently incubated overnight at 4 °C with primary antibodies specific to CDC20 (1:2000, catalog number: 10252-1-AP, Proteintech, Wuhan, Hubei, China) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2500, catalog number: ab9485, Abcam, Cambridge, MA, USA). The membranes were then washed with tris buffered saline tween-20 (TBST, catalog number: G2150, Servicebio, Wuhan, Hubei, China) and incubated with the secondary antibody (1:10000, catalog number: SA00001-2, Proteintech, Wuhan, Hubei, China) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence (ECL) detection reagent (Sigma–Aldrich Co. LLC., St. Louis, MO, USA).
Cells were seeded into 96-well culture plates at a density of
2
Cells were seeded in 6-well culture plates and cultured in complete media overnight. The following day, 15 µM pro-TAME alone or a combination of 10 µM pro-TAME with 25 µM Apcin was added to the culture media. After being treated with pro-TAME and Apcin for 72 h, cells were stained with Annexin V-Fluorescein Isothiocyanate/Propidium Iodide (FITC/PI) (BD Biosciences, Bedford, MA, USA) and analyzed using a flow cytometer (Beckman, DxFLEX, Brea, CA, USA) to evaluate the cell apoptosis.
Cells were seeded in 6-well culture plates and cultured until they reached
approximately 90% confluence. After a 24 h incubation in serum-free basal media
for starvation, the cell monolayers were scratched with a 200 µL pipette
tip to create linear wounds, and the media were replaced to fresh serum-free
basal media, with or without pro-TAME (15 µM). Images of
the wounds were captured under a light microscope (Olympus IX71, Olympus Corp.,
Tokyo, Japan) at 0 and 20 h after pro-TAME treatment. The
migration rates of the cells were determined as follows:
(width of wound at 0 h – width of wound at 20 h)/width of wound
at 0 h
Cells (1
The results are presented as the mean
GSE17025 contained 92 samples of EC and 12 samples of nonmalignant endometrium, while GSE39099 included data from 169 patients with EC and 20 patients with nonmalignant endometrium. A total of 4442 and 2447 DEGs were identified from GSE17025 and GSE39099, respectively. Among these, 832 genes were common to both datasets, including 305 upregulated and 527 downregulated DEGs (Fig. 1A,B). Upon analyzing the PPI network of the 832 genes, we identified 10 key genes that appeared in at least 7 out of the 12 algorithms within the CytoHubba plug-in, including KIF11, EGFR, MKI67, CDC20, FOXM1, RHOA, CHEK1, BIRC5, KIF23, and ZWINT (Fig. 1C). Additionally, the molecular complex module with the highest score of 48.423 was derived from MCODE plug-in (Fig. 1D), consisting of 53 genes. 8 of the 10 key genes, excluding EGFR and RHOA, were intersected with the module genes (Fig. 1C). As a hub gene, CDC20 has been reported to have oncogenic functions and was chosen for further investigation.
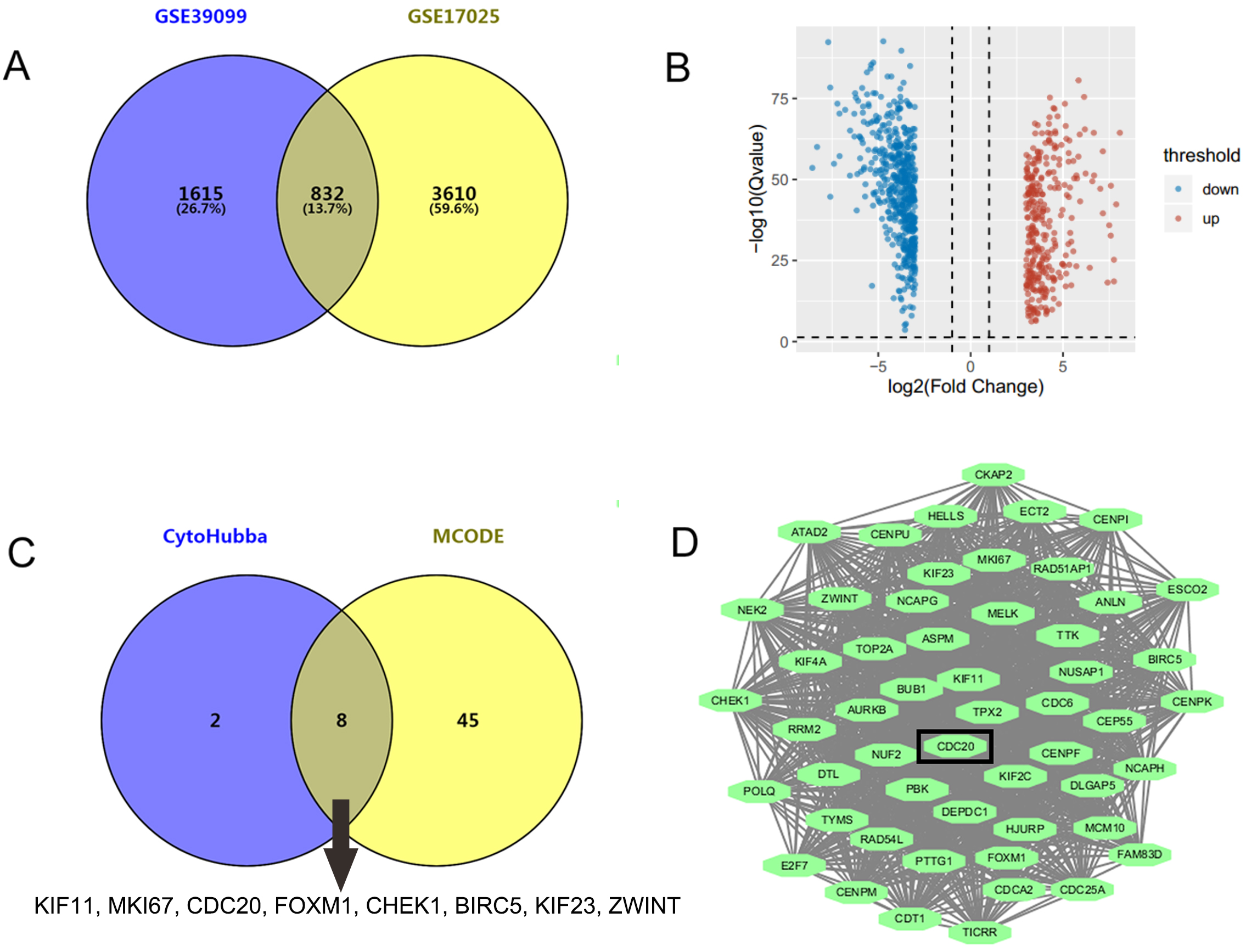 Fig. 1.
Fig. 1.
Identification of CDC20 as a novel target for EC treatment. (A)
A total of 832 genes were identified through the intersection of GSE17025 and
GSE39099 using the GEO2R online program, applying the screening criteria of
To investigate the potential roles of CDC20 in EC, we analyzed its expression levels using data from TCGA. The mRNA level of CDC20 was higher in the EC tissues compared to nonmalignant tissues (Fig. 2A). The histological morphology of nonmalignant and EC tissues was observed by HE staining. Compared with nonmalignant tissues, the crowded and densely-packed glands vary in size and are arranged without polarity in EC tissues (Fig. 2B). The immunohistochemical analysis indicated increased expression of CDC20 at protein level in EC tissues compared to nonmalignant tissues (Fig. 2B,C). These results suggest that CDC20 may play a significant role in EC.
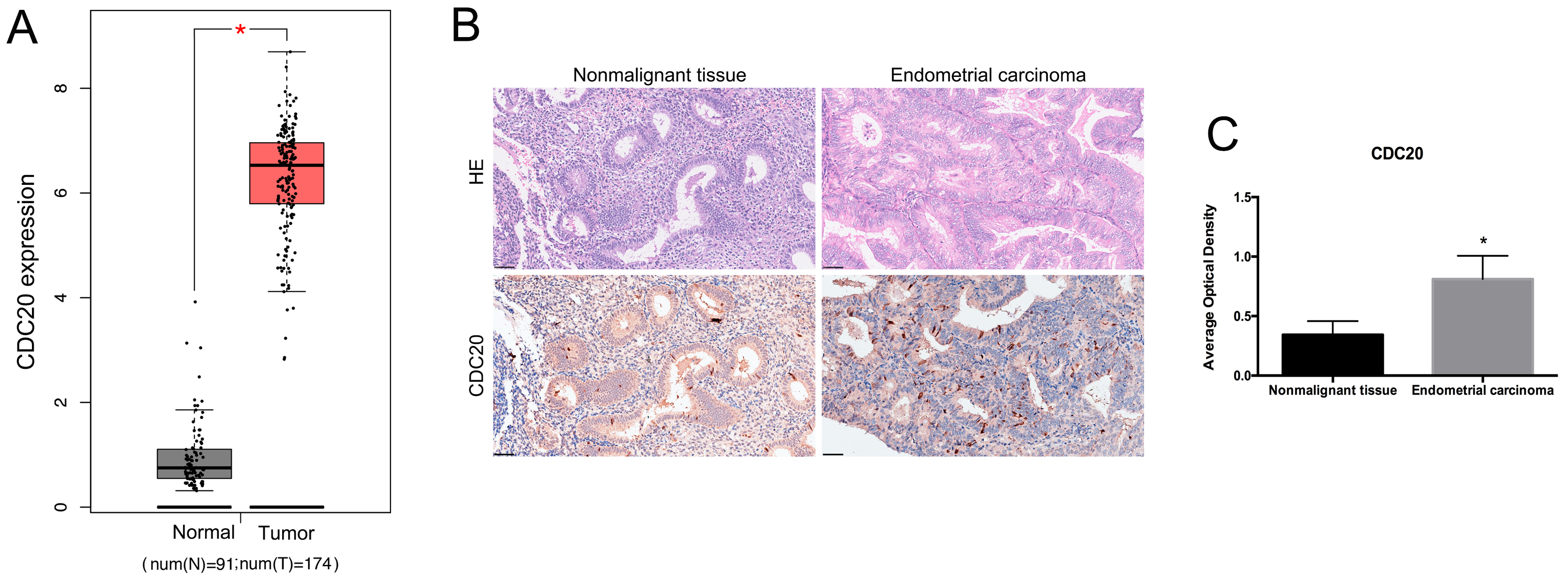 Fig. 2.
Fig. 2.
The expression of CDC20 in EC tissues. (A) Analysis of CDC20
mRNA levels in EC tissues and paired nonmalignant tissues from TCGA database.
*p
Western blot results revealed stable expression of CDC20 in
EC cell lines (Fig. 3A). The AN3CA and KLE cells were treated with increasing
concentrations of pro-TAME. The formula and structure of pro-TAME and Apcin are
shown in Fig. 3B,C. As shown in Fig. 3D, the proliferation of EC cells was
significantly suppressed by pro-TAME in a time- and
dose-dependent pattern. Treatment with 5–10 µM pro-TAME resulted in
slight growth inhibition in EC cells between 24 and 48 h. Low concentration (10
µM) of pro-TAME inhibited KLE cell proliferation significantly (cell
survival rate
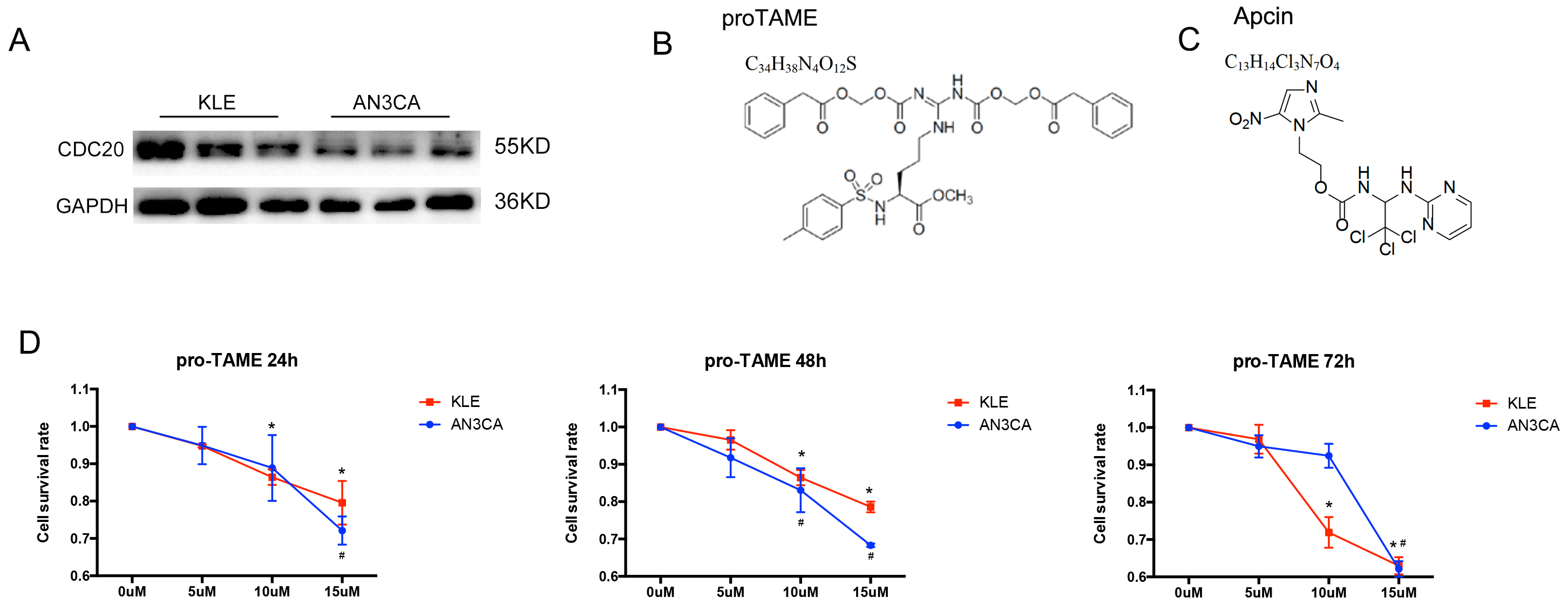 Fig. 3.
Fig. 3.
The effect of pro-TAME treatment on the growth of human EC
cells. (A) Validation of CDC20 protein levels by Western blot in AN3CA and KLE
cell lines. (B,C) The formula and structure of pro-TAME and Apcin. (D) A CCK-8
assay was conducted to detect EC cell proliferation following treatment with
increasing concentrations (0, 5, 10, and 15 µM) of pro-TAME at different
time point (24, 48 and 72 h). #p
Next, we conducted flow cytometry assay to detect cell apoptosis. Treatment with
high concentrations of pro-TAME (15 µM) for 72 h resulted in
significantly increased percentage of early apoptotic cells (lower right
quadrant, LR) and dead cells (upper right quadrant, UR), compared with the
control groups (p
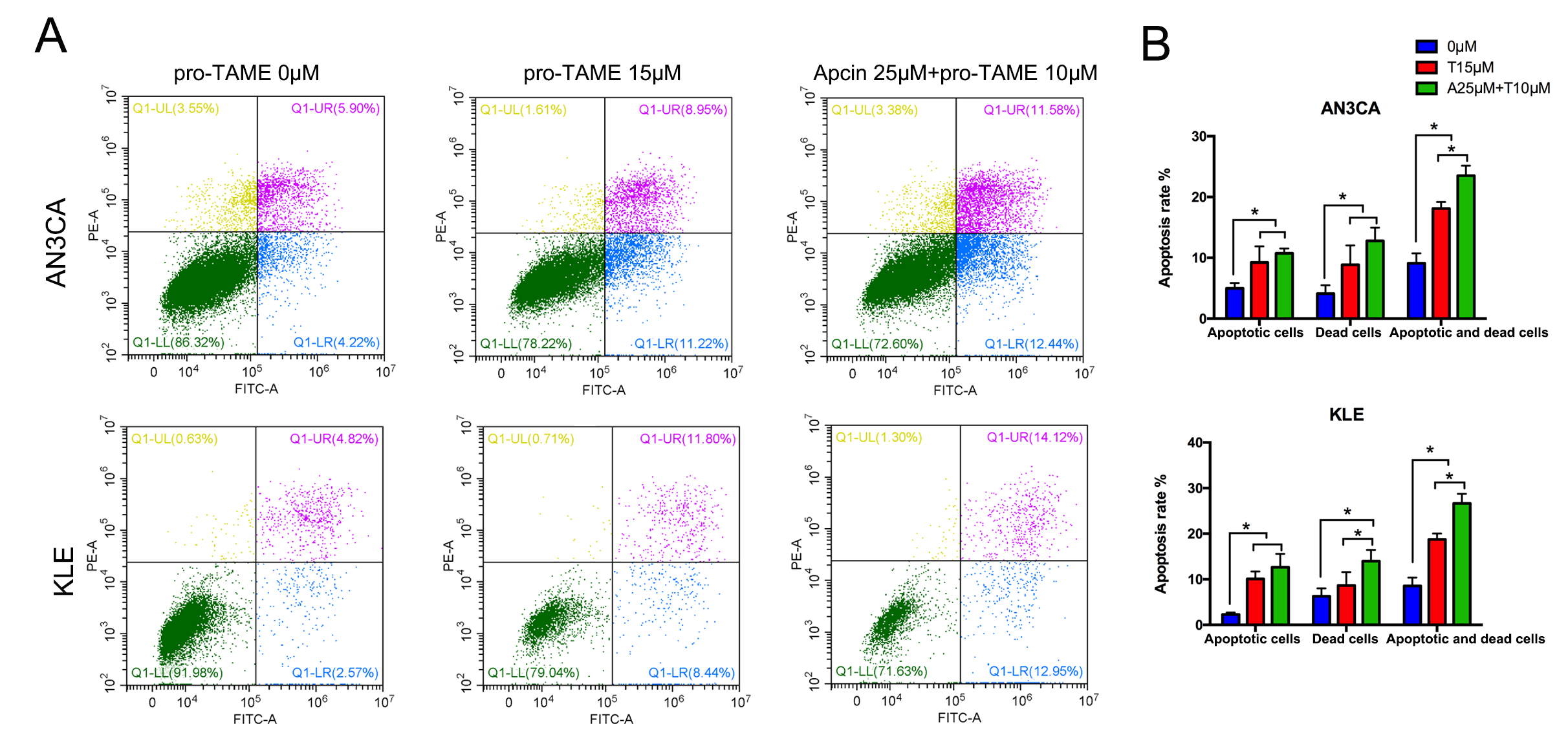 Fig. 4.
Fig. 4.
The effect of pro-TAME treatment on apoptosis of EC cells. (A)
EC cells were treated with either 15 µM pro-TAME alone or 10
µM pro-TAME in combination with 25 µM Apcin for 72 h.
Cell apoptosis was analyzed using flow cytometry. (B) Quantitative results for
apoptosis rates. *p
To explore the effects of pro-TAME treatment on metastasis ability of EC cells,
we conducted wound healing assay and invasion assay. No significant differences
were detected in the migration rates or the average numbers of migrated cells per
high-power field between pro-TAME-treated EC cells and control cells (p
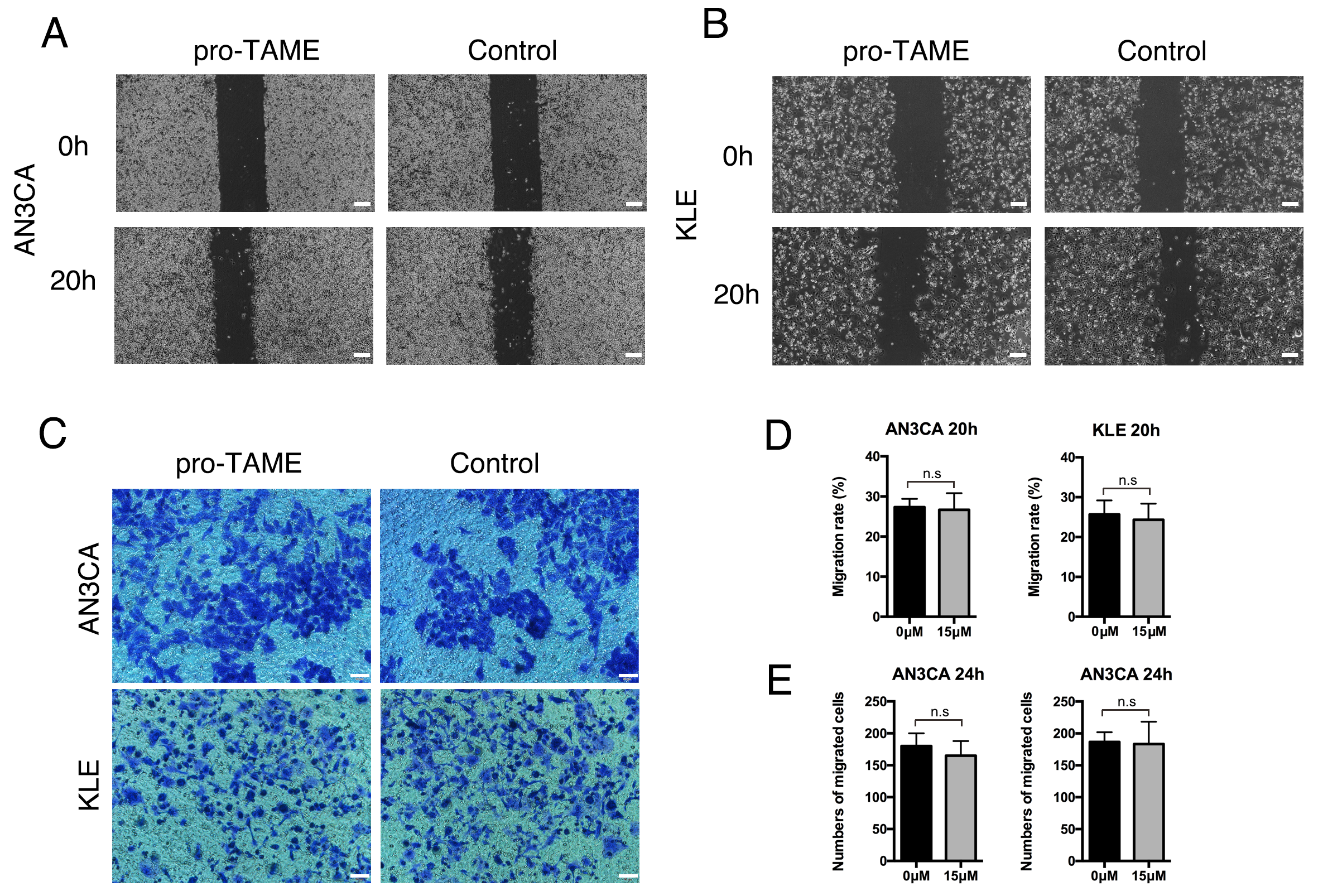 Fig. 5.
Fig. 5.
The effect of pro-TAME treatment on the migration and invasive ability of human EC cells. (A,B) The migration ability of pro-TAME-treated EC cells was assessed using a wound healing assay. (A) AN3CA cells. (B) KLE cells. The images were captured under a light microscope at 0 and 20 h. Scale bar, 200 µm. (C) The invasive ability of pro-TAME-treated EC cells was assessed using a transwell assay. The images were captured under a light microscope at 24 h. Scale bar, 50 µm. (D) Quantitative results for wound healing assay. (E) Quantitative results for transwell assay. n.s, not significant. pro-TAME, pro-Tosyl-L-Arginine Methyl Ester; EC, endometrial carcinoma.
Since pro-TAME and Apcin inhibit APC/CCDC20 through different mechanisms,
we next treated the EC cells with both inhibitors. Interestingly, the combination
of pro-TAME and Apcin significantly enhanced the inhibition of EC cell growth.
Furthermore, the administration of lower concentrations of pro-TAME
(5–10 µM) and Apcin (25–50 µM)
resulted in equal even stronger suppression of EC cells compared to the highest
concentration of each single inhibitor (15 µM pro-TAME or 100
µM Apcin) (Fig. 6). Moreover, the administration of both pro-TAME
(10 µM) and Apcin (25 µM) at lower concentrations
contributed to higher percentage of apoptotic and dead cells overall (p
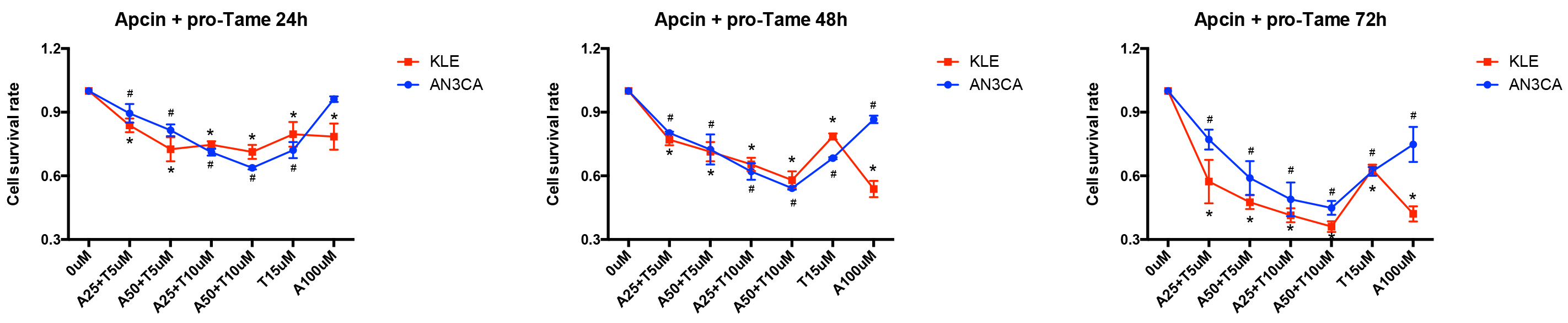 Fig. 6.
Fig. 6.
The effect of pro-TAME combined with Apcin on the growth of
human EC cells. A CCK-8 assay was conducted to assess EC cell proliferation
following treatment with various combinations of pro-TAME and Apcin at different
time point (24, 48, and 72 h). #p
EC is the most frequently occurring tumor in female reproductive system in the United States [22]. Although early symptoms, such as postmenopausal abnormal vaginal bleeding, can help diagnose EC at an early stage, often predicting a good prognosis [23], a recent research indicates that only 9% (95% confidence interval (CI), 8–11%) of patients with postmenopausal vaginal bleeding are diagnosed with EC [24]. When patients present with distant metastasis, the 5-year survival rate drops to just 9% [22]. Surgery is the primary treatment for patients diagnosed with stage I or II EC, while adjuvant therapy following surgical resection is an important adjunct for patients with advanced stages of the disease [25]. Chemotherapy is one of the main adjuvant treatments for EC patients, commonly involving the use of doxorubicin, cisplatin, cyclophosphamide, and paclitaxel. Considering the low survival rate of patients at advanced-stage EC, exploring novel therapeutic targets is both essential and meaningful.
Ubiquitination is closely associated with tumor development and progression. The E3 ubiquitin-protein ligase, speckle-type POZ protein (SPOP), promotes the ubiquitination of pyruvate dehydrogenase kinase 1 (PDK1), thereby repressing protein kinase B (AKT) kinase activity and its oncogenic functions [26]. Another E3 ubiquitin-protein ligase, F-Box Protein 16 (FBXO16), functions as a tumor suppressor in ovarian cancer by promoting the degradation of heterogeneous nuclear Ribonucleoprotein L (hnRNPL) [27]. Notably, ubiquitination-related genes can be applied to construct a novel prognostic model for EC [28]. Cancer is characterized by uncontrolled and excessive cell proliferation [29]. CDC20 is a well-known regulator of the cell cycle, primarily functioning by binding to the APC/C to facilitate the ubiquitination of downstream substrates [30]. Additionally, several mitotic proteins, such as Cyclin A, Mps1 and Cenp-F, have been identified as substrates of APC/CCDC20 for ubiquitination. Specifically, Cyclin A regulates S phase and G2/M transition [31]. Mps1 is involved in chromosome-microtubule attachments by activating the SAC [32]. Cenp-F functions at the kinetochore to mediate mitotic chromosome segregation [33].
TAME prevents CDC20 loading by antagonizing the Ile-Arg (IR)-tail interaction between CDC20 and the APC/C, leading to mitotic arrest [18]. In the absence of substrates, TAME ejects CDC20 from the APC/C and promotes auto-ubiquitination in the N-terminal region of CDC20 [19]. Apcin functions by binding to CDC20 and competitively inhibiting the ubiquitination of its substrates. It has been reported that the combined use of TAME and Apcin is more effective at blocking CDC20 binding to the APC/C than either compound used alone [21]. In this study, we also found that the ability of pro-TAME to inhibit proliferation and induce apoptosis in EC cells is synergistically amplified by co-addition of Apcin. This suggests that APC/C inhibition through the simultaneous disruption of multiple interactions between APC/C, CDC20, and substrate is a promising therapeutic strategy for EC.
The strength of this study lies in our identification of CDC20 as a novel therapeutic target for EC treatment. We also demonstrated for the first time that the CDC20 inhibitor pro-TAME can suppress tumor proliferation and induce apoptosis in EC cells. However, a limitation of this study is that it is a preliminary investigation into the effects of pro-TAME treatment on EC cells, and further mechanistic research is needed in the future.
This study is the first to investigate the antitumor properties of pro-TAME on EC cells. Our data indicate that inhibition of APC/CCDC20 by pro-TAME, in combination with Apcin, may represent a promising strategy for the treatment of EC that warrants further investigation.
The data are available from the corresponding author upon reasonable request.
The study was conceived and designed by KN and FF. KN and FF conducted the experiments and analyzed the data. KN wrote the manuscript with the help from FF. The final vision was approved by Both authors. Both authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
All experiments complied with the Declaration of Helsinki and were approved by the Institutional Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Approval Number: 3511). The normal endometrium and EC tissues were obtained from patients who underwent surgery at Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. All the patients or their families/legal guardians were provided with written informed consent.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.ceog5112274.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






