1 Department of Medical Ultrasonics, Medical Center Hospital of Qionglai City, 611530 Chengdu, Sichuan, China
†These authors contributed equally.
Abstract
Noninvasive biomarkers need identification to enhance the diagnostic accuracy of endometrial cancer (EC) detection. The diagnostic and prognostic significance of serum miR-222-3p in EC was investigated in this study to provide a reference for clinical diagnosis and treatment.
This prospective cohort study comprised 128 patients with EC and 65 patients with benign endometrial lesions (benign endometrial hyperplasia or polyps) admitted to Qionglai Medical Centre Hospital from January 2016 to December 2018. EC diagnosis was confirmed through endometrial biopsy and postoperative pathology. Serum miR-222-3p levels were quantified using the real-time reverse transcriptase-polymerase chain reaction (RT-qPCR). Pearson’s method was utilized to assess correlations between miR-222-3p and the resistance index (RI), pulsation index (PI), and mean blood flow velocity (MBFV). Diagnostic and prognostic assessments of miR-222-3p were conducted using receiver operating characteristic (ROC) curve analysis, Cox regression analysis, and Kaplan‒Meier curve analysis.
Serum miR-222-3p levels were higher in EC patients compared to controls and were correlated with RI, PI, and MBFV (p < 0.001). Serum miR-222-3p enhanced the sensitivity (69.53% vs. 96.88%) and specificity (92.31% vs. 96.92%) of transvaginal ultrasound for EC diagnosis. Furthermore, serum miR-222-3p showed significant associations with lymph node metastasis (p = 0.002), degree of differentiation (p = 0.023), and the International Federation of Gynecology and Obstetrics (FIGO) stage (p = 0.001) in EC patients. It also predicted poor prognosis and served as an independent prognostic predictor (p = 0.036).
The combination of miR-222-3p with transvaginal ultrasound enhanced the diagnostic efficiency of EC. Additionally, miR-222-3p holds promise as a biomarker for predicting the prognosis of EC patients.
Keywords
- endometrial cancer
- miR-222-3p
- transvaginal ultrasound
- clinical significance
Endometrial cancer (EC) is a malignant tumor of the reproductive system that affects women’s health worldwide, with its incidence gradually increasing. The main symptoms of EC include vaginal irregular bleeding and fluid discharge, and cachexia may develop in the advanced stage [1, 2]. Early detection and treatment can inhibit the infiltration of cancer cells into the deep muscle layer and prolong patient survival. Currently, the preoperative diagnosis of EC relies on endometrial biopsy, hysteroscopy, tumor marker tests, and imaging examinations [3, 4, 5, 6]. Endometrial biopsy is considered the most accurate method for diagnosing EC, but its invasive nature restricts its early diagnostic utility. Additionally, serum tumor markers such as carbohydrate antigen 125 (CA125) and human epididymal secretory protein E4 (HE4) hold some clinical value in early EC diagnosis, although their efficacy remains unsatisfactory [7, 8]. Transvaginal ultrasound, a common imaging technique involving vaginal probe exploration, has become pivotal in detecting lesions in the female reproductive system due to its simplicity, non-invasiveness, and repeatability [9, 10]. It demonstrates high sensitivity, specificity, and accuracy in diagnosing EC by clearly revealing lesions [11]. Angiogenesis in EC manifests as the presence of multiple blood vessels in the endometrium and the myometrium-endometrium junction. The proliferative vascular pattern of multiple vessels characterizes EC [12, 13, 14]. Doppler ultrasound parameters, including the resistance index (RI), pulsation index (PI), and mean blood flow velocity (MBFV), provide reliable information regarding changes in blood flow around the lesion [9, 15]. However, EC symptoms are diverse, lesions are mostly atypical, and early-stage myometrial infiltration depth is difficult to ascertain due to the absence of evident endometrial changes, leading to a high rate of misdiagnosis and underdiagnosis even with transvaginal ultrasound [10].
MicroRNAs (miRNAs) are potential biomarkers for disease diagnosis, and despite not yet being integrated into clinical applications, numerous clinical studies have highlighted their role in early disease monitoring and treatment determination. Several studies have indicated that miRNA dysregulation correlates with the onset and progression of EC, suggesting their potential for early EC diagnosis [16, 17, 18]. For instance, miR-15a-5p has been associated with EC aggressiveness and depth of muscle infiltration, showing promise as a biomarker for EC detection [19]. Additionally, elevated expression of miR-548ag in EC patients has been linked to reduced survival and enhanced tumor progression [20]. Other studies noted abnormal expression of miR-222-3p in EC [21, 22], implicating its potential role in disease progression and as a marker to enhance diagnostic accuracy in conjunction with transvaginal ultrasound.
In this study, a series of clinical data were analyzed to assess the diagnostic value and clinical significance of miR-222-3p in combination with transvaginal ultrasound for managing EC, offering a reference basis for the disease’s clinical diagnosis and treatment.
This was a prospective study relating to routine care. A total of 128 patients diagnosed with EC and treated at the Medical Center Hospital of Qionglai between January 2016 and December 2018 were enrolled in the study. Patients were included if they met the following criteria: (1) confirmation of EC diagnosis through endometrial biopsy (diagnostic curettage) and postoperative histologic examination; (2) first-time diagnosis without prior treatment of radiotherapy, chemotherapy, or targeted drugs; (3) expected survival period of more than 3 months; (4) underwent transvaginal ultrasound; and (5) had complete clinical data. Exclusion criteria comprised: (1) presence of leiomyoma, polyp, ovarian endometriosis, etc.; (2) severe liver or kidney dysfunction; (3) coexistence of other malignant tumors; and (4) pregnancy or breastfeeding. Patients in the control group were examined by hysteroscopy and tissues were taken from the lesions for pathologic examination to diagnose the disease. Additionally, the control group was included to ensure that their general information, such as age and medical history, was aligned with that of the EC patients. A total of 65 patients with benign endometrial lesions (benign endometrial hyperplasia, polyp) during the same period were ultimately included as controls. Informed consent was obtained from all participants, and the study received approval from the Medical Ethics Committee of the Medical Center Hospital of Qionglai City [No. (2016) 02]. A flow chart of the study is shown in Supplementary Fig. 1.
The clinical characteristics of the patients with EC were as follows. Diabetes
was defined as a random blood glucose concentration
All subjects underwent transvaginal ultrasound using a Voluson 730 Pro machine (General Electric Company, Milwaukee, WI, USA) while positioned in lithotomy, utilizing a probe frequency of 5–9 MHz. The vaginal probe was covered with a condom and inserted slowly into the vagina. Conventional 2D ultrasound was employed to assess endometrial thickness, mass echogenicity in the uterine cavity, and differences between lesions and surrounding myometrium. Color Doppler mode identified Doppler waveforms of blood flow near and within the lesion, with measurements taken from two vessels, each averaged from three readings for parameters, including RI, PI, and MBFV. Ultrasound characteristics indicative of malignant endometrial lesions included irregular endometrial thickening, unclear boundaries between local endometrium and myometrium, uneven echogenicity within the uterine cavity with prominent local blood flow signals, and irregular echogenicity of local myometrium without clear distinction from surrounding normal myometrium. These procedures were conducted by two sonographers, each with over a decade of experience. Detailed ultrasound features are provided in the Supplementary Material (Supplementary Table 1).
A 5-mL venous blood sample was collected from each
participant. Serum was obtained by centrifugation at 4000
Patients diagnosed with EC were monitored for survival status over a 60-month period. Follow-up assessments occurred every 3 months via telephone or outpatient clinic visits for the initial 24 months, and subsequently every 6 months.
SPSS 22.0 (IBM, Armonk, NY, USA) was used for data processing. Normally
distributed data are expressed as the mean
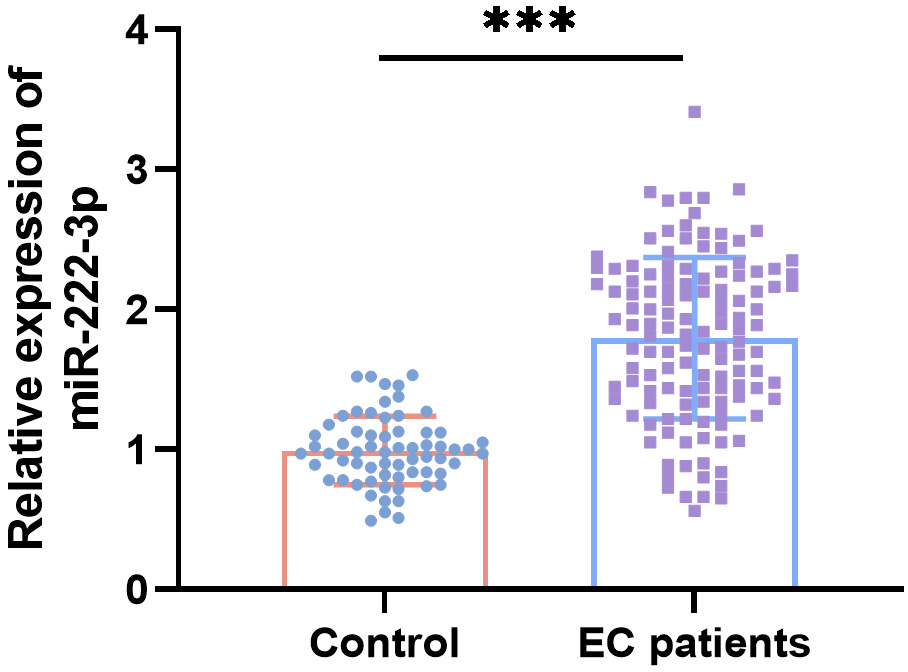
Baseline characteristics such as age, body mass index (BMI), history of hypertension or diabetes, parity, and menopausal history showed no significant differences between control subjects and EC patients (Table 1). Serum miR-222-3p levels were notably higher in EC patients compared to control subjects (Fig. 1). Compared to controls, EC patients exhibited significantly lower RI and PI values and higher MBFV values based on transvaginal ultrasound parameters (Table 2). Moreover, there were significant negative correlations between serum miR-222-3p levels and RI (r = –0.561) and PI (r = –0.620), and a positive correlation with MBFV (r = 0.536) (Table 3).
 Fig. 1.
Fig. 1.
Relative serum miR-222-3p levels in endometrial cancer (EC)
patients (n = 128) compared to control subjects (n = 65). ***p
| Characteristic | Control (n = 65) | EC (n = 128) | p value |
| Age (years), mean |
54.17 |
56.80 |
0.066a |
| BMI (kg/m2), mean |
24.14 |
24.90 |
0.106a |
| Hypertension (no/yes) | 46/19 | 76/52 | 0.121b |
| Diabetes (no/yes) | 48/17 | 81/47 | 0.141b |
| Parity, median (IQR) | 2 (1, 2) | 2 (1, 2) | 0.065c |
| Menopause (no/yes) | 26/39 | 41/87 | 0.272b |
Abbreviations: EC, endometrial cancer; SD, standard deviation; BMI, body mass
index; IQR, interquartile range. Significant difference at p
| Parameters | Control (n = 65) | EC (n = 128) | p value |
| RI | 0.63 |
0.36 |
|
| PI | 1.41 |
0.75 |
|
| MBFV (cm/s) | 9.21 |
14.73 |
Abbreviations: EC, endometrial cancer; RI, resistance index; PI, pulsation
index; MBFV, mean blood flow velocity. Significant difference at p
| Parameters | miR-222-3p | |
| r | p value | |
| RI | –0.561 | |
| PI | –0.620 | |
| MBFV | 0.536 | |
Abbreviations: RI, resistance index; PI, pulsation index; MBFV, mean blood flow
velocity. Significant difference at p
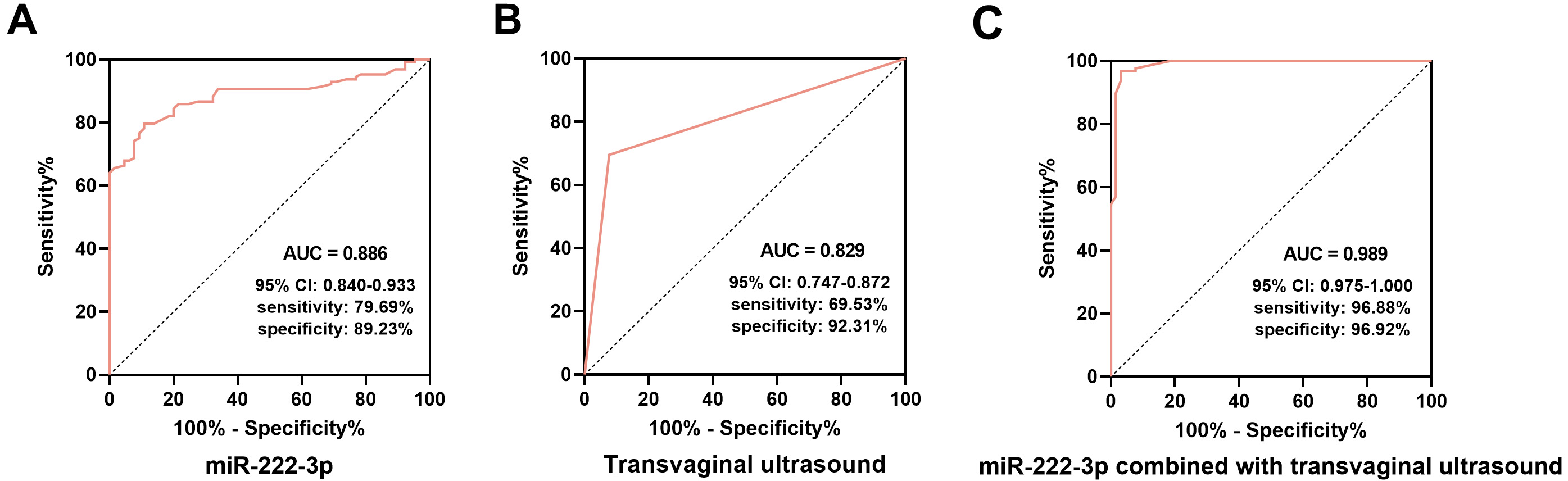
ROC curve analysis demonstrated that both miR-222-3p and transvaginal ultrasound were effective in diagnosing EC. The area under the ROC curve (AUC) for EC diagnosis using miR-222-3p alone was 0.886 (95% confidence interval (95% CI): 0.840–0.933), with sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 79.69%, 89.23%, 93.58%, and 69.05%, respectively (Fig. 2A). The AUC for EC diagnosis using transvaginal ultrasound alone was 0.829 (95% CI: 0.747–0.872) with sensitivity, specificity, PPV, and NPV of 69.53%, 92.31%, 94.68%, and 60.61% (Fig. 2B). When combined, miR-222-3p and transvaginal ultrasound yielded an AUC of 0.989 (95% CI: 0.975–1.000), with sensitivity, specificity, PPV, and NPV of 96.88%, 96.92%, 96.12%, and 93.75% (Fig. 2C).
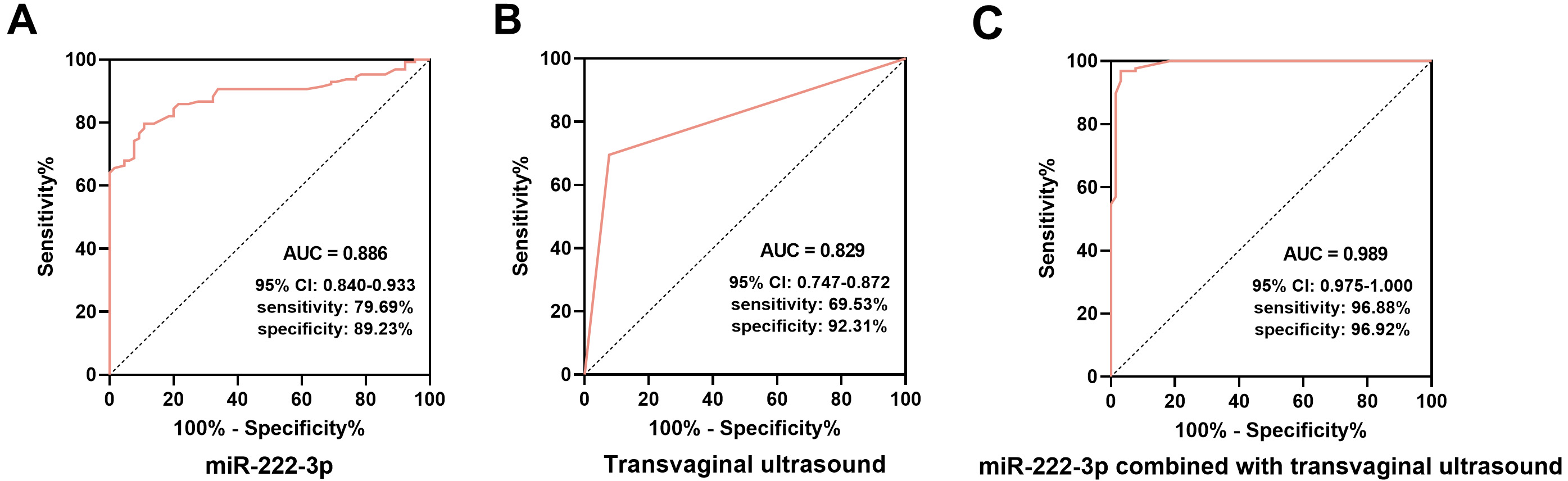 Fig. 2.
Fig. 2.
Diagnostic value of serum miR-222-3p and transvaginal ultrasound in EC. (A) The receiver operating characteristic (ROC) curve of serum miR-222-3p for the diagnosis of EC. (B) The ROC curve for the diagnosis of EC by transvaginal ultrasound. (C) The ROC curve of miR-222-3p combined with transvaginal ultrasound for the diagnosis of EC. AUC, area under the ROC curve; 95% CI, 95% confidence interval.
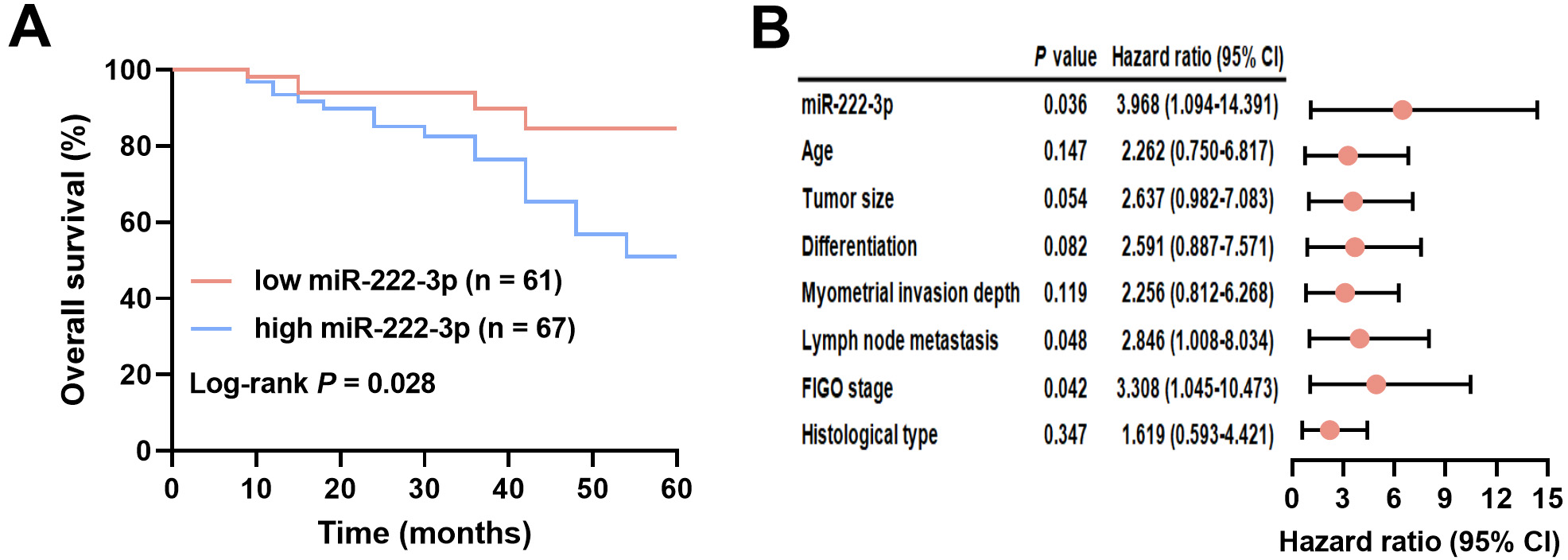
EC patients were divided into two groups based on miR-222-3p levels: low-miR-222-3p (n = 61) and high-miR-222-3p (n = 67), using a cutoff of 1.79, which was the mean miR-222-3p level. The miR-222-3p level did not show associations with age, myometrial invasion depth, tumor size, or histological type, but it correlated significantly with lymph node metastasis (p = 0.002), tumor differentiation (p = 0.023), and FIGO stage (p = 0.001) (Table 4). Follow-up analysis revealed a median overall survival of 24 months for EC patients. Kaplan‒Meier survival curves demonstrated significantly shorter overall survival in EC patients with high miR-222-3p levels compared to those with low miR-222-3p levels (log-rank test, p = 0.028; Fig. 3A). The results of the univariate Cox regression analysis are presented in the Supplementary Table 2. Furthermore, miR-222-3p (hazard ratio (HR) = 3.968, 95% CI = 1.094–14.391, p = 0.036), lymph node metastasis (HR = 2.846, 95% CI = 1.008–8.034, p = 0.048), and FIGO stage (HR = 3.308, 95% CI = 1.045–10.473, p = 0.042) were identified as independent prognostic factors for EC (Fig. 3B). See Supplementary Table 3 for a list of abbreviations.
| Characteristic | Cases (n = 128) | miR-222-3p | p value | ||
| Low (n = 61) | High (n = 67) | ||||
| Age (years) | 0.452 | ||||
| 69 | 35 | 34 | |||
| 59 | 26 | 33 | |||
| Tumor size (cm) | 0.650 | ||||
| 74 | 34 | 40 | |||
| 54 | 27 | 27 | |||
| Differentiation | 0.023 | ||||
| Well/moderate | 77 | 43 | 34 | ||
| Poor | 51 | 18 | 33 | ||
| Myometrial invasion depth | 0.080 | ||||
| 78 | 42 | 36 | |||
| 50 | 19 | 31 | |||
| Lymph node metastasis | 0.002 | ||||
| No | 88 | 50 | 38 | ||
| Yes | 40 | 11 | 29 | ||
| FIGO stage | 0.001 | ||||
| I–II | 80 | 47 | 33 | ||
| III | 48 | 14 | 34 | ||
| Histological type | 0.191 | ||||
| Endometrioid | 85 | 44 | 41 | ||
| Non-endometrioid | 43 | 17 | 26 | ||
Abbreviations: EC, endometrial cancer; FIGO, International Federation of
Gynecology and Obstetrics. Significant difference at p
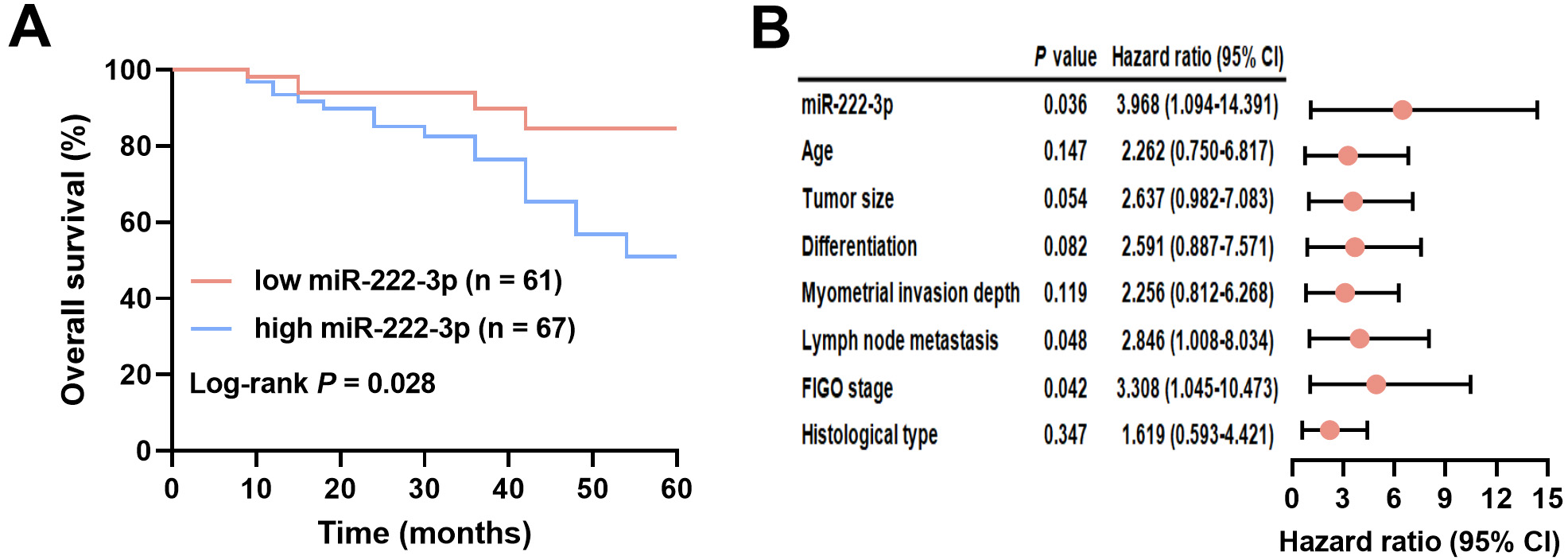 Fig. 3.
Fig. 3.
Clinical significance of serum miR-222-3p in EC prognosis. (A) Association of different miR-222-3p levels with survival of EC patients. (B) Analysis of factors associated with the prognosis of EC patients. FIGO, International Federation of Gynecology and Obstetrics; 95% CI, 95% confidence interval.
The clinical presentation of early EC is highly varied, posing challenges for accurate diagnosis. Transvaginal ultrasound often exhibits low sensitivity in detecting early EC due to factors such as small lesion size, inconspicuous infiltration, and minimal blood flow characteristics [23]. Therefore, combining it with other diagnostic methods can enhance diagnostic accuracy. miRNAs have been implicated in the initiation and progression of EC [24, 25]. For instance, miR-499 has been noted to be downregulated in EC, exerting tumor-suppressive effects through modulation of vav guanine nucleotide exchange factor 3 (VAV3) [26]. This study underscores the significance of serum miR-222-3p in both diagnosing and prognosticating EC. Given the relatively low incidence of endometrial cancer, efforts were made to include as many eligible patients as possible within the study’s timeframe, while maintaining a balanced sample size in accordance with previous research ratios. Ultimately, 128 EC patients and 65 individuals with benign endometrial hyperplasia or polyps comprised the control group. Some clinical characteristics like age, BMI, hypertension, and diabetes have been linked to EC development in a prior study [27]. However, in this study, these factors did not show statistically significant correlations with EC development, possibly due to variations in sample sources and size. The findings revealed a marked upregulation of serum miR-222-3p in EC patients compared to those with benign endometrial lesions. Regarding transvaginal ultrasound parameters, EC patients exhibited significantly lower RI and PI values and higher MBFV, which may correlate with accelerated cancer cell growth, vascular thickening, and increased angiogenesis [12].
Previous studies have demonstrated that Doppler indices (RI and PI) of the uterine arteries are notably lower in EC patients, suggesting their potential utility in EC screening [28, 29], which is consistent with our findings. Additionally, Doppler ultrasound has proven valuable in assessing EC stage, including deep myometrial infiltration and lymph node metastasis [30, 31, 32]. Interestingly, our study found correlations between serum miR-222-3p levels and Doppler ultrasound parameters RI, PI, and MBFV. This observation raises the hypothesis that miR-222-3p may contribute to the growth of EC.
Serum miRNAs are valuable for diagnosing and prognosticating various tumors due to their ease of sampling and reproducible detection [33]. Certain miRNAs are now recognized for their potential in diagnosing malignancies of the female reproductive system [34, 35]. For instance, serum miR-204-5p has been identified as downregulated in EC patients and linked to lymph node metastasis, suggesting its potential as an early diagnostic biomarker in clinical settings [36]. miR-222-3p has shown clinical significance in diagnosing pancreatic ductal adenocarcinoma, potentially enhancing diagnostic accuracy [37]. Moreover, elevated serum miR-222-3p has been proposed as a prospective biomarker for breast cancer screening [38]. In our study, miR-222-3p effectively distinguished benign endometrial lesions from EC and improved the accuracy and sensitivity of transvaginal ultrasound in diagnosing EC. Thus, miR-222-3p holds promise as a valuable biomarker for EC diagnosis.
A previous study reported that the levels of miR-21, miR-29b, and miR-27a in the peripheral blood of EC patients were significantly different according to different prognostic factors (e.g., lymph node involvement, histological grade, and FIGO stage) [39]. miR-222-3p has been demonstrated to play a role in the regulation of EC progression [22]. Additionally, miR-222-3p has been reported to be correlated with prognosis in many cancers [40, 41]. For example, serum miR-222-3p, which is linked to tumor metastasis and is a risk factor for patient prognosis, was upregulated in papillary thyroid cancer patients with lymph node metastasis [42]. The current study revealed that the upregulation of serum miR-222-3p correlated with patient FIGO stage, tumor differentiation, and lymph node metastasis. Elevated miR-222-3p levels predict shorter survival in EC patients, suggesting that higher miR-222-3p levels predict a greater risk of death. Furthermore, the serum miR-222-3p concentration was confirmed to be an independent predictor of EC prognosis. The above findings illustrate the clinical significance of serum miR-222-3p in EC and its potential as a prognostic biomarker for EC.
However, the current study’s reliance on a single-center and relatively small sample size may somewhat limit its findings. For instance, although histological type and tumor size are recognized prognostic factors for EC patients, their predictive value was not statistically significant in this study’s results. Secondly, since very few patients with benign endometrial hyperplasia and polyps undergo surgery, these patients included in this study were diagnosed through hysteroscopy by taking tissues from the abnormal areas for pathologic examination. Furthermore, the study did not account for the higher prevalence of benign endometrial lesions compared to EC, which resulted in a relatively small sample size of control patients. Therefore, further validation with larger sample sizes is warranted to confirm our findings. Atypical endometrial hyperplasia is known to carry a high risk of coexisting with or progressing to EC in a short timeframe [43]. Exploring miR-222-3p expression in different types of endometrial hyperplasia and its association with precancerous lesions should be prioritized in future research efforts. Additionally, given potential variations in miRNA expression profiles among different racial populations, future studies should investigate whether miR-222-3p exhibits differential expression across diverse racial groups.
Overall, this study highlights that combining miR-222-3p with transvaginal ultrasound enhances the diagnostic accuracy of EC and holds promise for clinical diagnosis. Furthermore, serum miR-222-3p emerges as a biomarker for predicting the prognosis of EC.
During the preparation of this work the authors used ChatGpt-3.5 in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
SRF, YPH and MZ designed the research study. JL, CLH and RJZ performed the research. SRF, YPH and MZ analyzed the data. SRF and YPH wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki. The experimental protocol was reviewed and approved by the Ethics Committee of Medical Center Hospital of Qionglai City [No. (2016) 02], and all the participating patients signed informed consent.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.ceog5112271.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.