1 Department of Ultrasound, Sichuan Provincial Woman’s and Children’s Hospital/The Affiliated Women’s and Children’s Hospital of Chengdu Medical College, 610041 Chengdu, Sichuan, China
2 Department of Ultrasound, The First Affiliated Hospital of Chengdu Medical College, 610500 Chengdu, Sichuan, China
†These authors contributed equally.
Abstract
The abnormal position or number of fetal kidneys accounts for 15%–20% of congenital abnormalities in the fetus, often resulting in oligo amniotic fluid or no amniotic fluid, thus affecting the development of fetal lung and resulting in a significant increase in the perinatal mortality of children. Therefore, detailed prenatal examination and evaluation of fetal kidneys should be carried out.
We retrospectively analyzed data on fetuses with abnormal kidney location or number, as diagnosed by prenatal ultrasound in our hospital from January 2014 to December 2021. And analysis of the image characteristics, combined with intracardiac and extracardiac malformations, abnormal appendage and pregnancy outcomes, and statistical analysis of the ratio of each type of combined intracardiac malformation, combined with extracardiac malformation, abnormal appendage and induced labor rate respectively.
(1) A total of 7953 fetal cases were systematically screened, of which 391 had an abnormal fetal kidney location or number. These included 50 cases of horseshoe kidney, 169 cases of pelvic ectopic kidney, 12 cases of crossed ectopic kidney, and 160 cases of renal absence. (2) The 391 fetuses included 35 cases with intracardiac malformation, 163 cases with extracardiac malformation, and 85 cases with abnormal appendages. (3) With regard to pregnancy outcomes, 324 fetuses were born healthy and showed no significant abnormalities up to 2 years after birth, whereas 67 pregnancies were terminated due to severe fetal malformations. (4) There was no statistical significance in the ratio of intracardiac malformations among the four groups of horseshoe kidney, pelvic ectopic kidney, crossed ectopic kidney, and renal absence (p > 0.05); there was statistical significance in the ratio of the four groups with extracardiac malformations (p < 0.05); there was statistical significance in the ratio of the four groups with abnormal appendage (p < 0.05). The ratio of induced labor rate of the four groups was compared, and the difference was statistically significant (p < 0.05).
Detailed prenatal ultrasonography can accurately diagnose fetal kidney location or number abnormalities, as well as fetal complications such as intra- and extra-cardiac malformations and abnormal appendages, providing important imaging information for subsequent clinical and eugenic care.
Keywords
- prenatal ultrasound
- horseshoe kidney
- ectopic kidney
- renal absence
The human embryonic kidney develops through three consecutive and slightly overlapping stages: the pro-kidney, the mesonephric, and the metanephric. The pro-kidney and the mesonephric gradually degenerate during the embryonic stage, with the true kidney developing from the metanephric [1]. Normal kidneys are located in the bilateral renal fossa. If fetal kidneys are not present in the renal fossa, their abnormal position or number should be taken into account. This often results in oligo-amniotic fluid or no amniotic fluid, thereby affecting development of the fetal lung and significantly increasing perinatal mortality of the child [2]. Abnormal renal location or number is manifested mainly as horseshoe kidney (HSK), pelvic ectopic kidney (PEK), crossed ectopic kidney (CEK), and renal absence (RA). During the 6th to 9th week of gestation, the kidneys ascend to the bilateral renal fossa located under the adrenal glands. The mechanism for this ascension remains unclear, but may be related to the growth and differentiation of the lumbosacral region. An ectopic kidney can arise when the ascension is abnormal, and may be related to abnormal metanephric cell migration, gene mutation, environmental factors, intrauterine factors (maternal factors and exposure to teratogens), and genetic factors [3, 4, 5, 6, 7]. PEKs occur when the kidneys fail to rise [8]. Very rarely, an ectopic kidney in the chest can occur if the kidney rises too quickly. The formation of HSKs occurs when the lower poles of the two kidneys fuse. This can arise during ascent when the kidney is blocked from reaching its normal position by the inferior mesenteric artery, leading to fusion of the lower part of the kidneys across the ventral aorta. CEKs develop when one kidney fuses with the opposite kidney and ascends diagonally to the opposite side. If one or both ureteral buds are absent from development, or if the buds fail to induce metanephric differentiation, this can result in the absence of one or both kidneys.
Prenatal ultrasonography is the first line of examination for the diagnosis of an abnormal position or number of fetal kidneys. The continuous improvement in ultrasound technology over recent years has led to an increasing number of studies on fetal kidney abnormalities, although these are not yet sufficiently comprehensive. In the present study, we retrospectively analyzed 391 cases with abnormalities in fetal kidney position or number, as diagnosed by prenatal ultrasonography at our hospital. These were analyzed in relation to intra- and extra-cardiac malformations, abnormal appendages, and pregnancy outcomes.
We retrospectively analyzed data for 391 fetuses with an abnormal renal position
or number, as diagnosed by prenatal ultrasound in our hospital from January 2014
to December 2021. The pregnant women were aged 20–45 years, with a mean age of
30.6
The ultrasound instruments included a GE Voluson E8 (GE Healthcare, Milwaukee, WI, USA), Samsung WS80A (Samsung Medison, Seoul, Korea), Philips EPIQ 7 (Philips Medical Systems, Bothell, WA, USA), Mindray Resona8S (Mindray, Shenzhen, Guangdong, China), and other color Doppler ultrasound diagnostic instruments with a probe frequency of 3.5–5.0 MHz. Systematic scans were performed on all fetuses. Kidney scans included the sagittal plane, transverse plane, and coronal plane. Careful observations were made regarding the fetal kidney location, number, size, etc. Cases with kidney abnormalities were examined in further detail for the presence of other abnormalities. The final diagnosis was confirmed by two doctors who were qualified for prenatal diagnosis. A total of 391 fetuses with an abnormal renal position or number detected by prenatal ultrasound were registered and managed. In cases of pregnancy termination, the fetus was subjected to pathological dissection. Pregnancies that continued were closely monitored. All cases were followed up.
SPSS 19.0 (IBM Corp, Armonk, NY, USA) was used for data analysis. Categorical
data was expressed as a percentage.
A total of 7953 fetal cases were systematically screened in our hospital from January 2014 to December 2021. This revealed 391 cases with abnormal fetal kidney location or number. Regarding pregnancy outcomes, 324 fetuses were born healthy and showed no significant abnormalities up to 2 years after birth, whereas 67 pregnancies were terminated due to severe fetal malformations.
Among the 50 cases with HSK, 8 (16%) had intracardiac abnormalities, 27 (54%) had extracardiac abnormalities, and 19 (38%) had appendage abnormalities. Moreover, 33 (66%) reached full-term, while 17 (34%) were terminated (Table 1).
| NO. | Intracardiac malformations | Extracardiac malformations | Abnormal appendages | Outcome |
|---|---|---|---|---|
| 1–2 | Left renal agenesis | Birth | ||
| 3–5 | Right renal agenesis | Birth | ||
| 6 | Right hydronephrosis, right ureteral cyst | Single umbilical artery | Birth | |
| 7 | Left diaphragmatic hernia, nasal bone dysplasia | Single umbilical artery, polyhydramnios | Induction of labour | |
| 8 | Bilateral choroid plexus cyst | Oligohydramnios | Birth | |
| 9 | Absence of right branch of portal vein, sacrococcygeal dysplasia of the spine, anal atresia | Single umbilical artery | Induction of labour | |
| 10–15 | Bilateral polycystic renal dysplasia | Oligohydramnios | Induction of labour | |
| 16 | Bilateral polycystic renal dysplasia | Single umbilical artery, Oligohydramnios | Induction of labour | |
| 17 | Pericardial effusion | Bilateral polycystic renal dysplasia, hydrops fetalis | Oligohydramnios | Induction of labour |
| 18 | Urorectal septum malformation sequence | Umbilical cyst | Induction of labour | |
| 19 | Hypoplastic left heart syndrome | Single umbilical artery | Birth | |
| 20 | Hemivertebra | Single umbilical artery | Birth | |
| 21 | Ventricular septal defect | Birth | ||
| 22 | Left microtia | Birth | ||
| 23 | complete transposition of the great arteries | Left diaphragmatic hernia | Single umbilical artery | Induction of labour |
| 24 | Right polycystic renal dysplasia, left hydronephrosis | Birth | ||
| 25 | Atrioventricular septal defect, coarctation of the aorta | Cervical lymphatic hygroma | Induction of labour | |
| 26 | Pericardial effusion | Cervical lymphatic hygroma | Induction of labour | |
| 27 | Bilateral polycystic renal dysplasia, arachnoid cyst | Oligohydramnios | Induction of labour | |
| 28 | Agenesis of the corpus callosum, cerebellar vermis dysplasia, broadened right lateral ventricle, nasal bone dysplasia | Single umbilical artery | Induction of labour | |
| 29 | Persistent truncus arteriosus, partial anomalous pulmonary venous drainage, absent ductus venous with venae hemiazygos draining into the left atrium | Spinal conus descends, left pleural effusion, peritoneal effusion, intracranial cyst | Single umbilical artery | Induction of labour |
| 30 | The left innominate vein demonstrates an anomalous course inferior to the aortic arch | Birth | ||
| 31–50 | None | None | None | Birth |
Among the 169 cases with PEK, 9 (5.3%) had intracardiac abnormalities, 89 (52.7%) had extracardiac abnormalities, and 20 (11.8%) had appendage abnormalities. Furthermore, 61 (36.1%) had a left PEK, 103 (60.9%) had a right PEK, and 5 (3.0%) had bilateral PEKs. In addition, 157 (92.9%) reached full-term, and 12 (7.1%) were terminated (Table 2).
| NO. | Intracardiac malformations | Extracardiac malformations | Abnormal appendages | Outcome |
|---|---|---|---|---|
| 1–15 | Left pelvic ectopic kidney, left polycystic renal dysplasia | Birth | ||
| 16–18 | Left pelvic ectopic kidney, left polycystic renal dysplasia, duplicate bladder | Birth | ||
| 19–20 | Left pelvic ectopic kidney, left hydronephrosis, left ureterectasis | Birth | ||
| 21 | Left pelvic ectopic kidney, left polycystic renal dysplasia, hypospadias | Induction of labour | ||
| 22 | Left pelvic ectopic kidney, abdominal cyst | Birth | ||
| 23–24 | Left pelvic ectopic kidney, hemivertebra | Birth | ||
| 25 | Left pelvic ectopic kidney | Oligohydramnios | Birth | |
| 26–27 | Left pelvic ectopic kidney | Single umbilical artery | Birth | |
| 28 | Left pelvic ectopic kidney, cystadenoma of the left lung | Birth | ||
| 29 | Ventricular septal defect | Left pelvic ectopic kidney, left polycystic renal dysplasia | Birth | |
| 30 | Left pelvic ectopic kidney, diaphragmatic hernia | Induction of labour | ||
| 31–32 | Persistent left superior vena cava | Right pelvic ectopic kidney, right renal agenesis | Single umbilical artery | Birth |
| 33–35 | Ventricular septal defect | Right pelvic ectopic kidney, right polycystic renal dysplasia | Birth | |
| 36 | Persistent left superior vena cava, aberrant right subclavian artery | Right pelvic ectopic kidney, right polycystic renal dysplasia | Single umbilical artery | Birth |
| 37 | Coarctation of the aorta | Right pelvic ectopic kidney | Birth | |
| 38–39 | Right pelvic ectopic kidney, cystadenoma of the left lung | Single umbilical artery | Birth | |
| 40–41 | Right pelvic ectopic kidney | Polyhydramnios | Birth | |
| 42–49 | Right pelvic ectopic kidney, right hydronephrosis | Birth | ||
| 50–81 | Right pelvic ectopic kidney, right polycystic renal dysplasia | Birth | ||
| 82 | Right pelvic ectopic kidney | Oligohydramnios | Birth | |
| 83 | Right pelvic ectopic kidney, duodenal atresia, right polycystic renal dysplasia, permanent right umbilical vein | Polyhydramnios | Induction of labour | |
| 84–85 | Right pelvic ectopic kidney | Single umbilical artery | Birth | |
| 86 | Right pelvic ectopic kidney, genital hermaphroditism, right polycystic renal dysplasia | Birth | ||
| 87 | Right pelvic ectopic kidney, cystic teratoma of abdominal cavity | Birth | ||
| 88 | Right pelvic ectopic kidney, double inferior vena cava, hemivertebra, right polycystic renal dysplasia | Birth | ||
| 89 | Right pelvic ectopic kidney, cleft lip and palate, open spina bifida | Induction of labour | ||
| 90 | Right pelvic ectopic kidney, right polycystic renal dysplasia | Single umbilical artery, oligohydramnios | Birth | |
| 91–93 | Right pelvic ectopic kidney, left polycystic renal dysplasia | Induction of labour | ||
| 94–97 | Bilateral pelvic ectopic kidney, bilateral polycystic renal dysplasia | Oligohydramnios | Induction of labour | |
| 98 | Persistent truncus arteriosus | Bilateral pelvic ectopic kidney, bilateral polycystic renal dysplasia, the partial absence of sacrococcygeal centrum | Oligohydramnios | Induction of labour |
| 99–129 | None | Left pelvic ectopic kidney | Birth | |
| 130–169 | None | Right pelvic ectopic kidney | Birth |
Among the 12 cases with CEK, 2 (16.7%) had intracardiac abnormalities, 2 (16.7%) had extracardiac abnormalities, and 3 (25.0%) had appendage abnormalities. Moreover, 5 (41.7%) had a left CEK and 7 (58.3%) had a right CEK. Pregnancy was terminated in two of the 12 CEK cases (16.7%), as shown in Table 3.
| NO. | Intracardiac malformations | Extracardiac malformations | Abnormal appendages | Outcome |
|---|---|---|---|---|
| 1 | Ventricular septal defect | Right crossed ectopic kidney | ||
| 2 | Right crossed ectopic kidney, right polycystic renal dysplasia | Single umbilical artery | Induction of labour | |
| 3 | Right crossed ectopic kidney, right microtia | Induction of labour | ||
| 4 | Right crossed ectopic kidney | Polyhydramnios | Birth | |
| 5–7 | Right crossed ectopic kidney | Birth | ||
| 8 | Aberrant right subclavian artery | Left crossed ectopic kidney | Birth | |
| 9 | Left crossed ectopic kidney | Single umbilical artery | Birth | |
| 10–12 | Left crossed ectopic kidney | Birth |
Among the 160 cases with RA, 16 (10.0%) had intracardiac malformations, 45 (28.1%) had extracardiac malformations, and 43 (26.9%) had appendage abnormalities. Furthermore, 67 (41.9%) had a left RA, 80 (50.0%) had a right RA, and 13 (8.1%) had bilateral RA. Of the 160 fetuses with RA, 124 (77.5%) reached full-term and 36 (22.5%) were terminated (Table 4).
| NO. | Intracardiac malformations | Extracardiac malformations | Abnormal appendages | Outcome |
|---|---|---|---|---|
| 1 | Persistent left superior vena cava | Left renal absence | Single umbilical artery | Birth |
| 2 | Persistent left superior vena cava | Left renal absence, cleft lip and palate | Induction of labour | |
| 3 | Right aortic arch with aberrant left subclavian artery | Left renal absence | Single umbilical artery | Birth |
| 4–10 | Left renal absence | Single umbilical artery | Birth | |
| 11–12 | Left renal absence, fetal growth restriction | Induction of labour | ||
| 13–14 | Left renal absence, right duplicate kidney | Birth | ||
| 15 | Left renal absence, nasal bone dysplasia, hypospadias | Polyhydramnios | Induction of labour | |
| 16 | Left renal absence, abdominal cyst | Birth | ||
| 17 | Left renal absence, right polycystic renal dysplasia | Induction of labour | ||
| 18 | Left renal absence, cloacal ectropion (omphalocele, bladder ectropion, spinal sacrococcygeal closed spina bifida, meningocele, anal atresia) | Single umbilical artery | Induction of labour | |
| 19 | Left renal absence, omphalocele, abdominal aorta and left iliac aneurysm | Single umbilical artery, umbilical cyst | Induction of labour | |
| 20 | Left renal absence, micrognathia | Birth | ||
| 21 | Left renal absence, hydrocephalus | Induction of labour | ||
| 22 | Left renal absence, nasal bone dysplasia, bilateral clubfoot | Single umbilical artery | Birth | |
| 23 | Left renal absence, duplicate bladder | Birth | ||
| 24 | Left renal absence, hemivertebra | Birth | ||
| 25 | Left renal absence, anal atresia | Induction of labour | ||
| 26 | Left renal absence, duplication | Birth | ||
| 27 | Left renal absence, right hydronephrosis | Birth | ||
| 28 | Ventricular septal defect, persistent left superior vena cava, coarctation of the aorta | Right renal absence, scoliosis | Single umbilical artery | Induction of labour |
| 29 | Mesocardia, of the aorta left superior vena cava | Right renal absence | Single umbilical artery | Birth |
| 30 | Dextroversion of the heart, single atrium, single ventricle, persistent truncus arteriosus | Right renal absence, cleft lip and palate | Induction of labour | |
| 31 | Atrioventricular septal defect, aberrant right subclavian artery | Right renal absence, right lower abdominal cyst | Induction of labour | |
| 32 | Tetralogy of Fallot, atrioventricular septal defect | Right renal absence, left polycystic renal dysplasia | Oligohydramnios | Induction of labour |
| 33 | Double outlet right ventricular, pulmonary stenosis, atrioventricular septal defect | Right renal absence, micrognathia, permanent right umbilical vein | Single umbilical artery | Induction of labour |
| 34 | Tetralogy of Fallot, persistent left superior vena cava | Right renal absence | Single umbilical artery | Induction of labour |
| 35 | Ventricular septal defect | Right renal absence | Birth | |
| 36–37 | Persistent left superior vena cava | Right renal absence | Birth | |
| 38 | Right renal absence, distal ureteral cyst | Birth | ||
| 39 | Right renal absence, bilaterial clubfoot, left polycystic renal dysplasia, fetal growth restriction | Oligohydramnios | Induction of labour | |
| 40 | Right renal absence, absence of corpus callosum, broadened right lateral ventricle, nasal bone dysplasia | Single umbilical artery | Induction of labour | |
| 41 | Right renal absence, cleft lip and palate | Induction of labour | ||
| 42 | Right renal absence | Umbilical edema | Birth | |
| 43 | Right renal absence, hemivertebra with partial rib absence, scoliosis | Induction of labour | ||
| 44–46 | Right renal absence, right lower abdominal cyst | Birth | ||
| 47 | Right renal absence, hypospadias, anal atresia | Single umbilical artery | Induction of labour | |
| 48–49 | Right renal absence, left polycystic renal dysplasia | Oligohydramnios | Induction of labour | |
| 50–52 | Right renal absence, left duplicate kidney | Single umbilical artery | Birth | |
| 53 | Right renal absence, thymus dysplasia | Single umbilical artery, polyhydramnios | Induction of labour | |
| 54 | Right renal absence | Single umbilical artery | Birth | |
| 55 | Right renal absence, hemivertebra | Single umbilical artery | Birth | |
| 56 | Ventricular septal defect, pulmonary stenosis | Bilateral renal absence | Single umbilical artery, oligohydramnios | Induction of labour |
| 57 | Atrioventricular septal defect, pulmonary valve absence syndrome, intracardiac total anomalous pulmonary venous drainage, pericardial effusion | Bilateral renal absence, fetal growth restriction | Oligohydramnios | Induction of labour |
| 58 | Ventricular septal defect, hypoplastic left heart syndrome | Bilateral renal absence, enlarged cisterna magna | Oligohydramnios | Induction of labour |
| 59 | Bilateral renal absence, spinal disalignment | Oligohydramnios | Induction of labour | |
| 60 | Bilateral renal absence | Single umbilical artery, oligohydramnios | Induction of labour | |
| 61 | Bilateral renal absence, holocephaly with hydrocephalic anencephaly, cleft lip and palate | Oligohydramnios | Induction of labour | |
| 62 | Bilateral renal absence, absence of left lower extremity | Single umbilical artery | Induction of labour | |
| 63 | Bilateral renal absence, open spina bifida, right clubfoot | Oligohydramnios | Induction of labour | |
| 64–68 | Bilateral renal absence | Oligohydramnios | Induction of labour | |
| 69–108 | None | Left renal absence | Birth | |
| 109–160 | None | Right renal absence | Birth |
Comparison of 391 fetuses with abnormal renal location or number combined with intracardiac and extracardiac malformations, abnormal appendages and pregnancy outcomes.
There was no statistical significance in the ratio of
intracardiac malformations among the four groups of horseshoe kidney, pelvic
ectopic kidney, crossed ectopic kidney, and renal absence (p
| Tape | n | Intracardiac malformations | Extracardiac malformations | Abnormal appendages | Induced labor |
| Horseshoe kidney | 50 | 8 | 27 | 19 | 17 |
| Pelvic ectopic kidney | 169 | 9 | 89 | 20 | 12 |
| Crossed ectopic kidney | 12 | 2 | 2 | 3 | 2 |
| Renal absence | 160 | 16 | 45 | 43 | 36 |
| 6.867 | 26.690 | 20.072 | 25.245 | ||
| p | 0.076 | 0.000 | 0.000 | 0.000 |
p
Ultrasound findings for normal fetal kidneys. The left and right kidneys were first identified according to the fetal position, and sagittal section scans were then performed on both sides of the spine along the long axial plane of the fetal spine. The probe was then rotated 90° and transverse section scans were performed on the vertical spine. Renal cavity emptiness (Fig. 1), adrenal supine signs, and renal displacement are important clues for the diagnosis of abnormalities in the position and number of fetal kidneys. Color Doppler is also helpful for the display of bilateral renal arteries.
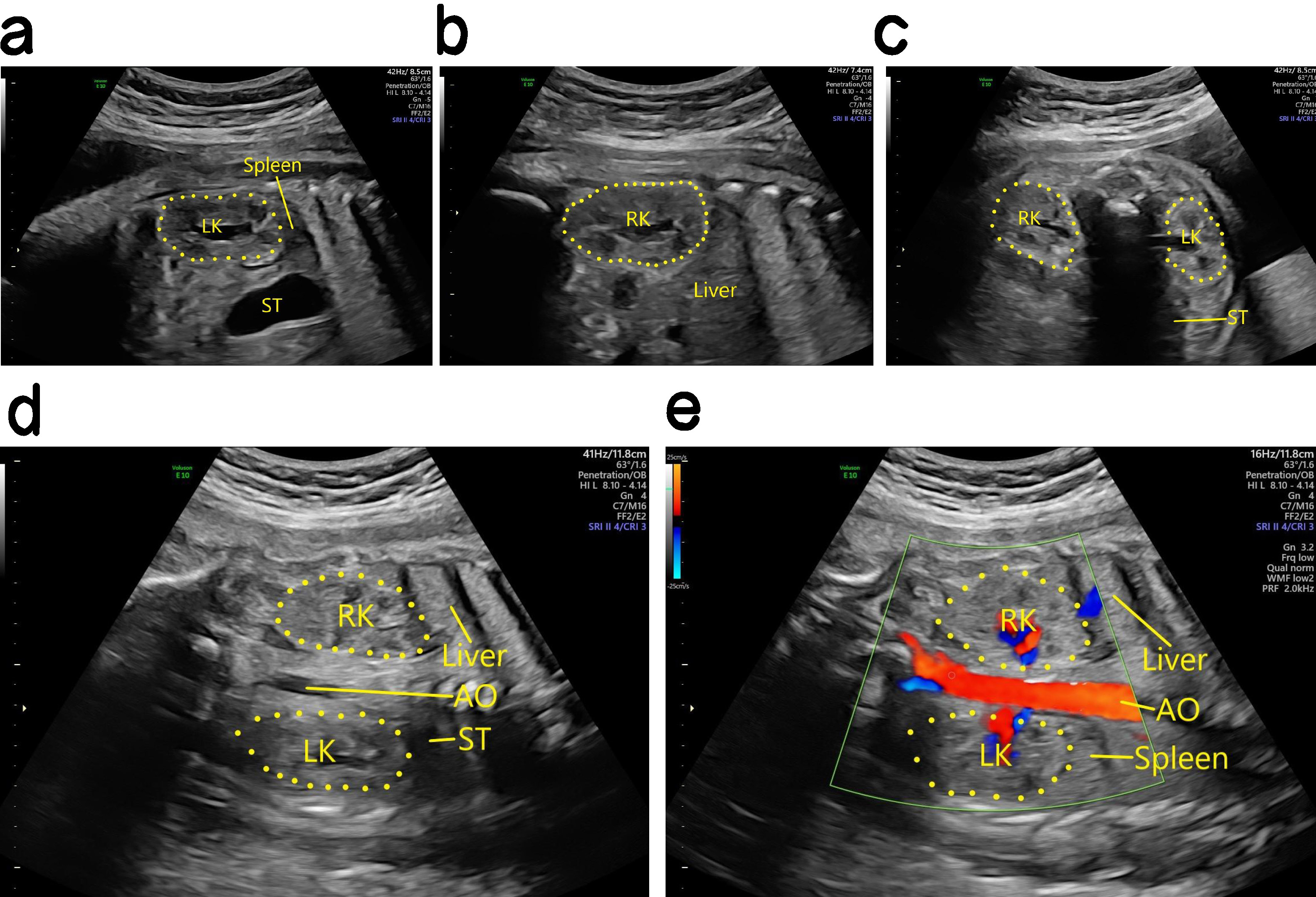 Fig. 1.
Fig. 1.
Antenatal ultrasound of the normal fetal kidney. (a) Two-dimensional (2D) ultrasound imaging of the left kidney in the sagittal section. (b) 2D ultrasound imaging of the right kidney in the sagittal section. (c) 2D ultrasound imaging of bilateral kidneys in the transverse section. (d) 2D ultrasound imaging of bilateral kidneys in the coronal section. (e) Blood flow imaging of the two kidneys in the coronal section. LK, left kidney; RK, right kidney; ST, stomach; AO, aorta.
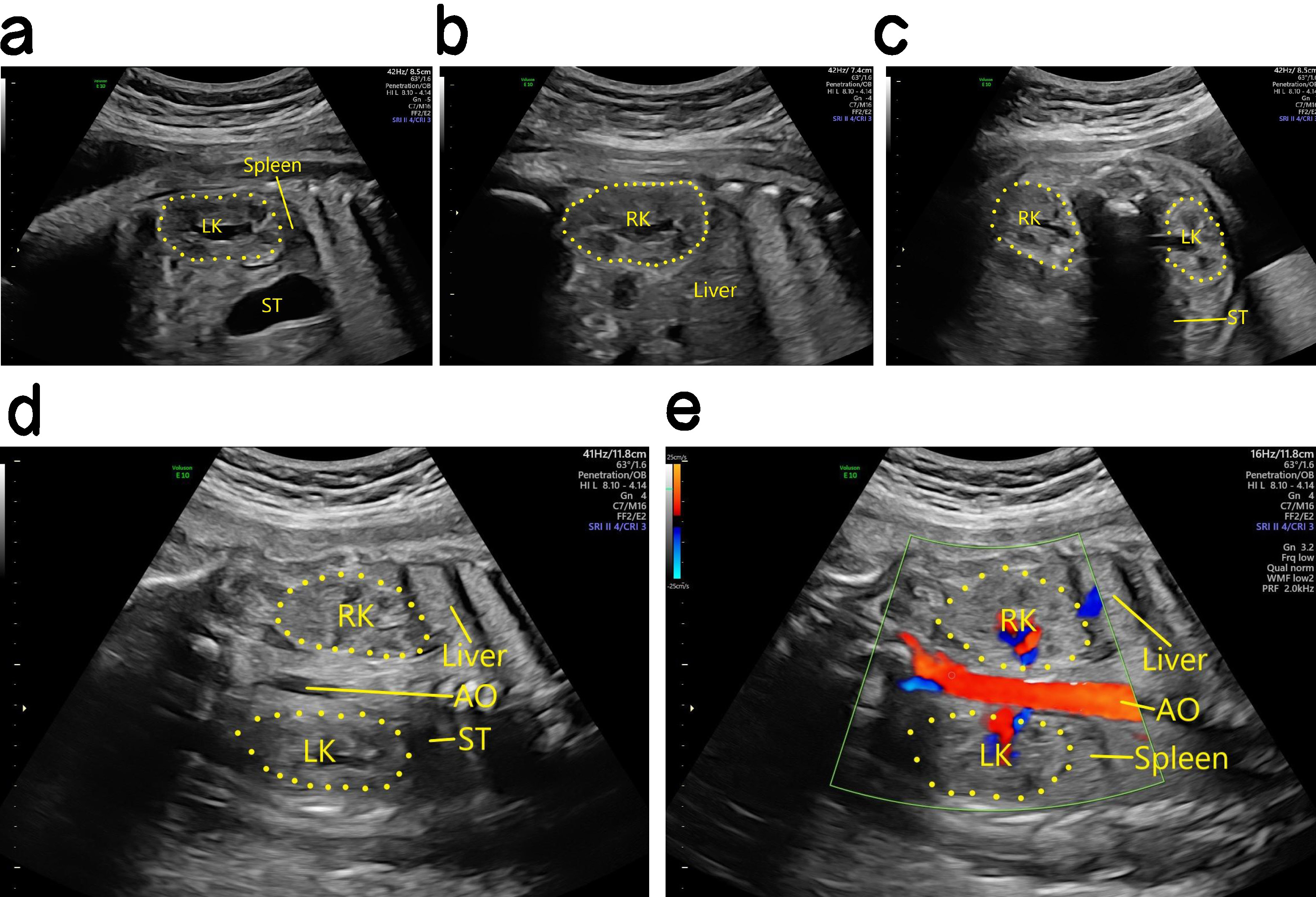
Ultrasound findings with HSK. The position of the two kidneys was lower than that of the normal kidney, and the kidney appeared abnormal. The lower pole substance of the two kidneys was fused at the midline. The site of fusion was in front of the inferior vena cava and the abdominal aorta, and resembled a “horse shoe” in cross section (Fig. 2). The diagnosis of HSK is easy to miss in cases with urinary system malformation. Kidney scans should therefore be observed in multiple sections, with cross sections and coronal sections being critical for the diagnosis of HSK. The application of high-frequency linear array probes can also improve the accuracy of HSK diagnosis [9].
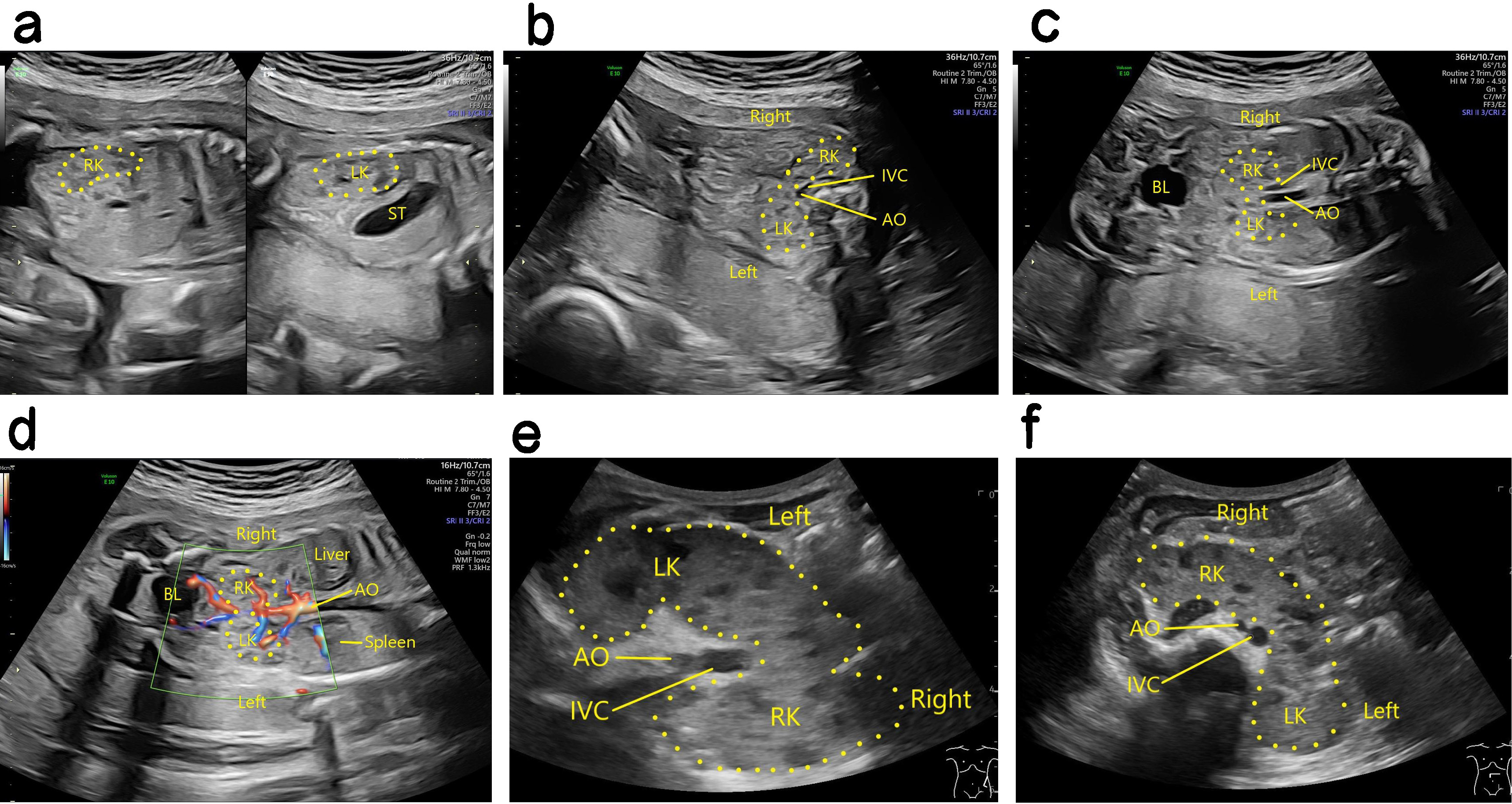 Fig. 2.
Fig. 2.
Antenatal and postnatal ultrasound of HSK. (a) The left panel is a 2D image showing the right kidney of a fetal HSK in the sagittal section, while the right panel is a 2D image showing the left kidney of a fetal HSK in the sagittal section. (b) 2D image showing fusion of the inferior pole parenchyma of a fetal HSK in front of the inferior vena cava and abdominal aorta in the transverse section. (c) 2D image showing two fetal HSKs in the coronal section. (d) Blood flow image showing a fetal HSK in the coronal section. (e) 2D image showing a HSK after birth in the coronal section. (f) 2D image showing a HSK after birth in the transverse section. HSK, horseshoe kidney; LK, left kidney; RK, right kidney; ST, stomach; AO, aorta; IVC, inferior vena cava; BL, bladder.
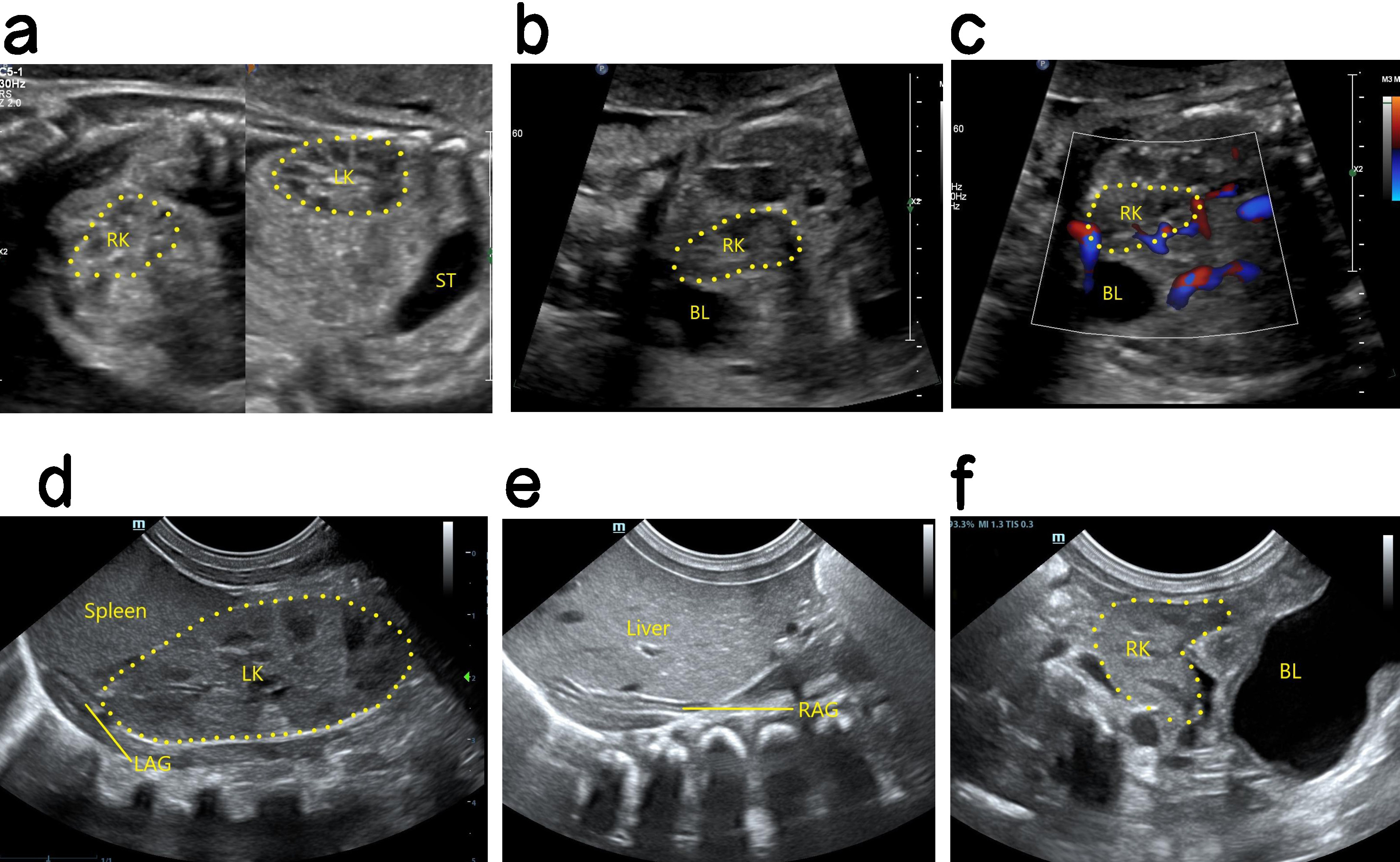
Ultrasound findings with PEK. No renal echo was shown in the lumbar renal fossa area on one side, the ipsolateral adrenal gland showed a “supine sign”, the contralateral kidney was enlarged, and the ectopic kidney image was displayed in the pelvic cavity (Fig. 3). When the renal fossa was found to be empty, the adrenal gland was relatively enlarged and lay flat in front of the psoas major muscle in the renal fossa area. Careful examination of the fetal abdomen, pelvic cavity to the renal fossa and then to the chest cavity is required to determine whether there is an ectopic kidney. Most PEKs are smaller than normal kidneys, and the echo is similar to that of the surrounding intestine. Hence, PEKs are sometimes difficult to detect by ultrasound, and careful scanning of multiple sections and angles is required.
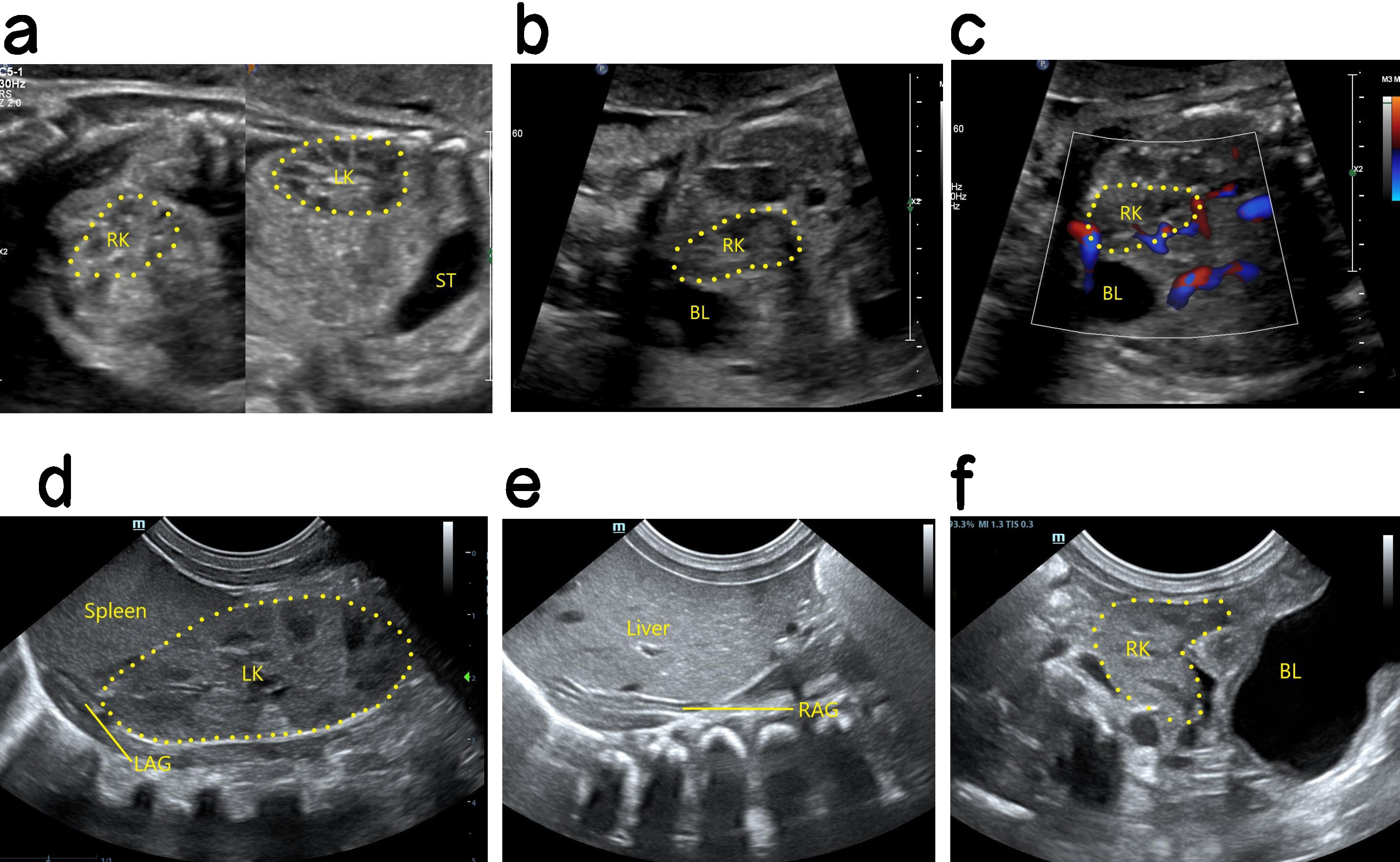 Fig. 3.
Fig. 3.
Antenatal and postnatal ultrasound of a right PEK. (a) The left panel shows a 2D image of the fetal right kidney in the right pelvic cavity. The right panel shows a 2D image of the fetal left kidney in sagittal section. The left kidney is in the normal position and is larger than the right kidney. (b) 2D image of the right PEK in oblique section. (c) Blood flow image of the right PEK in oblique section. (d) 2D postnatal image of the left normal kidney in coronal section, showing a normal position and enlargement. (e) Coronal 2D image showing that the right kidney area of the newborn was empty, and no normal right kidney was seen. The right adrenal gland is supine. (f) Oblique 2D image showing location of the right kidney in the right pelvic cavity after birth. PEK, pelvic ectopic kidney; LK, left kidney; RK, right kidney; ST, stomach; RAG, right adrenal gland; LAG, left adrenal gland; BL, bladder.
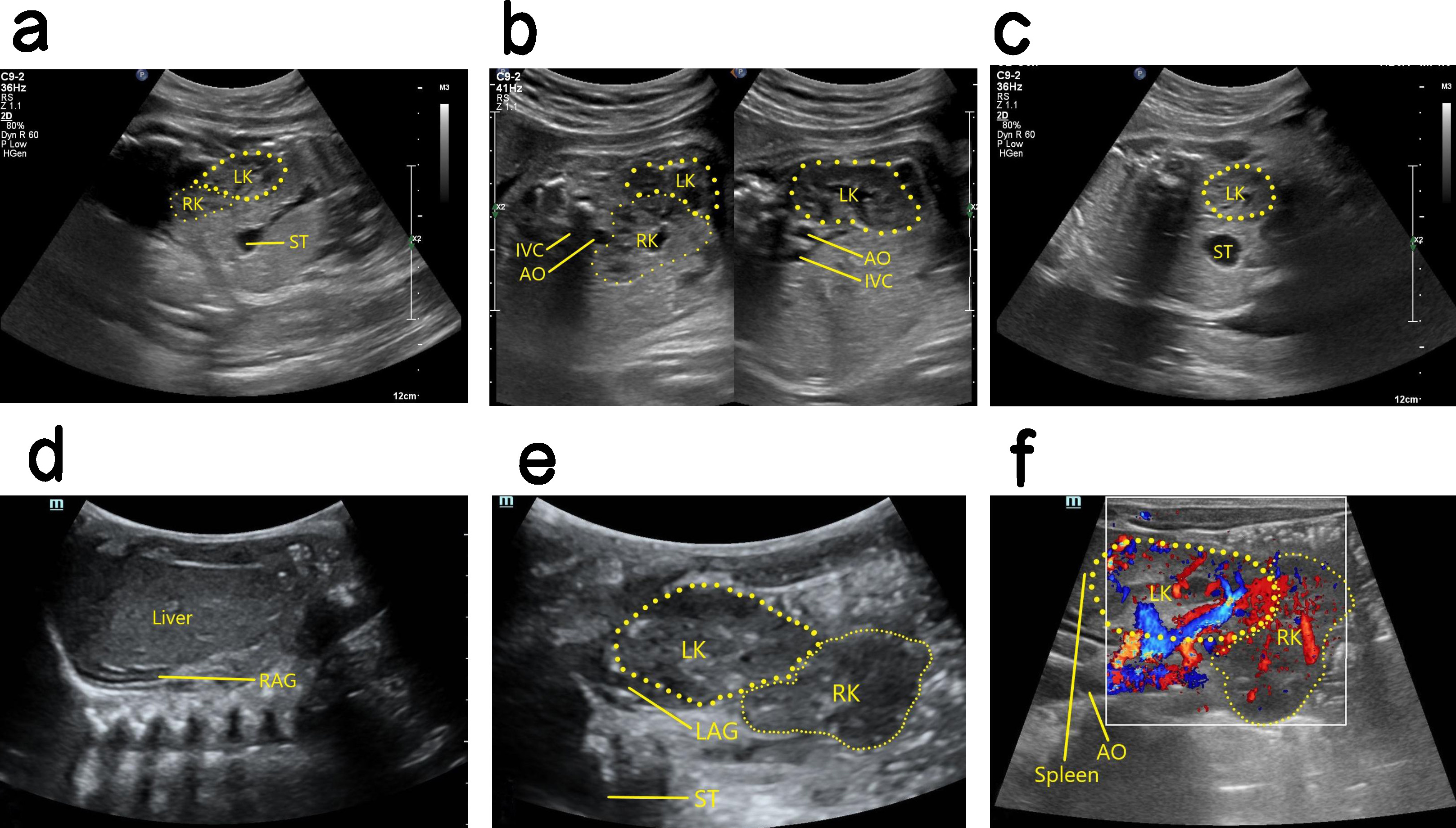
Ultrasound findings with CEK. The kidney was not observed in the renal bed area on one side, and the ipsolateral adrenal gland showed a “supine sign”. Furthermore, the contralateral kidney was significantly enlarged, and the CEK was smaller than the normal kidney and had poor rotation. Usually, the CEK was located on the bottom side of the normal kidney, and its upper pole was fused with the lower pole of the normal kidney (Fig. 4). Ectopia occurs more often in the left kidney than in the right kidney. Moreover, the ureter that drains the ectopia crosses the spine and returns to the original side, where it descends into the triangulation and then into the bladder.
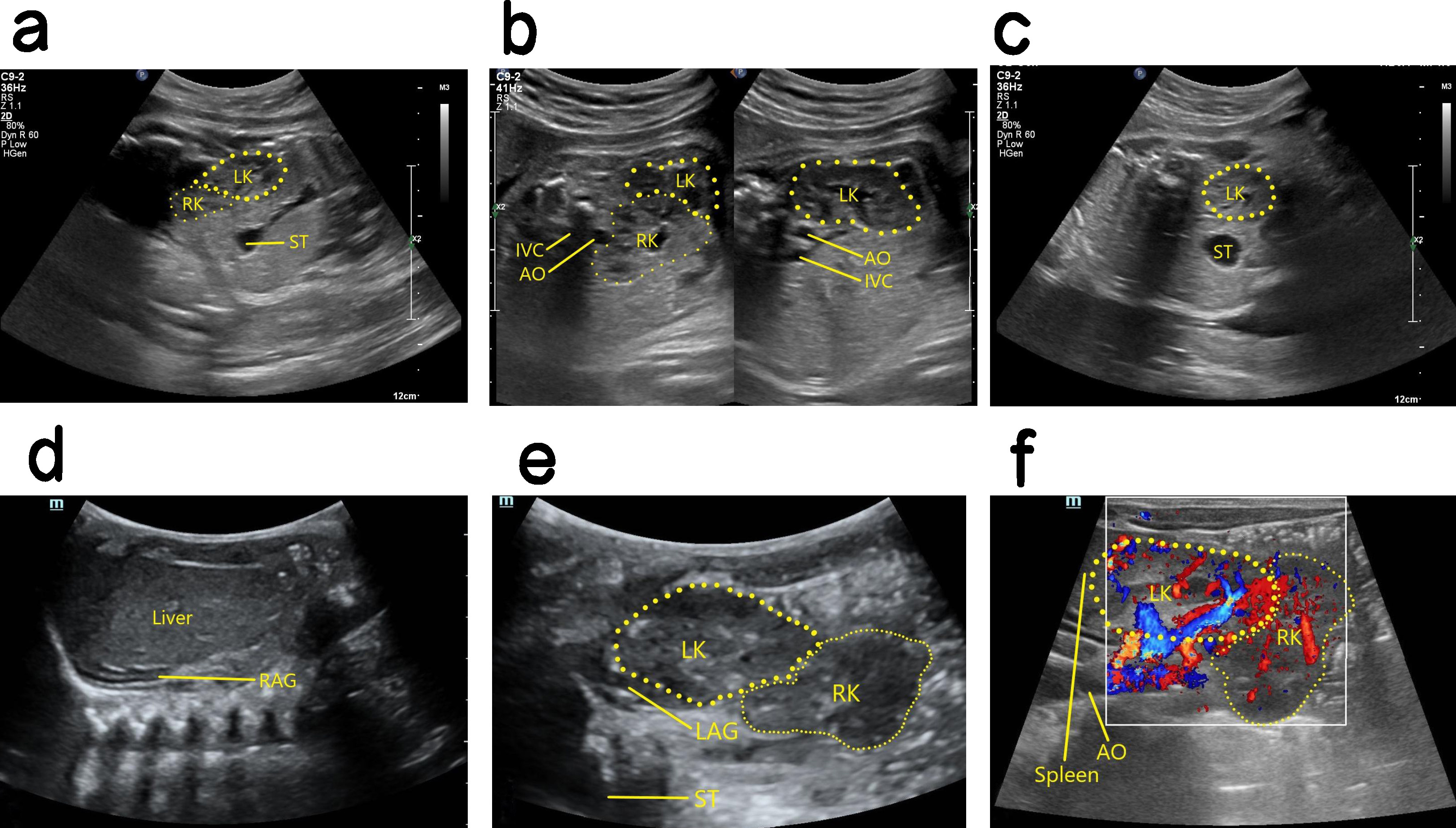 Fig. 4.
Fig. 4.
Antenatal and postnatal ultrasound of right CEK. (a) 2D image of sagittal section showing cross over of the fetal right kidney to the lower pole of the left kidney, with its upper pole fused to the lower pole of the left kidney. (b) The left panel is a 2D image in oblique section showing fusion of the upper pole of the right kidney with the lower pole of the left kidney. The right panel is a non-standard oblique 2D image showing the intact left kidney. (c) 2D image in transverse section showing the fetal left kidney in a normal position, whereas the right kidney cannot be seen in the right renal bed area. (d) 2D image in coronal section revealing the right renal bed area in this case did not show the right kidney after birth, and the right adrenal gland showed a “supine sign”. (e) 2D postnatal image in oblique section showing the right kidney cross to the bottom side of the left kidney, and the upper pole of the right kidney fused with the lower pole of the left kidney. (f) Blood flow image in oblique section after birth. CEK, crossed ectopic kidney; LK, left kidney; RK, right kidney; ST, stomach; AO, aorta; IVC, inferior vena cava; RAG, right adrenal gland; LAG, left adrenal gland.
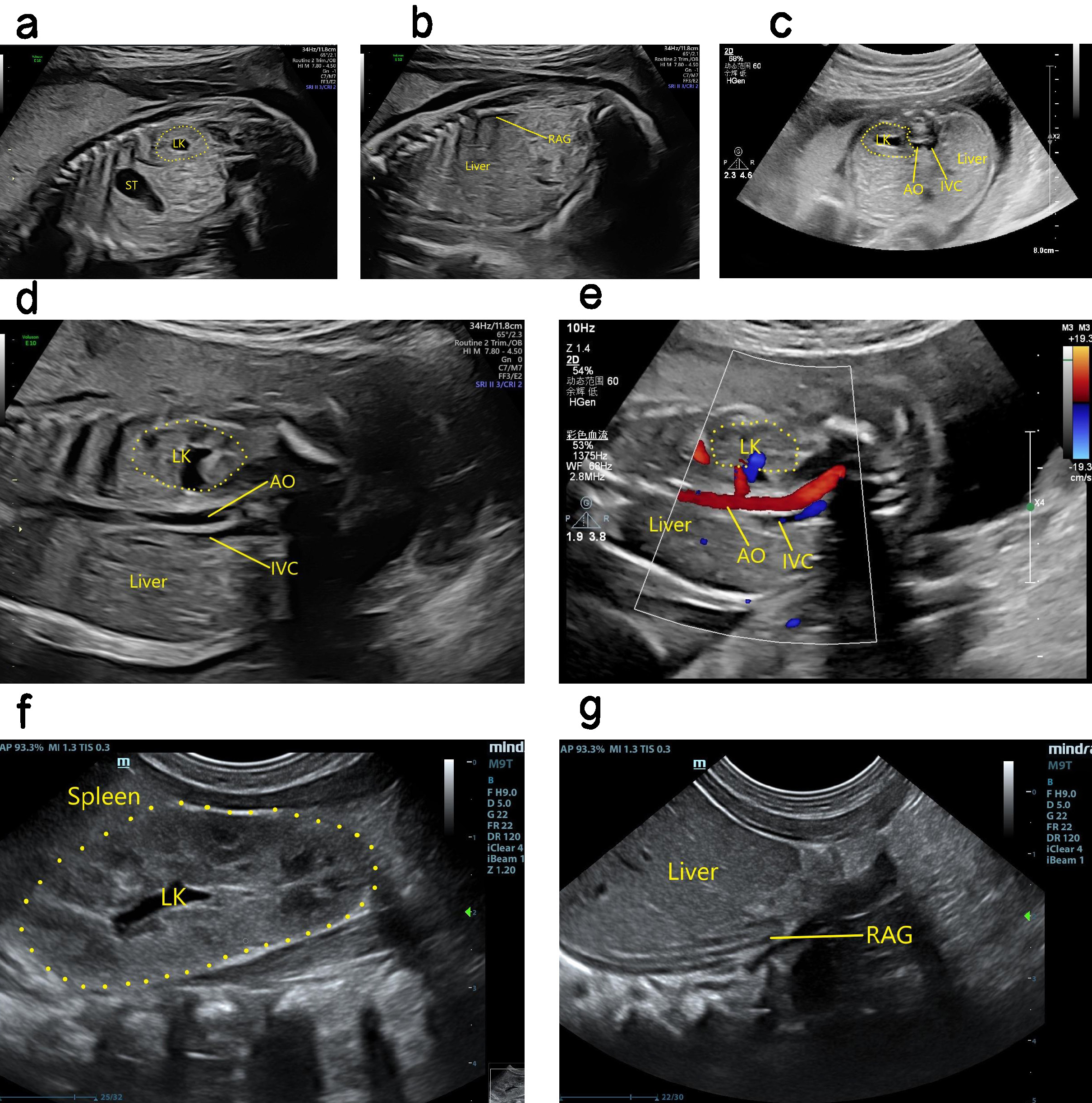
Ultrasound findings with RA. The kidney was not apparent during ultrasound examination, and color Doppler could not detect any kidney blood flow. The adrenal gland was “supine”, and the possibility of an ectopic kidney was excluded. RA was therefore considered (Fig. 5). Ultrasound can determine fetal renal function by assessing the volume of amniotic fluid, as well as through careful observation of the bladder for evidence of urine production. In cases with oligohydramnios and difficulty in performing ultrasound scans, a comprehensive assessment can be obtained by fetal magnetic resonance imaging (MRI) [10]. Perfusion of the amniotic cavity can also be performed to improve image quality and assist with diagnosis [11].
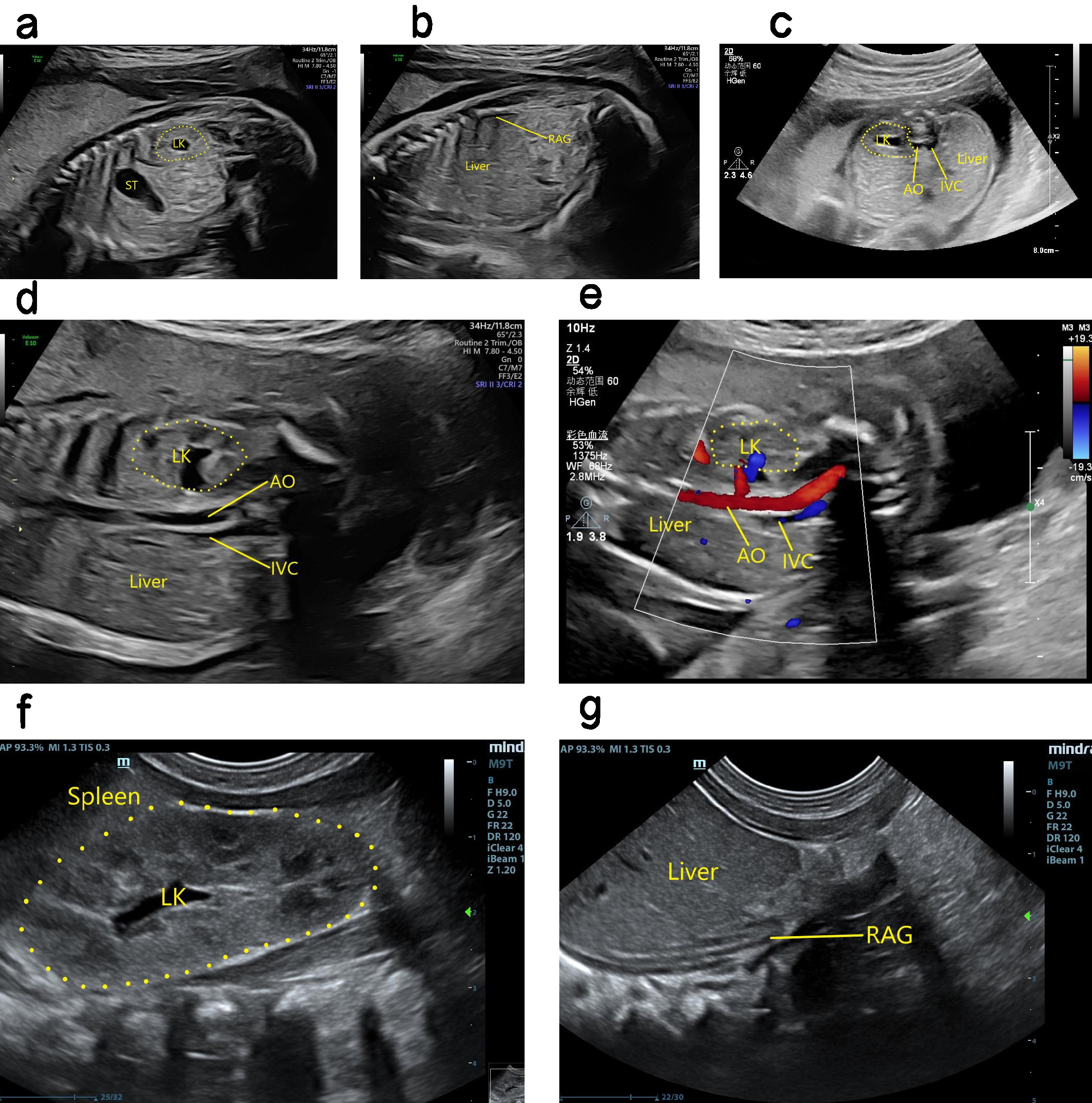 Fig. 5.
Fig. 5.
Antenatal and postnatal ultrasound showing absence of right kidney. (a) 2D image in sagittal section showing the fetal left kidney in its normal position. (b) 2D image in sagittal section showing absence of the fetal right kidney, and the right adrenal gland showing a “supine sign”. (c) 2D image in transverse section showing the normal position of the fetal left kidney. The right kidney was not seen in the right renal fossa area of the fetus. (d) 2D image of coronal section showing the enlarged left kidney of the fetus, with absent right kidney. (e) The fetal left renal artery can be seen in the coronary section, but not the right renal artery. (f) 2D coronal section image showing the left kidney in its normal position after birth, as well as its enlargement. (g) 2D coronal section image showing absence of the right kidney after birth, and a “supine sign” of the right adrenal gland. LK, left kidney; ST, stomach; AO, aorta; IVC, inferior vena cava; RAG, right adrenal gland.
The abnormal location or number of fetal kidneys is the most common disease of the urinary system, with up to one-third of cases complicated by related intra- and extra-cardiac abnormalities [12]. The pathogenesis of this condition is still unclear.
The abnormal position or number of fetal kidneys may be associated with malformations in the cardiovascular, central nervous, and genitourinary systems [13, 14]. The study included 35 cases of intracardiac malformations and 163 cases of extracardiac malformations. Intracardiac malformations of horseshoe kidney are mainly pericardial effusion, and extracardiac malformations are mainly unilateral or bilateral renal dysplasia. Pelvic ectopic kidney with intracardiac malformations are mainly ventricular septal defect and persistent left superior vena cava. Pelvic ectopic kidney with ectopic polycystic dysplasia was the main complication of extrinsic malformations. There were 2 cases of crossed ectopic kidney with ventricular septal defect and right subclavicular vagal artery, and 2 cases of crossed ectopic kidney with ipsilateral polycystic dysplasia kidney and ipsilateral microtia. Renal deficiency combined with intracardiac malformations are mainly persistent left superior vena cava, ventricular septal defect, tetralogy of Fallot, aortic arch constriction, etc. Extracardiac malformations can be abnormalities of digestive tract, urinary system, central nervous system, etc. There was no statistically significant difference in the ratio of horseshoe kidney, pelvic ectopic kidney, crossed ectopic kidney and renal absence combined with intracardiac malformations, while there was statistically significant difference in the ratio of extrinsic malformations among the four types of patients. HSK was previously associated with heart, lung, bone and gastrointestinal tract abnormalities [15]. PEK is most often associated with genitourinary system malformations, with the most common being renal dysplasia and hypospadias. PEKs are usually small and sclerotic, and have been associated with vaginal atresia, hymen atresia, and uterine dysplasia [16]. PEK has an increased likelihood of being associated with other malformations, and the decreased production of amniotic fluid increases the possibility of multi-system malformations. Ectopic kidney in the chest has been reported [17, 18]. Although extremely rare, this may be associated with the diagnosis of an abnormal location or number of fetal kidneys. No cases of ectopic kidney in the chest were found in the current study, possibly due to the small sample size. CEK is closely related to reproductive system malformations such as hypospadias, cryptorchidism and vaginal dysplasia. Moreover, CEK often occurs in combination with kidney diseases such as polycystic dysplasia kidney, hydronephrosis and kidney stones [19]. KIF14 gene mutation can lead to a severe syndrome associated with microcephaly and the absence of kidneys [20], while pAX2 gene mutation can lead to syndromes involving abnormalities of the optic nerve and kidneys [21]. Renal deficiency is also closely related to heart function and central nervous system functions [22]. Double-kidney deficiency accompanied by oligohydramnios is associated with poor fetal prognosis [23]. In most cases, the cause of perinatal death is secondary to pulmonary hypoplasia [24]. If fetal kidney abnormalities are detected, other organs should be carefully investigated. In particular, the cardiovascular, central nervous and urogenital systems should be examined to determine whether other malformations are also present. In addition, the contralateral kidney should be carefully examined in order to evaluate the prognosis.
In the current study, 85 of the 391 fetuses (21.7%) were associated with abnormal appendages. These included one case with an umbilical cyst, one with an umbilical edema, and three with hyper amniotic fluid. The remainder showed a single umbilical artery, oligo amniotic fluid, or both. These findings indicate that an abnormal location or number of fetal kidneys often occurs in combination with a single umbilical artery and oligohydramnios. In this study, the ratio of abnormal appendages such as horseshoe kidney, pelvic ectopic kidney, crossed ectopic kidney and renal absence was compared, and the difference was statistically significant. When a single umbilical artery or oligohydramnios are observed during ultrasound examination, the focus should then be on determining whether the fetal kidneys are abnormal.
In this study, CEK case 1 (shown in Table 3) underwent interventional repair for a ventricular septal defect at one year of age, and was healthy at two years of age. Case 3 with left RA (shown in Table 4) had a right aortic arch and abnormal right subclavian artery, but did not receive surgical treatment after birth. This child was followed up until the age of 2 years, and no symptoms such as dyspnea or dysphagia were found. Case 22 with left renal agenesis (shown in Table 4) was complicated with bipedal varus. Rehabilitation training was carried out one month after birth, and no obvious abnormality was detected during follow-up at the age of 2 years. Case 35 with right RA (shown in Table 4) was complicated with a ventricular septal defect. This child underwent interventional repair for the ventricular septal defect one year after birth, and was followed up at the age of 2 years. The other children with intra- or extra-cardiac malformations did not receive related treatments after birth. Follow-up was conducted mainly by calling the family to enquire about their child’s health, and during the attendance of children at our hospital for re-examination.
The prognosis for fetuses with an isolated unilateral kidney and abnormal location is better, but regular follow-up should be performed after birth. If other malformations are also present, the prognosis and pregnancy outcome will depend mainly on the severity of the accompanying malformation. In this study, the induced labor rates of horseshoe kidney, pelvic ectopic kidney, crossed ectopic kidney, and renal absence were compared, and the differences were statistically significant. Bilateral renal dysplasia leads to porter sequence and early death due to lung dysplasia, and is also the most common cause of chronic renal failure in children [25]. In the present study, all patients in which the fetus showed bilateral RA chose to terminate the pregnancy. Moreover, all such cases were combined with oligohydramnios, possibly due to genetic factors, environmental factors, and other unknown causes. It has been reported that CD34+ cells in the maternal blood can be used as a potential marker to predict the development of fetal kidney malformation [26]. Fetal growth disorders such as intrauterine growth restriction and long gestational age can greatly impact fetal kidney development. Abnormal kidney development may lead to hypertension and chronic kidney disease [27].
Prenatal chromosome microarray analysis and exome sequencing can identify the genetic causes of fetal kidney abnormalities. A high incidence of genetic defects has been found at the chromosome (aneuploid), subchromosome (microdeletion/microduplication) and single gene (point mutation) levels in cases with fetal kidney abnormalities. One of the most common pathogenic copy number variants is 17q12 microdeletion [28]. In the current study, only 77 chromosomes were examined, 10 of which were abnormal. In all cases, the chromosome analysis was performed without further genetic diagnosis, and hence additional analysis is needed in future work.
Prenatal ultrasound is the preferred examination technique for the detection of an abnormal location or number of fetal kidneys. In addition to careful examination of bilateral kidneys, comprehensive and systematic scans should also be performed to provide detailed imaging information for prenatal consultation and postnatal follow-up.
The dataset analyzed in the current study is available from the corresponding author upon reasonable request.
LH conceived the study, conducted the literature review and drafted the manuscript. JZ and HW analyzed and interpreted the data. LH and CZ proposed and designed the study. LH revised the manuscript and supervised the study. XZ assisted with the collection and analysis of data. RW and YL analyzed and interpreted the data, and proof-read and revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of the Sichuan Provincial Maternity and Child Health Care Hospital (Ethics No. 20211216-265). Written informed consent was obtained from participants prior to the study.
The authors express their gratitude to all those who helped with the writing of this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.