1 College of Clinical Medical, Jining Medical University, 272000 Jining, Shandong, China
2 Department of Obstetrics, Affiliated Hospital of Jining Medical University, Jining Medical University, 272000 Jining, Shandong, China
Abstract
Preeclampsia is a pregnancy-specific disease, which is easy to cause adverse outcomes in mother and child. Effective prediction of preeclampsia have important clinic al significance. This retrospective study aimed to investigate the utility of thrombin time during the first trimester as a predictive marker for preeclampsia.
We meticulously examined the clinical characteristics of a cohort comprising 222 pregnant individuals with mild preeclampsia, 315 with severe preeclampsia, and 396 healthy pregnant women. Subsequently, we conducted both univariate and multiple regression analyses to discern variations in quantitative variables across these groups and to ascertain any discernible associations between thrombin time and the incidence of preeclampsia. Furthermore, we assessed the predictive performance of thrombin time by utilizing the receiver-operating characteristic (ROC) area under the curve (AUC).
Thrombin time exhibited a statistically significant prolongation in the preeclampsia cohort compared to the healthy pregnancy cohort (p < 0.05). This significance was maintained after adjusting for maternal age and gestation at testing in the logistic regression model. The AUC for thrombin time was found to be 0.953, with a commendable specificity of 97.28% and sensitivity of 92.48% in predicting preeclampsia.
Our findings provide compelling evidence of a noteworthy association between prolonged thrombin time in the first trimester and an elevated risk of preeclampsia. The robust positive correlation underscores the potential of prolonged thrombin time as a predictive marker for the development of preeclampsia. Nevertheless, it is crucial to emphasize that further experimental studies are imperative to elucidate the underlying pathogenesis of thrombin time in the progression of preeclampsia.
Keywords
- preeclampsia
- thrombin time
- coagulation function
- predictive value
Preeclampsia (PE) is defined by pregnancy-induced hypertension with proteinuria occurring after the 20th week of gestation [1]. Globally, it affects approximately 2–8% of pregnant women and is responsible for over 500,000 fetal and neonatal fatalities and over 70,000 maternal deaths, positioning it as the second leading cause of maternal mortality worldwide [2, 3]. Despite being the most prevalent pregnancy complication, the exact pathogenesis and definitive early screening indicators for diagnosing preeclampsia remain elusive. Preeclampsia arises from insufficient trophoblast invasion, resulting in placental ischemia. This ischemia and hypoxia trigger the release of placental inflammatory mediators, which can result in vascular endothelial damage and disruption of the coagulation, anticoagulation, and fibrinolytic systems [4, 5, 6]. Several studies have indicated a correlation between thrombin time (TT) and an elevated risk of various conditions, including preeclampsia, gestational diabetes, and endometriosis [7, 8]. Given these considerations, it is plausible that thrombin time could be related to the initiation and progression of preeclampsia. However, the precise nature of the relationship between thrombin time and preeclampsia remains ambiguous. Moreover, as a readily accessible screening test primarily utilized for detecting fibrinogen abnormalities [9], TT offers a convenient and swift alternative. Our aim was to ascertain its potential as a predictive biomarker for preeclampsia.
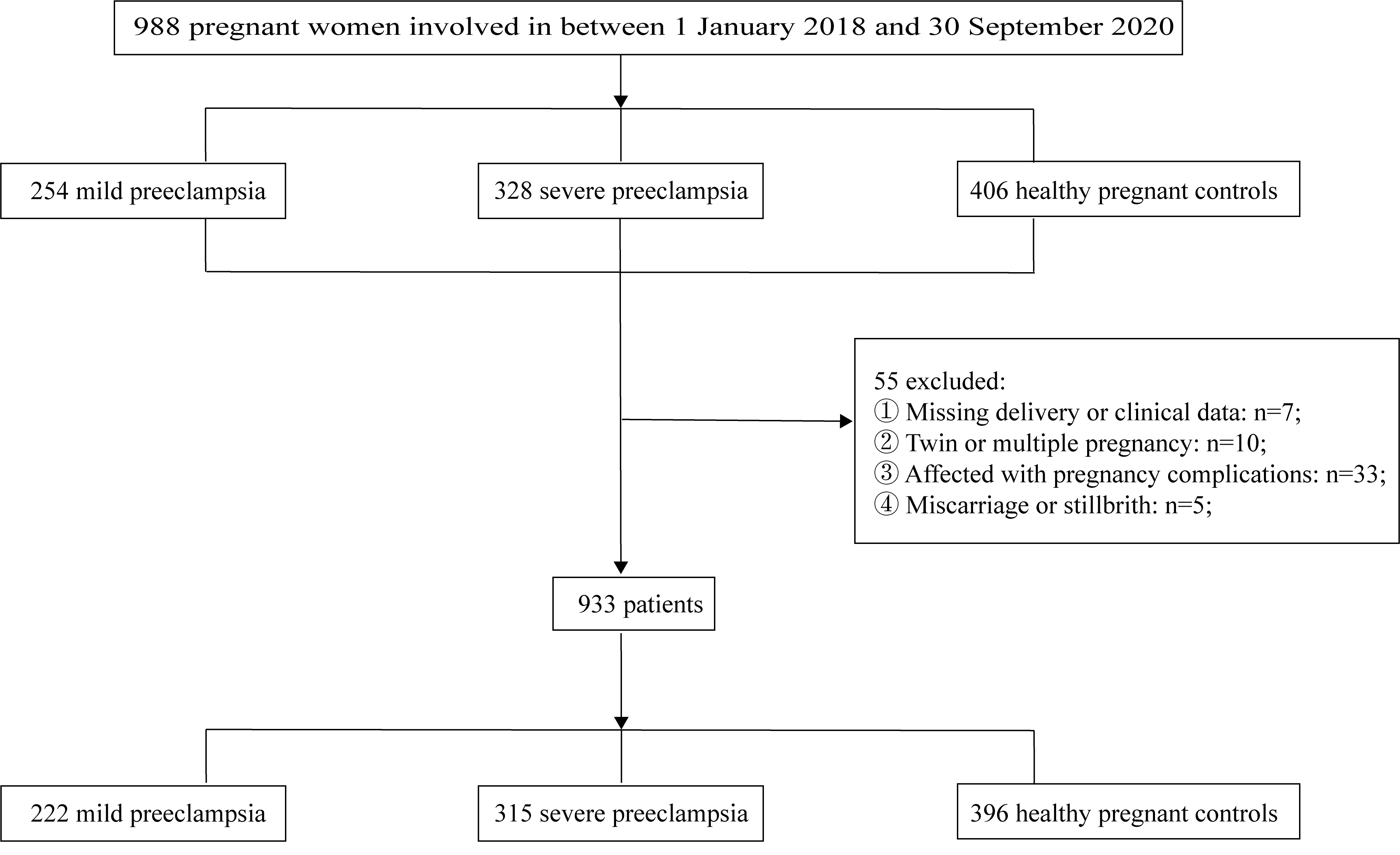
This retrospective study encompassed a cohort of 988 patients who received
treatment at the Obstetric Outpatient Clinic and Inpatient Department of the
Affiliated Hospital of Jining Medical University during the period spanning from
1st January 2018 to 30th September 2020. Among these patients, 933 patients were
meticulously screened and subsequently categorized into three groups: 222 women
with mild preeclampsia, 315 women with severe preeclampsia, and 396 healthy
women, in accordance with the criteria outlined in the 2002 Practice Bulletin of
the American College of Obstetricians and Gynecologists (ACOG) [10]. The
diagnostic criteria for preeclampsia include the following parameters: systolic
blood pressure
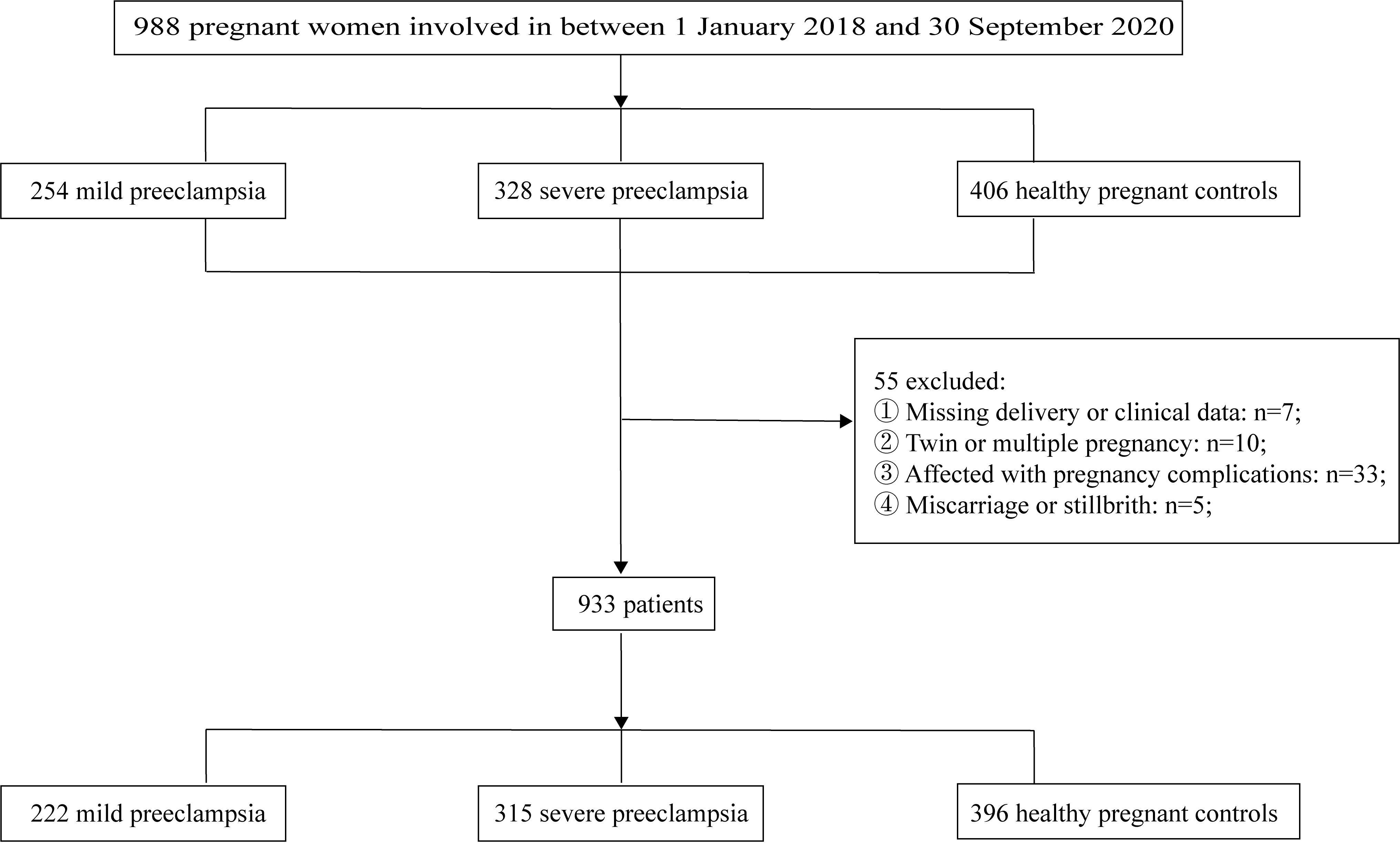 Fig. 1.
Fig. 1.
Flowchart of the study population.
We gathered demographic and medical history information, including age, gestation at testing, body mass index (BMI), and delivery week, from the Haitai Medical Record Information System. Blood samples were obtained in anticoagulant tubes between the 16th and 24th weeks of gestation and were subjected to coagulation function testing within a 2-hour timeframe.
For data analysis, we employed the R software (http://www.R-project.org, 4.3.1 version, The R
Foundation, Vienna, Austria) and Empower Stats software
(http://www.empowerstats.com, 4.2 version, X&Y Solutions, Inc, Boston, MA, USA). Univariate
analysis and multiple regression were conducted on the dataset. The receiver-operating characteristic (ROC) and the area under the curve (AUC) were
generated to assess the potential predictive utility for preeclampsia.
Quantitative factors were presented as
In this study, our sample of 933 pregnant women was stratified into three
distinct categories: 396 healthy pregnant women constituted the control group,
while 222 pregnant women were diagnosed with mild preeclampsia, and 315 pregnant
women exhibited severe preeclampsia. Table 1 provides an overview of the
essential clinical characteristics and selected coagulation routine indicators,
including thrombin time, across these three groups. Throughout the course of
gestation, parameters such as systolic blood pressure, diastolic blood pressure,
and gestation at testing displayed elevations in both the mild and severe
preeclampsia groups when compared to the control group, there are significant
differences among the three groups (p
| Characteristics | Controls (n = 396) | Mild preeclampsia (n = 222) | Severe preeclampsia (n = 315) | p-value |
| Age (years) | 29.64 |
30.68 |
31.91 |
|
| BMI (kg/m2) | 23.01 |
23.12 |
22.99 |
0.974 |
| Systolic blood pressure (mmHg) | 120.41 |
165.55 |
179.63 |
|
| Diastolic blood pressure (mmHg) | 73.14 |
103.47 |
112.27 |
|
| Gestation at delivery (weeks) | 38.76 |
36.17 |
34.08 |
|
| Gestation at testing (weeks) | 14.29 |
15.47 |
17.43 |
|
| Fibrinogen* (g/L) | 4.25 |
4.34 |
5.18 |
0.014 |
| Prothrombin time* (s) | 10.72 |
10.90 |
11.12 |
0.107 |
| Thrombin time (s) | 13.55 |
17.84 |
16.55 |
Results in the table:
In the univariate analysis, as presented in Table 2, we identified systolic
blood pressure, diastolic blood pressure, and thrombin time as statistically
significant risk factors associated with the development of preeclampsia (odds
rratio (OR): 1.41, 1.53 and 5.70; 95% confidence interval (CI): 1.30–1.52,
1.40–1.67 and 4.06–8.01, p
| Covariate | OR (95% CI) | p-value |
| Age (years) | 1.05 (1.03, 1.08) | |
| Systolic blood pressure (mmHg) | 1.41 (1.30, 1.52) | |
| Diastolic blood pressure (mmHg) | 1.53 (1.40, 1.67) | |
| Thrombin time (s) | 5.70 (4.06, 8.01) | |
| Fibrinogen (g/L) | 1.04 (0.98, 1.10) | 0.2230 |
p
CI, confidence interval; OR, odds ratio.
| Variable | Non-adjusted | Adjusted | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Thrombin time (s) | 5.70 (4.06, 8.01) | p |
5.84 (4.07, 8.39) | p |
Results in table: OR (95% CI) p-value. p
Adjusted: adjusted for gestation at testing and age.
OR, odds ratio.
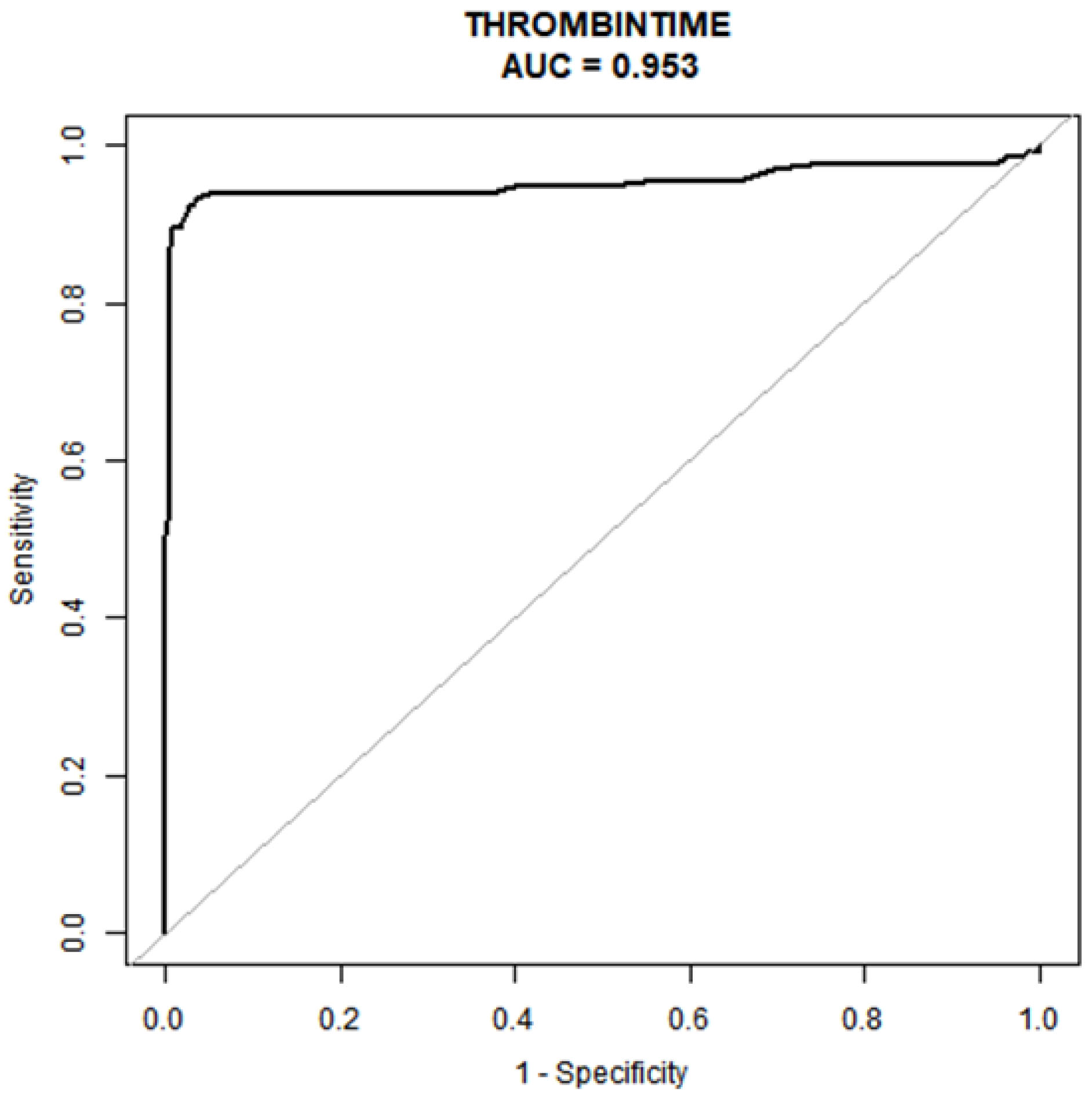
In Table 4 and Fig. 2, we present a comprehensive statistical analysis aimed at identifying models with superior predictive capabilities. ROC curve was meticulously constructed to assess the predictiveprowess of the early pregnancy thrombin time test for preeclampsia. Our analysisunveiled that the early pregnancy thrombin time test indeed exhibits robust predictive potential for preeclampsia, boasting an impressive AUC of 0.953 (95% CI: 0.92–0.98). Moreover, ityielded an optimal TT prediction threshold of 15.05 seconds, achieving animpressive specificity of 97.28% and a sensitivity of 92.48% in predicting preeclampsia.
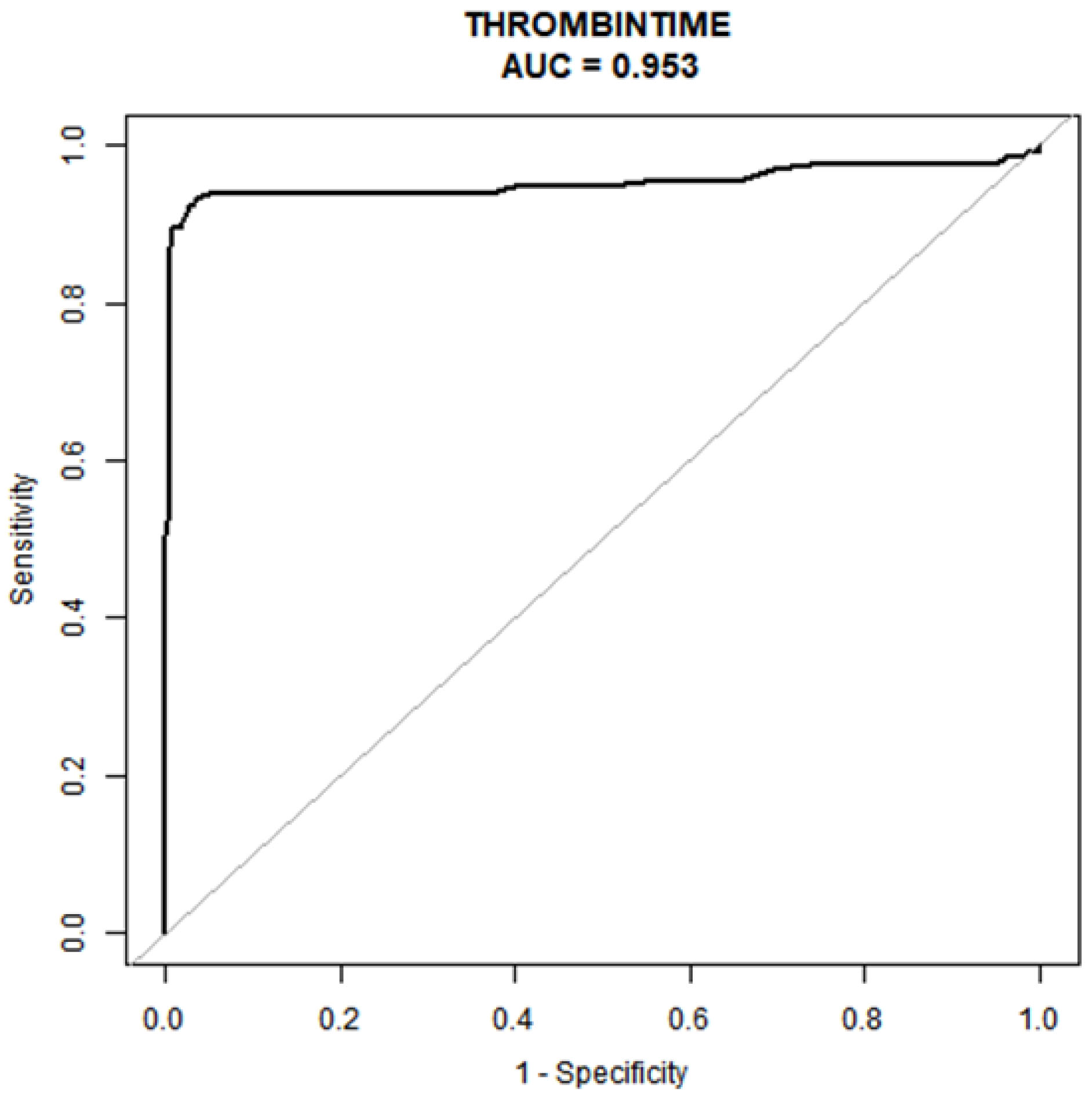 Fig. 2.
Fig. 2.
Area under the ROC curve. The determination of the optimal cut-off point is contingent on the maximization of the combined values of sensitivity and specificity. AUC, area under the curve; ROC, the receiver-operating characteristic.
| Test | ROC area (AUC) | p | 95% CI low | 95% CI up | Best threshold | Specificity | Sensitivity |
| Thrombin time(s) | 0.9527 | 0.9213 | 0.9842 | 15.0500 | 0.9728 | 0.9248 |
Preeclampsia is characterized by its challenging preventability, significant risks, and complex clinical presentation as a prevalent idiopathic disorder in obstetrics [11]. In our retrospective study, preeclampsia was divided into mild preeclampsia and severe preeclampsia. Our classification of severe preeclampsia aligns with the concept of preeclampsia with severe features as outlined in the ACOG (American College of Obstetricians and Gynecologists) guidelines [10]; however, the ISSHP (International Society for the Study of Hypertension in Pregnancy) guidelines recommend that preeclampsia should not be classified as mild or severe because the condition can deteriorate rapidly and without warning [12]. Although the guidelines are slightly different, the distinction between mild and severe preeclampsia is clinically advantageous, as severe cases are associated with serious complications such as HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, placental abruption, eclampsia, hypertensive crises, multiple organ dysfunction, and significant fetal growth restriction [13]. This differentiation is essential for guiding management strategies, therapeutic modalities, timing and mode of delivery, and prenatal care for patients with preeclampsia, ultimately aiming for favorable outcomes for both the mother and fetus [10]. In light of the imperative need to forestall the swift deterioration of maternal organ function, early pregnancy termination frequently becomes a necessary intervention [14]. Therefore, the discovery of reliable predictors for early intervention, prior to the development of serious complications, becomes exceedingly crucial. In our study, we meticulously scrutinized coagulation routines in both preeclampsia-afflicted and non-preeclampsia pregnant individuals, both before and after the 20th week of gestation. Our analyses, including univariate and multiple regression examinations, discerned a robust association between the extension of thrombin time and the condition of patients afflicted with preeclampsia. A further examination of the ROC curve unveiled a substantial AUC of 0.953, complemented by commendable sensitivity and specificity values of 0.9248 and 0.9728, respectively, for predicting preeclampsia. This substantiates the assertion that the prolongation of thrombin time is intimately linked to preeclampsia and holds the potential to serve as a novel predictor for this condition. The extension of thrombin time is associated with pathological anticoagulation states such as hypofibrinogenemia or signifies hyperfibrinolysis and fibrinogen depletion [15, 16]. Plausible rationales for the prolonged thrombin time in the preeclampsia cohort encompass: firstly, while Williams VK et al. [17] demonstrated an elevation in fibrinogen concentration in the preeclampsia group, a substantial reduction in both the rate and peak thrombin generation was noted in the early preeclampsia group compared to their healthy pregnant counterparts [18]. This indicates that thrombin generation, instigated by low-dose tissue factor (TF), undergoes attenuation in the preeclampsia group. Secondly, an alternative explanation can be derived from the markedly lower fibrinogen levels observed in pregnant women afflicted with preeclampsia, in comparison to their healthy counterparts [19, 20]. Serum fibrinogen beta chain levels, a byproduct of fibrinogen degradation, demonstrated elevations even prior to the diagnosis of preeclampsia, with significant surges following diagnosis. Collectively, these findings underline a significant depletion of fibrinogen in cases of preeclampsia [21, 22]. Consequently, a reduction in either fibrinogen or thrombin may account for the elongation of thrombin time in the context of preeclampsia.
The precise pathogenesis of preeclampsia remains enigmatic. In the course of a typical pregnancy, cytotrophoblasts traverse the inner third of the myometrium, penetrating deeply into the innermost layer of the maternal spiral arteries [23]. Subsequently, they integrate with vascular smooth muscle cells, thereby preparing for adequate placental perfusion and optimal fetal development [24]. Conversely, the prevailing theory regarding preeclampsia’s pathogenesis, as proposed by scholars, focuses on the frequently superficial and incomplete invasion of cytotrophoblasts, leading to suboptimal placental perfusion. This in turn induces placental ischemia and hypoxia, resulting in an influx of various detrimental factors into the maternal bloodstream [25]. These factors instigate damage to vascular endothelium, initiating a cascade of coagulopathy [25, 26]. In cases of severe endothelial damage, the liberation of tissue and coagulation factors stimulates both the endogenous and exogenous coagulation systems [27]. This can culminate in a significant elevation in plasma TF concentrations, serving as a catalyst for the extrinsic coagulation pathway in individuals with preeclampsia [28]. Numerous factors within the coagulation cascade may contribute to heighten thrombin generation, ultimately promoting the conversion of fibrinogen into fibrin [29]. Notably, the accumulation of fibrin within the glomeruli has been thoroughly documented in preeclampsia, accentuating a more pronounced hypercoagulable state compared to normal pregnancy [28, 29, 30].
Furthermore, recent investigations have spotlighted the long non-coding RNA, Growth Arrest-Specific 5 (GAS5), as a significant contributor in preeclampsia. GAS5, a lengthy non-coding RNA, has been linked to trophoblast cell proliferation, migration, and invasion and its expression has been found to be upregulated in placentas from preeclampsia-afflicted patients [20]. The invasive characteristics of trophoblast cells resemble those of tumor cells [31]. GAS5 expression typically decreases in tumor tissues due to the excessive invasion of tumor cells [32], whereas it significantly increases in the placentas of pregnant women with preeclampsia, particularly in cases of early-onset preeclampsia. Zheng D et al. [20] have reported a positive correlation between elevated GAS5 levels and prolonged TT, alongside reduced fibrinogen levels. This correlation is consistent with the documented extension of thrombin time in pregnant women with preeclampsia. Interestingly, some investigations have unearthed a hypercoagulable state in preeclampsia, which is not entirely congruent with our findings [33]. It is posited that, as the severity of preeclampsia escalates, heightened activation of the coagulation cascade may lead to the depletion of coagulation components such as fibrinogen or a reduction in thrombin. This, in turn, could culminate in a protracted thrombin time, thus imbuing the utility of prolonged thrombin time as a potential predictor of preeclampsia with meaningful significance.
Currently, although some studies are endeavoring to explore potential biomarkers for early-onset preeclampsia, such as neuroserpin, sFlt-1 (soluble fms-like tyrosine kinase-1), and PIGF (Placental Growth Factor) [34, 35], thrombin time offer a more cost-effective and easily accepted option for patients. However, it is important to recognize the limitations inherent in our study. Due to its retrospective design, we were unable to directly monitor the dynamic evolution of fibrinogen and thrombin in relation to thrombin time throughout pregnancy, which would be possible in experimental studies.
Our research has demonstrated a significant linear correlation between the prolongation of thrombin time and the onset of preeclampsia. Consequently, thrombin time presents potential as a potential predictor for assessing the occurrence of preeclampsia. Future studies could benefit from the implementation of a randomized controlled trial to investigate the dynamic alterations in fibrinogen and thrombin throughout the development of preeclampsia.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
YTL, YLC, HS, and XYZ gathered the essential data and samples. YTL, FGW, and PL conducted the data analysis. YTL authored the manuscript. FGW and DMM designed the research study, while DMM provided oversight and supervision throughout the study. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (Ethics Approval Number: 2024-08-C011).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This project was supported by the National Natural Science Foundation of China (Grant No:82201876), Natural Science Foundation of Shandong Province (Grant No:ZR2021QH114), Natural Science Foundation of Shandong Province (Grant No:ZR2021LZY001), China Postdoctoral Science Foundation (2023M731307), Research Fund for Academician Lin He New Medicine (Grant No:JYHL2021MS24), Postdoctoral Program in the Affiliated Hospital of Jining Medical University (Grant No:322155), Incubation Program of High-level Scientific Research Projects in Jining Medical University (Grant No:JYGC2021KJ007), the Key Research and Development Program of Jining Science (2023YXNS070).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.