1 Department of Thyroid and Breast Surgery, The Affiliated Cangnan Hospital of Wenzhou Medical University, 325800 Wenzhou, Zhejiang, China
2 Department of Thyroid and Breast Surgery, The Third Affiliated Hospital of Wenzhou Medical University, 325200 Wenzhou, Zhejiang, China
Abstract
To study the correlation between the expression of immune factors and the prognosis of surgical treatment of plasma cell mastitis (PCM) and to analyze its predictive value for the prognosis of patients.
89 female patients with PCM treated in our hospital from June 2020 to September 2022 were divided into good prognosis group (69 cases) and poor prognosis group (20 cases). Logistic regression was used to analyze the influencing factors of poor prognosis of surgical treatment for PCM, and to explore the correlation between these immune factors and the prognosis of surgical treatment for PCM. Draw the receiver operating characteristic (ROC) curve to analyze the predictive value of the above indexes for the prognosis of patients with PCM.
After 4 weeks of treatment, the levels of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) were significantly lower than before treatment (p < 0.05), and the level of interleukin 10 (IL-10) was significantly higher than before treatment (p < 0.05). At the time of admission, there was no significant difference in the clinical data and the levels of TNF-α, IL-6 and IL-10 between the two groups (p > 0.05). After 4 weeks of treatment, the indexes of TNF-α and IL-6 in the poor prognosis group were higher than those in the good prognosis group (p < 0.05). Logistic regression analysis showed that the levels of TNF-α (odds ratio (OR) = 1.551, 95% confidence interval (95% CI): 1.276–1.886) and IL-6 (OR = 1.082, 95% CI: 1.046–1.119) were increased, which were risk factors for the prognosis of PCM (OR >1). Correlation analysis showed that TNF-α and IL-6 were negatively correlated with the prognosis of PCM, while IL-10 was positively correlated with the prognosis of PCM. ROC curve analysis showed that the areas under the curve for TNF-α, IL-6 and IL-10 to predict the prognosis of surgical treatment of PCM were 0.896, 0.931 and 0.709 respectively.
The expression of immune factors such as TNF-α, IL-6 and IL-10 is closely related to the prognosis of surgical treatment of PCM, which has high predictive value for its prognosis.
Keywords
- plasma cell mastitis
- tumor necrosis factor α
- interleukin 6
- interleukin 10
- prognosis
- relevance
- predicted value
Plasma cell mastitis (PCM) is a rare inflammatory breast disease, which is
characterized by the infiltration of a large number of plasma cells in breast
tissue. The disease is usually manifested by breast mass, pain or body
temperature discomfort [1]. Although the pathogenesis is not fully understood,
factors such as abnormal immune system and autoimmune diseases are considered to
be related to this. Because of the complexity of its pathogenesis, the diagnosis
and treatment of the disease face many challenges in clinic. Especially in the
prognosis of treatment, the recurrence rate is often high. In recent years, the
role of immune factors in a variety of inflammatory diseases has been gradually
revealed, and the expression level of immune factors is closely related to the
prognosis of many diseases [2]. At present, the urgent research direction is to
explore the correlation between the expression of immune factors and the
prognosis of surgical treatment of PCM, and how to predict the
effect of surgical treatment by using the expression of immune factors. In PCM, immune factors such as tumor necrosis factor
89 female patients with PCM treated in our hospital from June 2020 to September 2022 were followed up after operation, and were divided into good prognosis group (69 cases) and poor prognosis group (20 cases) according to the evaluation criteria of curative effect. Inclusion criteria: ① female patients diagnosed as PCM by breast ultrasound, galactography, molybdenum target X-ray and biopsy [5]; ② both patients and their families signed an informed commitment letter. Exclusion criteria: ① those who were treated with hormones, immunosuppressants and other related drugs one month before admission; ② patients with malignant tumor diseases.
After ultrasonic examination and location, patients were disinfected and covered with towels. Then 2% lidocaine (China Suicheng Pharmaceutical Co., Ltd., Xinzheng, Henan, China) was used for infiltration anesthesia, and simple tumor resection or segmental mastectomy was performed. During the operation, the wounds were washed with hydrogen peroxide and diluted iodophor, and the drainage was standardized after the operation. After 4 weeks of treatment, the patient came to our hospital for follow-up review.
The clinical indexes of the two groups were observed, including the stage of
onset and the location of lesions. At the time of admission and after 4 weeks of
treatment, 5 mL venous blood was taken from the patients, and the supernatant was
centrifuged at 3000 r/min for 10 min, then stored in the refrigerator at –20
°C for later use. The immune factors TNF-
Cure: fistula and lump disappeared completely; improvement: the mass is obviously reduced, without pain and fever, and the fistula is close to closure; unhealed: the lump did not disappear and the fistula persisted; total effective = cure + improvement. All patients were evaluated 4 weeks after operation. Patients who were cured and improved were included in the good prognosis group, and the rest were included in the poor prognosis group.
(1) Follow-up for 4 weeks, and compare the levels of immune factors
TNF-
Sample size calculation basis, in which µα = 0.83,
µβ = 0.36, and the sample size required for calculation and
research is 89 cases. SPSS 26.0 (IBM Corp.,
Armonk, NY, USA) statistical software was used for analysis. The measurement data
were expressed by (
Four weeks after the baseline data of the two groups were compared, 20 patients
(22.47%) had poor prognosis and 69 patients (77.53%) had good prognosis. The
clinical data of the two groups were compared as follows, and there was no
significant difference (p
| Variable | Poor prognosis group (n = 20) | Good prognosis group (n = 69) | t/ |
p | |
| Age (years) | 31.65 |
32.16 |
0.327 | 0.743 | |
| BMI (kg/m2) | 22.45 |
22.36 |
0.142 | 0.886 | |
| Onset side | Unilateral | 17 (85.00) | 61 (88.41) | - | 0.705* |
| Bilateral | 3 (15.00) | 8 (11.59) | |||
| Clinical stages | Sinus period | 9 (45.00) | 32 (46.38) | - | 0.899* |
| Abscess stage | 5 (25.00) | 17 (24.64) | |||
| Mass stage | 4 (20.00) | 15 (21.73) | |||
| Overflow period | 2 (10.00) | 5 (7.25) | |||
| Nipple state | Normal | 5 (25.00) | 18 (26.08) | 0.358 | 0.836 |
| Mild depression | 11 (55.00) | 41 (59.42) | |||
| Complete depression | 4 (20.00) | 10 (14.50) | |||
| Marital status | Be unmarried | 17 (85.00) | 57 (82.61) | - | 1.000* |
| Married | 3 (15.00) | 12 (17.39) | |||
| Menstrual condition | Law | 5 (25.00) | 17 (24.64) | - | 1.000* |
| Indiscipline | 15 (75.00) | 52 (75.36) | |||
| Course of disease (days) | 32.24 |
33.26 |
1.566 | 0.121 | |
| TNF- |
72.19 |
73.24 |
0.556 | 0.572 | |
| IL-6 (pg/mL) | 348.27 |
346.95 |
0.138 | 0.890 | |
| IL-10 (ng/mL) | 96.44 |
95.87 |
0.117 | 0.906 | |
*Fisher’s precision probability test. BMI, body mass index; TNF-
After 4 weeks of treatment, the levels of TNF-
| Factor | On admission | After 4 weeks of treatment | t | p |
| TNF- |
73.00 |
39.58 |
34.145 | |
| IL-6 (pg/mL) | 347.25 |
208.35 |
26.704 | |
| IL-10 (ng/mL) | 96.00 |
136.81 |
11.071 |
After 4 weeks of treatment, the poor prognosis group had higher levels of
TNF-
| Factor | Poor prognosis group (n = 20) | Good prognosis group (n = 69) | t | p |
| TNF- |
46.35 |
37.62 |
7.870 | |
| IL-6 (pg/mL) | 248.34 |
196.76 |
8.709 | |
| IL-10 (ng/mL) | 121.23 |
141.32 |
2.820 | 0.005 |
Taking the prognosis of surgical treatment of PCM as the
dependent variable (good prognosis = 0; poor prognosis = 1). Taking
TNF-
| Factor | Standard error | Wald | p | OR | 95% CI | |
| TNF- |
0.439 | 0.100 | 19.414 | 1.551 | 1.276–1.886 | |
| IL-6 | 0.079 | 0.017 | 20.976 | 1.082 | 1.046–1.119 | |
| IL-10 | –0.026 | 0.010 | 6.852 | 0.009 | 0.974 | 0.955–0.993 |
OR, odds ratio; 95% CI, 95% confidence interval.
Pearson correlation analysis showed that TNF-
| Factor | Prognosis of surgical treatment of plasma cell mastitis | |
| Determinant coefficient | p | |
| TNF- |
–0.645 | |
| IL-6 | –0.682 | |
| IL-10 | 0.289 | 0.006 |
The prognosis of surgical treatment of PCM was taken as a state
variable (good prognosis = 1; poor prognosis = 0), using TNF-
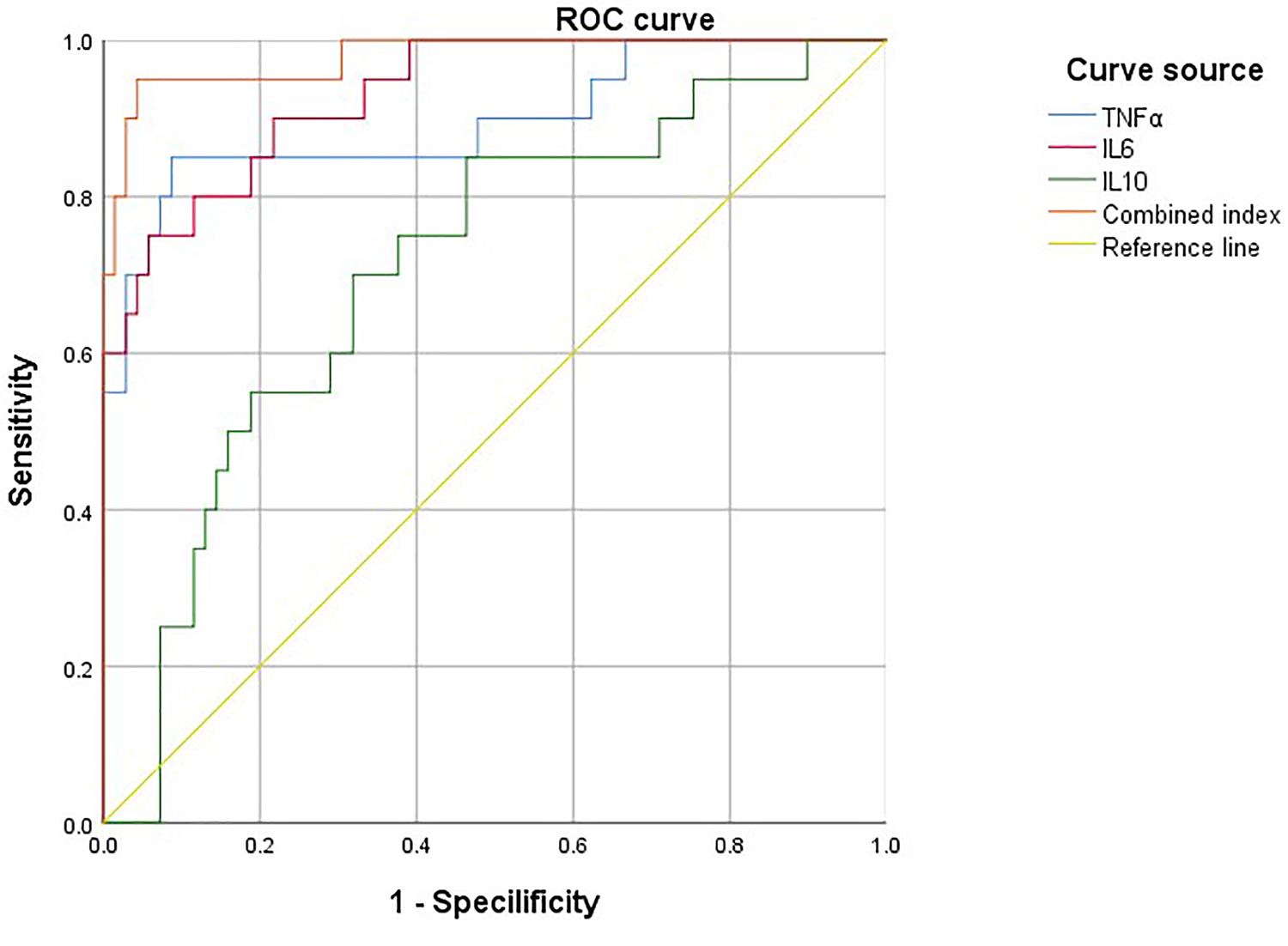 Fig. 1.
Fig. 1.
ROC curve of TNF-
| Factor | AUC | p | 95% CI | Specificity | Sensitivity | Youden’s index |
| TNF- |
0.896 | 0.802–0.991 | 0.928 | 0.750 | 0.678 | |
| IL-6 | 0.931 | 0.873–0.989 | 0.957 | 0.650 | 0.607 | |
| IL-10 | 0.709 | 0.005 | 0.583–0.836 | 0.850 | 0.522 | 0.372 |
| Combined index | 0.945 | 0.881–0.990 | 0.961 | 0.649 | 0.610 |
AUC, area under the ROC curve; 95% CI, 95% confidence interval.
PCM, also known as plasma cell mastitis, is a rare chronic inflammatory disease. This disease is usually caused by nipple invagination and difficulty in excreting mammary duct secretions, which may cause abnormal mammary secretion. It causes chemical inflammatory reaction, and with the continuous progress of the disease, it forms a local mass, which causes great trouble to women’s physical and mental health. The disease usually occurs in middle-aged and elderly women and is related to the abnormal function of autoimmune system. Surgical treatment is one of the commonly used methods to treat PCM at present, which mainly includes local excision, mass excision or partial mastectomy. The main purpose of surgery is to remove inflammatory tissue, relieve the pain and discomfort of patients and prevent the spread of lesions [6].
At home and abroad, the evaluation of surgical prognosis mainly focuses on the survival rate, recurrence rate and quality of life of patients. According to study [7], the vast majority of patients with PCM after surgical treatment can obtain good therapeutic effect, with low postoperative recurrence rate and significantly improved quality of life. However, one study has pointed out that [8], a few patients may have recurrence after operation or need further surgical treatment. In addition, with the deepening of the understanding of the disease, some experts and scholars began to explore more conservative or personalized treatment strategies, such as drug treatment and course adjustment [9], in order to achieve better treatment results and lower recurrence rate. In any case, the study on the prognosis of surgical treatment of PCM has always been one of the focuses of medical and scientific research fields. Because the etiology of PCM is unclear, it brings great difficulties to the diagnosis and treatment of the disease. At present, most scholars consider that the disease is related to the immune system dysfunction of patients, so most scholars do a series of clinical study from the perspective of patients’ immune system [10].
Immune factor refers to various molecules and cellular components
that play a role in identifying, coping with and destroying foreign substances
(such as pathogens, viruses, bacteria, etc.) or abnormal cells in the body (such
as cancer cells) in the immune system. Immune factors include cytokines, growth
factors and other signal molecules. According to modern medical research,
cytokines are mainly divided into two categories: pro-inflammatory factors and
anti-inflammatory factors. The normal immune function of the body needs the
balance of these two factors, and once they change, the immune response of the
body will also be affected [11]. Besides IL-6, TNF-
Similarly, the expression level of TNF-
Different from IL-6 and TNF-
Through Pearson analysis and drawing ROC curve, it can be concluded that the
above indexes have good predictive value for the prognosis after surgical
treatment. Therefore, in patients with poor prognosis after surgical treatment,
we can consider monitoring the changes of immune indexes such as IL-6,
TNF-
To sum up, the expression of immune factors is closely related to the postoperative prognosis of PCM. Monitoring the changes of immune factors after operation can not only provide a direct indication of patients’ recovery status, but also help predict the recurrence and long-term results of the disease. Therefore, in clinic, the detection and regulation of immune factors may become an important means to improve the prognosis of patients with PCM. Future research needs to further explore the exact mechanism between specific immune factors and the prognosis of surgical treatment in order to provide patients with more personalized and effective treatment programs.
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
Conceptualization: SL and XL. Methodology: SL. Data curation: SL. Writing-original draft: SL. Writing-review and editing: XL. Supervision: XL. Both authors contributed to the article and approved the submitted version. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was reviewed and approved by the Ethics Committee of The Affiliated Cangnan Hospital of Wenzhou Medical University, Approval No. 2024078, and informed consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

