1 Torrens University, 5000 Adelaide, SA, Australia
2 National Institute of Complementary Medicine Health Research Institute, Western Sydney University, 2145 Sydney, NSW, Australia
3 School of Medicine, University of Wollongong, 2522 Wollongong, NSW, Australia
†These authors contributed equally.
Abstract
The aim of this narrative review is to summarize studies examining the relationship between microparticulate air pollution (MAP) and polycystic ovary syndrome (PCOS).
PCOS is increasingly regarded as an evolutionary mismatch disorder that manifests in women due to exposure to a range of lifestyle and environmental factors. Although the underlying causes of PCOS remain debated, environmental factors such as endocrine-disrupting chemicals (EDC), may contribute to its pathogenesis due to their well-documented hormonal and metabolic effects. MAP is another significant environmental exposure that has been associated with a variety of chronic diseases, as well as adverse hormonal and metabolic effects, including PCOS. Chronic low-grade inflammation and insulin resistance (IR) are key pathophysiological features of PCOS that have been associated with inhalation and ingestion of MAP.
Our search identified four studies that systematically examined the relationship between MAP and PCOS. One population-based cohort study showed an increased risk of PCOS with increased exposure to various air pollutants, including MAP. A second population-based study showed a decreased risk of PCOS with increasing levels of exposure, while a longer duration of exposure was associated with an increased risk of PCOS. A third study found that conception rates were lower in women with PCOS exposed to second-hand smoke (SHS) compared to non-exposed women. In contrast, a fourth study reported that exposure to air pollutants was not associated with reduced pregnancy outcomes. These data suggest that both the concentration and duration of exposure to MAP may be important factors, and that reproductive outcomes could be affected by exposure to MAP through second-hand cigarette smoke.
Preliminary data suggest that MAP may contribute to an increased risk of PCOS, although the available evidence remains inconsistent. Nevertheless, the current evidence supports advising women to avoid exposure to SHS and MAP whenever possible. This review highlights the need for further research on the effects of MAP in women with PCOS.
Graphical Abstract
Keywords
- polycystic ovary syndrome
- microparticulate air pollution
- second-hand smoke
- pregnancy
- insulin resistance
- inflammation
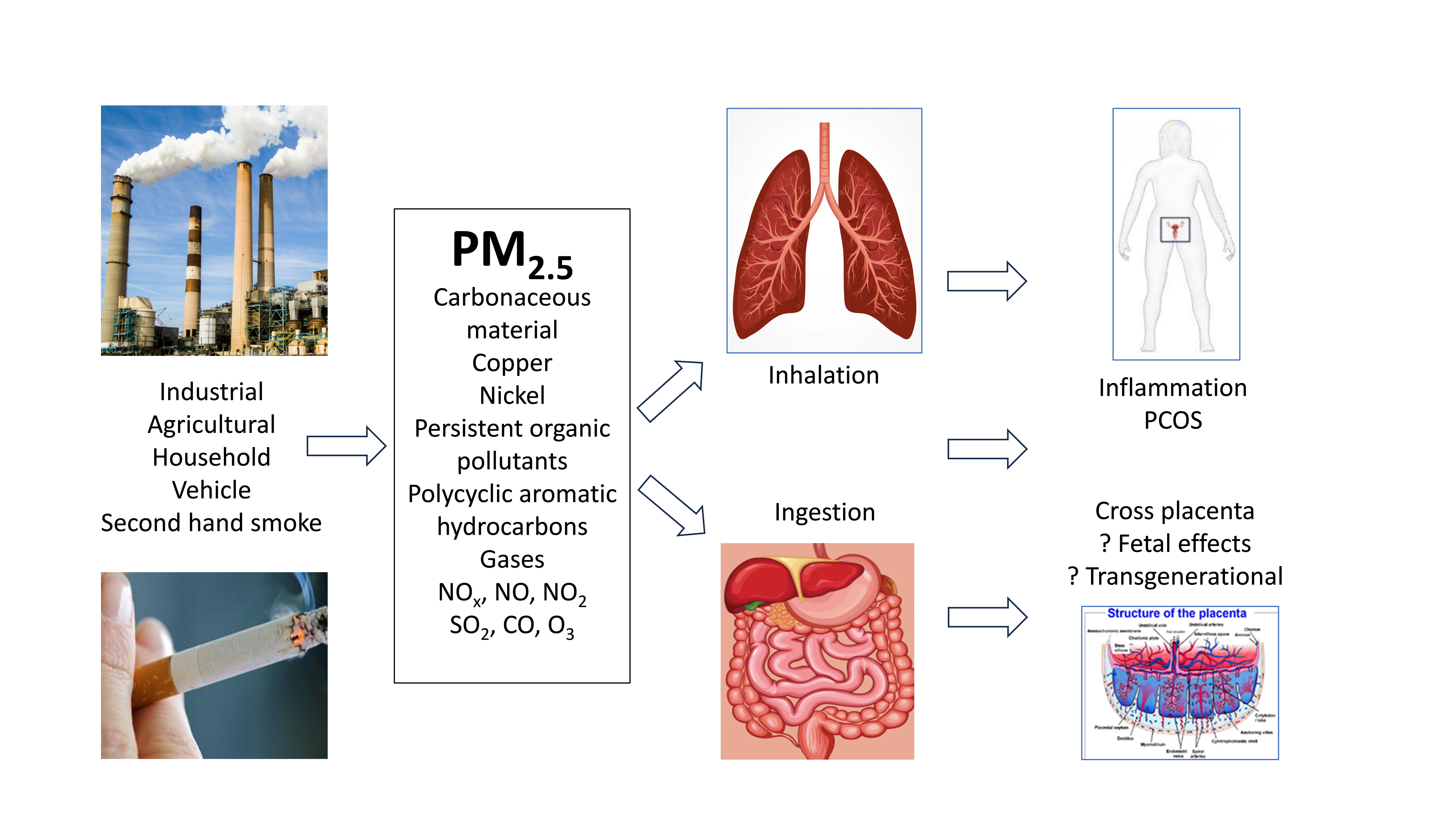
Polycystic ovary syndrome (PCOS) is a complex multisystem disorder that arises from exposure to a variety of environmental and lifestyle factors in women with a genetic predisposition [1, 2]. PCOS is increasingly recognized as an evolutionary mismatch disorder, where ancient adaptive survival mechanisms that were advantageous to ancestral populations have become maladaptive in a modern environment [1, 2, 3, 4]. The core pathophysiological features of PCOS include chronic low-grade systemic inflammation, insulin resistance (IR), hyperandrogenism, and microbiome disruption [4]. A range of lifestyle and environmental factors have been implicated in the pathogenesis of PCOS, including diet, exercise, sleep disruption, stress, disturbances to the gastrointestinal microbiome, and exposure to endocrine-disrupting chemicals (EDC) [1, 5]. Recent evidence has identified microparticulate air pollution (MAP) as a factor that may dysregulate internal homeostasis, leading to changes that initiate and exacerbate the pathophysiology of PCOS [6, 7, 8].
PCOS is a metabolic and endocrine condition affecting 10–13% of women of reproductive age, and its prevalence is increasing worldwide [9, 10, 11]. PCOS is associated with an increased risk of subfertility, preeclampsia, fetal growth restriction, miscarriage, preterm labour, stillbirth, anxiety, depression, and eating disorders. PCOS can progress to a range of other conditions such as obesity, gestational diabetes, type 2 diabetes (T2DM), metabolic syndrome, cardiovascular disease, and cancer [12, 13, 14]. Therefore, PCOS makes a significant contribution to the chronic disease epidemic [15]. While previous research has predominantly focused on lifestyle factors such as diet and exercise, emerging evidence suggests that environmental risk factors such as EDC and MAP may also contribute to the risk of PCOS [6, 16, 17, 18].
MAP is a global threat to health and well-being and has been identified as a possible contributing factor to several chronic conditions, including PCOS. The Global Burden of Disease Study has ranked air pollution as the fourth leading cause of death among females worldwide, and the third cause of disability-adjusted-life-years [19]. Previous studies estimate that MAP is responsible for 4–9 million premature deaths worldwide each year [20]. The widespread presence of MAP has been identified as a contributing factor to increased risk of chronic disease, including respiratory illnesses such as asthma and chronic obstructive airway disease [21], T2DM [7], cardiovascular disease [22], dementia [23], autoimmune diseases [24], and infertility [25]. Additionally, MAP has been associated with reduced life expectancy [26].
Recent observational study has explored the influence of MAP on pulmonary-induced chronic systemic inflammation, suggesting it may contribute to the pathogenesis of PCOS [6]. One proposed mechanism in the pathogenesis of PCOS involves diet-induced increased gastrointestinal permeability, which promotes chronic inflammation, IR, hyperandrogenism, and the characteristic endocrine and metabolic features of PCOS [1, 5, 27]. Chuang et al. [8] reported that exposure to air pollution causes chronic inflammatory changes in blood samples from healthy college students. Zhang et al. [7] found that long-term exposure to air pollution was associated with elevated fasting insulin levels and surrogate markers of IR. Li et al. [28] found that exposure to second-hand smoke (SHS) was associated with elevated androgen levels in women with PCOS. It has been suggested that similar pathways may be disrupted in women with PCOS who are exposed to MAP [6].
Familial and twin studies suggest that PCOS is a heritable condition [10, 29, 30]. Genome-wide association studies have identified gene loci associated to insulin action, androgen biosynthesis, hormone receptors, and ovarian function [1, 31, 32]. These findings have subsequently been further supported by Mendelian randomization studies [33, 34]. PCOS is therefore characterized as a polygenic syndrome arising from interactions between the environmental factors and susceptible gene variants. Compelling evidence suggests that the heritability of PCOS involves in utero developmental epigenetic programming influenced by a range of maternal, nutritional, and environmental factors [35, 36]. MAP may also have an impact on fetal epigenetic programming and play a role in the transgenerational transmission of PCOS [37]. This study aimed to review evidence from studies investigating the associations between MAP and PCOS.
The literature search was performed according with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched PubMed, Cochrane, Web of Science, and Scopus databases for articles published in English, using a combination of Medical Subject Heading (MeSH) and keywords, including polycystic ovary syndrome, polycystic ovarian syndrome, PCOS, microparticulate air pollution, and air pollution.
The search included original articles (prospective observational studies, retrospective cohort studies, and case-control studies) as well as review articles. Studies were reviewed and summarized if they reported outcomes related to PCOS and MAP exposure. The results are presented as a narrative review, as combining the results in a systematic review or meta-analysis was not feasible due to the degree of heterogeneity and the small number of studies identified.
The literature review identified 235 records from the PubMed, Web of Science, Scopus, and Cochrane databases. 45 duplicates were removed, and 190 reports were assessed by screening titles and abstracts. 12 full-text articles were reviewed, with 8 articles excluded. Ultimately, 4 studies met the inclusion criteria. Details of the selection process are shown in the PRISMA flow diagram (Fig. 1).
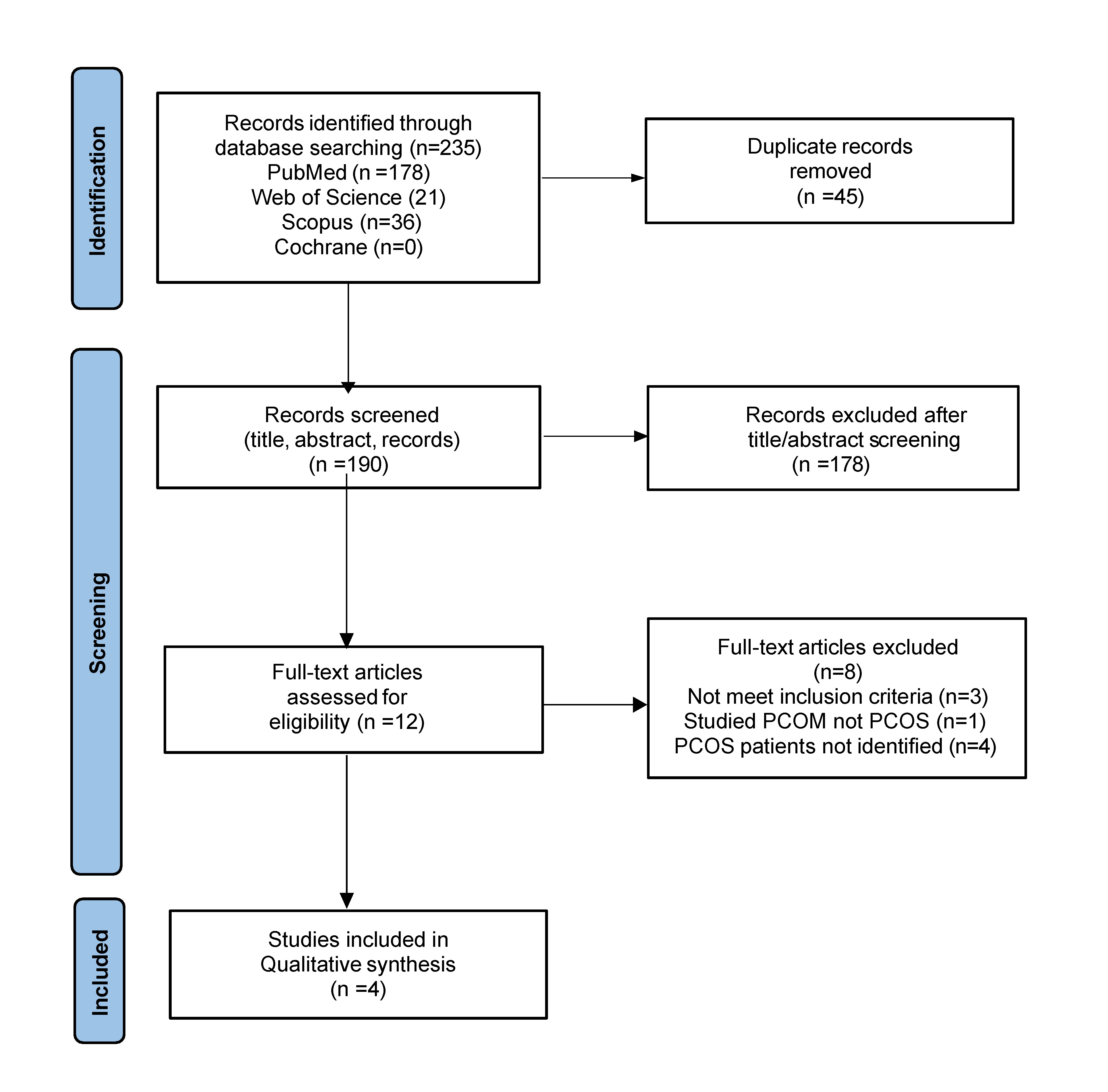 Fig. 1.
Fig. 1. PRISMA flow diagram of the study selection process. PCOM, polycystic ovary morphology; PCOS, polycystic ovary syndrome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The study design, exposures studied, and main findings of 4 studies included in the narrative review are summarized in Table 1 (Ref. [6, 28, 38, 39]). The specific characteristics of each of study included are reviewed and discussed in detail in section 4.
| Source | Study design | Exposure | Main findings |
| Lin et al. (2019) Taiwan [6] | Retrospective-cohort study | SO2 | Women exposed to high pollutant concentrations had a high-risk of new-onset PCOS (N = 2072) |
| Population-wide databases | NOx, NO, NO2 | ||
| Exposure concentration | PM2.5 | ||
| Kim et al. (2022) Korea [38] | Retrospective-cohort study Concentration and duration | SO2 | Risk of PCOS increased with exposure duration but not concentration (N = 237,582) |
| CO, NO2, O3 | |||
| PM2.5, PM10 | |||
| Li et al. (2018) China [28] | Prospective observational study Secondary analysis of RCT | Husband’s SHS | Increased testosterone and FAI, and decreased SHBG in PCOS women exposed (N = 500) |
| Zhu et al. (2022) China [39] | Retrospective-cohort study Exposure concentration | SO2 | Air pollution not associated with pregnancy outcomes in women with PCOS (N = 1652) |
| CO, NO2, O3 | |||
| PM2.5, PM10 |
SO2, sulfur dioxide; NOx, nitrogen oxides; NO, nitrogen monoxide; NO2, nitrogen dioxide; PM2.5, suspended particulates; PM10, coarse particles; CO, carbon monoxide; O3, ozone; FAI, free androgen index; RCT, randomized controlled trial; SHS, second-hand smoke; SHBG, sex hormone binding globulin; PCOS, polycystic ovary syndrome.
A large population cohort analysis investigated the risk of new-onset PCOS in relation to various levels of air pollutants [6]. Data were extracted from 2 population databases: the Taiwan Longitudinal Health Insurance Database (LHID), which covers over 95% of Taiwan’s residents, and the Taiwan Air Quality Monitoring Database (TAQMD). Women with a prior diagnosis of PCOS were excluded. A total of 91,803 female participants were enrolled and followed until their first diagnosis of PCOS. They used the International Classification of Diseases (ICD) to identify women diagnosed with PCOS. A total of 2072 women were diagnosed with PCOS during the study period, after a mean follow-up time of 7.76 (
The TAQMD provides data from 78 air quality monitoring stations distributed across Taiwan. The study utilized data on daily concentrations of sulfur dioxide (SO2), nitrogen oxides (NOx), nitrogen monoxide (NO), nitrogen dioxide (NO2), and suspended particulates (PM2.5). The investigators integrated daily concentrations of air pollutants corresponding to residential addresses to calculate the annual average exposure to air pollutants. The concentrations of air pollutants were divided into four quartiles. A multivariate Cox proportional hazards regression model was used to adjust for confounding factors such as living area, urbanization level (categorized by population density), monthly income, and occupational class (white-collar, blue-collar, other).
Women in the highest quartile (fourth) of exposure had a significantly increased risk of developing PCOS compared to women in the lowest quartile (first) of exposure for all air pollutants studied. The increased risk was 10.31 times for SO2 (95% confidence interval (CI) = 8.35–12.7), 3.37 times for NOx (95% CI = 2.86–3.96), 4.18 times for NO (95% CI = 3.57–4.89), 7.46 times for NO2 (95% CI = 6.38–8.71), and 3.56 times for PM2.5 (95% CI = 3.05–4.15). The authors concluded that women exposed to high concentrations of air pollutants, including microparticulate matter, faced an increased risk of developing PCOS.
This retrospective population-based cohort study examined the relationship between the level and duration of air pollution exposure and the risk of developing PCOS [38]. Data were extracted from the Korean National Health Information Database (NHID), which provides comprehensive medical services to all Koreans. The investigators used the Korean Informative Classification of Disease to identify women newly diagnosed with PCOS. A total of 237,582 PCOS cases were analyzed using a spatial prediction model to assess individual-level exposure to air pollutants.
Exposure to air pollutants was determined using nationwide real-time data on outdoor pollutants from 355 monitoring stations provided by the Ministry of Environment Korea. Monthly average concentrations were used to calculate the risk of developing PCOS associated with 1-year, 2-year, and 3-year exposure to PM10, PM2.5, ozone (O3), carbon monoxide (CO), SO2, and NO2. The residences of participants were matched to of air pollutant concentrations, and logistic regression analysis was used to investigate the effects of exposure duration and air pollutant levels on the risk of developing PCOS.
The annual age-adjusted incidence of PCOS increased progressively from 6.7% to 11.97% over the 5-year study period. The risk of PCOS significantly increased with 3-year exposure to PM2.5, O3, and NO2, compared to 1-year average exposure. The adjusted odds ratio (OR) for 3-year exposure to PM2.5, compared to 1-year exposure was 1.32 (95% CI = 1.27–1.37). However, despite the increased risk of PCOS with 1-year, 2-year, and 3-year exposures, the risk of PCOS actually decreased as the concentration of PM2.5 increased. In contrast to the PM2.5 results, the risk of PCOS increased with higher concentrations of NO2, SO2, and CO. The authors concluded that the risk of PCOS increased with the duration of exposure to air pollutants, including PM2.5, but was not increased by exposure to higher concentrations of PM2.5.
These data contrast with the findings of Lin et al. [6] who reported an increased risk of developing PCOS with rising exposure concentrations of all air pollutants, including PM2.5. The authors suggested that the differences may be due to methodological approaches, as the Lin et al. [6] study used average daily air pollution concentrations prior to PCOS diagnosis, whereas they used monthly average concentrations. Nevertheless, the similarities and differences between the Taiwan and Korean studies demonstrate the challenges of assessing disease risk using population databases and air quality monitoring systems. The reported differences may be due to multiple factors, including differences in PCOS diagnosis, compounding variables, database accuracy, duration of air pollutant exposure, methods of measuring PM2.5, combined effects of multiple air pollutants in different geographic areas, and the assessment models used for data analysis.
In conclusion, the combined results of these two large population-based studies raise concerns about the impact of both the duration and concentrations of multiple air pollutants, including fine particulate matter (PM), on the risk of developing PCOS. This is a significant concern when considered in the context of the extensive body of research linking air pollution to the pathogenesis of other chronic diseases. These findings support the need for further studies and precautionary measures to mitigate the future risk posed by increasing air pollution in women at risk of developing PCOS.
This study investigated the influence of SHS exposure from husbands smoke on metabolic, endocrine, fertility, and obstetric outcomes in women with PCOS [28]. This prospective observational study involved 500 women, comparing 271 women exposed to SHS with 229 women who were not exposed. The data was a secondary analysis from the PCOS Acupuncture and Clomiphene randomized controlled trial, conducted at 27 hospitals in mainland China.
The study participants were Chinese women with PCOS undergoing ovulation induction for anovulatory infertility. PCOS was defined using the Rotterdam criteria, and SHS exposure was defined as living with a partner who was a daily chronic smoker for at least 6 months. The exposure group was divided into high (
Women in the SHS-exposed group had significantly higher total serum testosterone and free androgen index (FAI) and lower sex hormone binding globulin (SHBG) compared to non-exposed women. Further subgroup analysis demonstrated a dose-response effect, with women in the high-exposure SHS group showing significantly higher total testosterone (1.8 vs. 1.5 nmol/L, p
Cigarette smoke is an aerosol containing over 5000 chemicals, including noxious gases, and volatile chemicals, including microparticulate matter and nicotine [40]. The PM component of cigarette smoke consists of thousands of chemicals, including alkaloids, polycyclic aromatic hydrocarbons, tobacco-specific nitrosamines, polonium-210, nickel, cadmium, arsenic, and lead [41]. Nicotine has been shown to inhibit aromatase, the enzyme responsible for converting testosterone to estrogens [42, 43]. This may be one mechanism contributing to the observed increase in androgens in the current study. These findings warrant further investigation, as altered aromatase activity is known to contribute to hyperandrogenism in women with PCOS [4, 44].
The findings of Li et al. [28] therefore support previous research on the risk of SHS exposure for a large section of the community. It is estimated that worldwide, 65% of non-smoking adults and 40% of children are exposed to SHS [45]. According to a World Health Organization (WHO) report, SHS exposure causes 1.2 million deaths per year among non-smokers [46]. The percentage of women exposed to SHS is estimated to be 70% in China [47] and nearly 50% in the United States [48]. Households with smokers have been found to have higher concentrations of PM compared to non-smoking households [49]. Exposure to SHS has also been associated with increased risks of implantation failure and reduced live birth rates in infertile women [50].
In summary, the study of Li et al. [28] found that biochemical hyperandrogenism and metabolic syndrome were more common, and conception rates were lower, in women with PCOS and anovulatory infertility exposed to SHS. These data are consistent with a large body of epidemiological research showing a range of adverse effects of SHS in women. The authors recommended that partners of infertile women with PCOS be advised to quit smoking.
Zhu et al. [39] performed a retrospective cohort study involving 1652 women with PCOS, compared to a control group of 12,543 women with tubal factor or male factor infertility who were receiving in vitro fertilization (IVF) treatment. The investigators evaluated the average daily concentrations of PM2.5, PM10, SO2, NO2, CO, and O3 during 6 different exposure periods of the IVF cycle. Daily concentration data were obtained from the National Urban Air Quality Real-time Publishing Platform at the nearest monitoring station to each patient.
The study examined differences in ultrasound-determined clinical pregnancy rates 35 days after embryo transfer, and live birth rate, defined as at least one infant born alive after 24 weeks of gestation and surviving beyond 28 days. The results indicated that exposure to air pollutants was not associated with reduced clinical pregnancy rates or live birth rates in women with PCOS. In contrast, women in the control group with tubal or male factor infertility were found to have lower clinical pregnancy and live birth rates.
However, there were significant differences between the study and control populations. The women with PCOS were younger, had more primary infertility, a higher body mass index (BMI), fewer embryos transferred, and fewer fresh embryo transfers. The investigators repeated the sensitivity analysis after adjusting for age, BMI, and first embryo transfer cycle, and found no significant differences compared to the original analysis. The authors identified several limitations in their investigation, including its retrospective nature, the lack of personal ambient air pollution exposure data, and various other potential confounders. Nevertheless, it is concerning that women without PCOS experienced negative pregnancy outcomes related to air pollutant exposure.
Previous studies have shown that ambient PM can adversely affect sperm quality [51] and other pregnancy outcomes in the general population [39, 52]. Epidemiological data have linked air pollution exposure to miscarriage, preterm birth [53, 54], low birthweight [53], and preeclampsia [55]. Proximity to the source of air pollution is also a factor contributing to adverse pregnancy outcomes. However, no studies have found an association between adverse pregnancy outcomes and MAP in women with PCOS. The findings of Zhu et al. [39] are consistent with the reported adverse effects of air pollution on pregnancy and highlight the need for further investigation and precautionary measures with women with PCOS.
An increasing body of epidemiological research has identified components in air pollution, including microparticulate matter, as risk factors for the development of chronic disease. PCOS is a lifelong condition that can lead to a variety of chronic metabolic diseases, which have been associated with MAP exposure. This review has identified preliminary evidence suggesting that MAP may be a risk factor in PCOS. Both the concentration and duration of exposure to PM2.5 may be important. In addition, reproductive outcomes during infertility treatment may be impacted by exposure to PM2.5 from air pollution, from second-hand cigarette smoke, and ambient air pollution.
Air pollution is an increasing global problem that has been associated with a wide variety of human diseases, including PCOS [6, 28, 38, 39, 56, 57, 58]. Air pollution consists of a range of components, including gases (SO2, NOx, NO, NO2) and PM2.5of different sizes. PM is classified by the particle size into PM10 (diameter
MAP has been associated with menstrual irregularities [61], infertility [25, 28], adverse pregnancy outcomes [39], an increased incidence of PCOS [6, 38], and has also been linked to transgenerational transmission and infant weight gain [62, 63]. Human studies have shown that metabolites of tobacco, such as cotinine, can be found in ovarian follicular fluid and may potentially affect the developing egg [50, 61, 64]. An in vitro study have demonstrated that diesel exhaust particles can exhibit both estrogenic and androgenic activity, raising concerns about potential adverse hormonal effects in women’s health [65]. MAP has also been associated with chronic systemic inflammation [7] and IR [66, 67], both of which are known core pathophysiological components of PCOS [1, 4]. All of aforementioned health-related effects can occur in women with PCOS, raising significant concerns that MAP may exacerbate both the clinical and biochemical components of PCOS.
Large systematic reviews confirm an important role for chronic systemic inflammation in the pathogenesis and pathophysiology of PCOS [68, 69]. Acute inflammation is an evolutionary conserved physiological survival mechanism that occurs in response to exposure to non-infectious agents (such as trauma, reactive oxygen species (ROS), uric acid, cytokines, exosomes) and infectious agents [70]. Optimal health is achieved when a balance between pro- and anti-inflammatory processes restores homeostasis. Chronic inflammation can be caused by factors such as poor-quality diet, disturbance of the microbiome (dysbiosis), EDC, oxidative stress, advanced glycation end-products, neuroendocrine imbalance due to stress, and hyperandrogenism [71]. Chronic low-grade systemic inflammation can occur when persistent stimuli lead to the failure of homeostatic mechanisms, and is a cornerstone of PCOS pathophysiology [4]. MAP may act as a persistent chronic inflammatory stimulus and is increasingly recognized as a potential risk factor in the pathophysiology of PCOS [6, 28, 38].
PM can deeply penetrate the respiratory passages and lungs, to lodge in the alveoli [72]. Finer particles can translocate across the alveolar epithelial cells, where they are absorbed by submucosal immune cells [59]. Immune activation can cause oxidative stress, DNA damage, respiratory and systemic inflammation, and changes in the biochemistry and cellular physiology [73]. MAP has also been associated with chronic inflammation, endothelial injury, respiratory disease, and cardiovascular disease [72, 74, 75]. Episodic PM2.5 exposure has been associated with endothelial cell apoptosis, an antiangiogenic plasma profile, and elevated levels of monocytes and T-lymphocytes [74]. MAP was also associated with elevated levels of microparticles in endothelial cells and an increase in systemic antiangiogenic cytokines in healthy adults [74].
IR is characterized by decreased tissue sensitivity and cellular response to insulin and is present in the majority of women with PCOS [76, 77]. The gold standard for assessing IR is the hyperinsulinemic-euglycemic clamp test [78]. A systematic review of hyperinsulinemic-euglycemic clamp studies reported that women with PCOS experience a 27% reduction in insulin sensitivity [79]. IR can be caused by multiple factors, including a high-glycemic diet, metabolic intermediates such as diacylglycerol, hyperinsulinemia, insulin receptor antagonists, hormones, oxidative stress, advanced-glycation end-products, and inflammatory cytokines [80, 81]. Physiological IR has an evolutionary adaptive role, benefiting women with PCOS by responding to environmental challenges (such as infection, starvation) and internal states (such as pregnancy, puberty, physical and psychological stress) [82, 83, 84]. The implementation of IR results in the mobilization of fatty acids from adipose tissue, increased hepatic gluconeogenesis, glucose release from the liver, and redistribution of energy to the brain and immune system [80]. Chronic activation of this response can become pathological and lead to T2DM, metabolic syndrome, metabolic-associated liver disease, and cardiovascular disease, all of which are associated with PCOS [14, 85, 86].
IR is a central component in the pathophysiology of PCOS and has been associated with MAP in both animal and human studies [66, 87]. MAP may contribute to the development of IR through various biological mechanisms [7] and may therefore be involved in the pathophysiology of PCOS [6]. Air pollutants such as NO and PM have been positively associated with IR in various populations. This association has been observed in elder individuals, assessed by serum insulin level and the homeostasis model assessment index [88], as well as in children (evaluated using the Pollution Standard Index) [89]. Epidemiological research has shown an association between air pollution and IR in gestational diabetes [66], T2DM [90], and obesity [91, 92]. Niemann et al. [93] have published a comprehensive review detailing the biological mechanisms underlying IR. The authors highlight the central role of ROS in inhibiting insulin signaling and causing mitochondrial dysfunction, which can contribute to the development of IR. The production of ROS can result from a number of nutritional and environmental causes, including MAP. Further studies into the role of MAP in the development of IR, and the effectiveness of mitigation strategies, should be a research priority.
Both gaseous and PM in air pollution can have endocrine-disrupting effects [94]. Volatile and semi-volatile organic compounds found in both indoor and outdoor air environments can act as EDC [95]. These compounds, including phthalates, bisphenol A, parabens, and triclosan, are known to disrupt estrogen and androgen pathways and have all been associated to PCOS [96, 97, 98, 99, 100]. EDCs released into the indoor environment from activities such as cooking and smoking, including polycyclic aromatic hydrocarbons, can also act as EDCs [101]. This may be one mechanism contributing to the elevated androgen levels found in women with PCOS who are exposed to SHS. Prenatal exposure to cigarette smoke has been linked to childhood obesity and may have transgenerational effects that contribute to the development of PCOS [102]. Cigarette smoking has also been shown to alter the gastrointestinal microbiome, which is believed to play a central role in the pathogenesis of PCOS [103].
The dysbiosis theory of PCOS pathogenesis proposes that a poor-quality diet leads to an imbalance in the gastrointestinal microbiome, disrupts intestinal barrier integrity, and activates submucosal macrophages. This process initiates a cascade of changes that result in systemic inflammation, IR, and hyperandrogenism, contributing to the observed features of PCOS [5, 27]. Oral ingestion of airborne PM from contaminated food has been shown to alter the gastrointestinal microbiome, disrupt the intestinal barrier, and modify systemic immune function [104, 105, 106]. Glyphosate, a known EDC, is a widely used herbicide in domestic gardens and farming areas, and has been detected in outdoor air [107]. Glyphosate may play a role in the pathogenesis of PCOS by altering gastrointestinal microbiome function and via other endocrine-disrupting and metabolic effects [108]. Oral ingestion of polycyclic aromatic hydrocarbons carried on PM can be metabolized by the gut microbiome into EDCs with estrogenic effects [109]. In summary, multiple potential endocrine-disrupting effects of MAP may be involved in the pathogenesis and pathophysiology of PCOS.
Rodent studies have also demonstrated adverse effects of maternal PM2.5 exposure on fetal and placental development [110]. Bové et al. [111] demonstrated the accumulation of black carbon particles on the fetal side of the placenta. The average particle count was higher in mothers with high exposure compared to those with lower levels of PM exposure. These data suggest that atmospheric PM can be transported across the placenta, potentially explaining the detrimental health effects of in-utero exposure to PM, including adverse developmental programming. In summary, these reports suggest that PM2.5 may contribute to placental dysfunction and adverse pregnancy outcomes in women with PCOS.
Recent research in human and animal models has shown that there is dysregulation in noncoding RNA networks that may contribute to the development of PCOS [112]. Women with PCOS have been found to exhibit abnormal expression of microRNAs in ovarian theca and granulosa cells, serum, and adipose cells [113]. An in vitro study have shown that MAP can invoke an inflammatory response by altering non-coding RNAs [114]. Although direct studies linking air pollution, non-coding RNAs, and PCOS are not available, it is plausible that air pollution could influence the expression of non-coding RNAs and affect the development and progression of PCOS. Further research is needed to explore these interactions and their implications for the management and prevention of PCOS.
Both individual and public health strategies have been proposed to minimize exposure to MAP [115]. Individual strategies include behavioral approaches such as ensuring good indoor ventilation, avoiding high-traffic areas for living and commuting, using HEPA air purifiers, avoiding SHS exposure, replacing scented cleaning products with natural products [116], as well as nutritional practices to increase consumption of a healthy plant-based diet rich in antioxidants (vitamins A, B6, C, D, E, and omega-3 polyunsaturated fatty acids) [117]. Public health strategies to reduce air pollution in urban areas, along with educational campaigns to limit pollution sources, should be a priority [115]. Reproductive-age women should be counselled about the potential risks related to air pollution and advised to limit their exposure to areas with high pollutant concentrations and to avoid smoking. Women with PCOS-associated infertility and their partners should be counseled about the risks of SHS exposure on both fertility and pregnancy outcomes. Partners of infertile women with PCOS should be advised and supported to quit smoking.
Future research on the health effects of air pollution should include cohorts of women with PCOS, who make up 10–13% of reproductive-age women. This is important because they represent a significant link to progressive chronic diseases and the future health of their offspring. Research towards decreasing exposure to pollutants and promoting smoking cessation among pregnant women and their partners should be prioritized. The potential risk of air pollution on the incidence of PCOS, as well as on fertility and pregnancy outcomes, should be highlighted in discussions aimed at mitigating the risks associated with anthropogenic induced air pollution and climate change.
This review has several limitations. Only 4 studies were identified despite a comprehensive literature search. The identified studies demonstrate heterogeneity in both their objectives and reported outcomes. As a result, each study was discussed separately to elaborate a detailed evaluation of its methodology and results for individual evaluation and discussion. The findings of Lin et al. [6], which indicate an increased risk of PCOS with higher concentrations of air pollutants, contrast with those of Kim et al. [38], who reported a decreased risk of PCOS with higher concentration of PM2.5. This discrepancy may stem from methodological differences, as previously discussed, or it could indicate a true lack of effect. However, the progressive increase in incidence of PCOS with higher quartiles of exposure reported by Lin et al. [6], coupled with the increased risk of PCOS with longer exposure durations reported by Kim et al. [38], underscores the need for further investigation and highlights the need for precautionary measures. Another limitation of the review is that all 4 studies were performed in Asian populations. There is substantial variation in the prevalence and phenotypic presentation of PCOS in women of different ethnicities, which may limit the generalizability of the reported findings [118].
MAP has been found to play a significant role in development of various metabolic-associated chronic diseases. Preliminary data from the present review suggest that MAP may contribute to an increased risk of PCOS, alongside other established lifestyle, nutritional, and environmental risk factors. The small number of studies identified limits the ability to draw definite conclusions. Nevertheless, these findings underscore the increasing global concern regarding the impacts of air pollution and climate change on human health. This review highlights the urgent need for further research into the effects of MAP in women with PCOS. Mitigation strategies to reduce individual exposure to MAP and SHS should be considered when counselling women with PCOS. Women with PCOS, as well as their partners, should be advised to quit smoking.
NS, JP and VK were responsible for conceptualisation and study design. NS and JP performed the literature search and wrote the original manuscript. All authors contributed to critically revising and making editorial changes to the manuscript. All authors have read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to express our appreciation to those who have helped us during the writing of this manuscript. We would like to thank Michele Parker for her English language editing advice. We would also like to thank the peer-reviewers for their encouragement, opinions and suggestions, which have helped improve the quality of the manuscript.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

