1 Department of Gynecology and Obstetrics, Wuxi No.2 People’s Hospital, 214000 Wuxi, Jiangsu, China
Abstract
Gestational diabetes mellitus (GDM) is a common complication of pregnancy that has a certain impact on maternal and infant health. The aim of this study was to investigate the diagnostic value of microRNA-204-5p (miR-204-5p) in GDM by analyzing its differential expression between GDM patients and healthy individuals and to assess its predictive potential for the occurrence of poor maternal and infant outcomes in GDM patients.
GDM patients (107; GDM group) and healthy pregnant women (92; control group) were recruited for this prospective study. GDM patients meet the relevant criteria of the International Association of Diabetes and Pregnancy Study Groups (IADPSG), which are categorized into good and poor outcome groups on the basis of maternal and infant pregnancy outcomes. Serum miR-204-5p expression was quantified via the reverse transcription quantitative polymerase chain reaction (RT-qPCR). The diagnostic potential of miR-204-5p in GDM patients was analyzed by receiver operating characteristic (ROC) curves and further assessed for its ability to predict the occurrence of poor maternal and infant outcomes in GDM patients. The difference in clinical indicators between GDM patients and healthy pregnant women was determined through Student's t-test, and the clinical indicators of GDM patients in the good outcome group and the poor outcome group were evaluated. The potential of miR-204-5p as a risk factor for poor maternal and infant outcomes in GDM patients was evaluated by binary logistic analysis.
miR-204-5p expression was decreased in GDM patients compared to healthy pregnant women. The area under the curve (AUC) for distinguishing the healthy people from GDM patients was 0.918, with a sensitivity and specificity of 78.5% and 90.2%. Additionally, miR-204-5p expression in GDM patients in the poor outcome group was lower than that in the good outcome group. The AUC reflecting the potential of miR-204-5p in predicting poor maternal and infant outcomes in GDM patients was 0.855, with a sensitivity of 66.7% and a specificity of 90.5%. miR-204-5p is a risk factor for poor maternal and infant outcomes in GDM patients.
Serum miR-204-5p expression is low in GDM and has a high diagnostic potential for predicting the occurrence of GDM and poor maternal and infant pregnancy outcomes. It is expected to be a biomarker for the risk of GDM.
Keywords
- gestational diabetes mellitus
- miR-204-5p
- diagnosis
- poor maternal and infant outcomes
Gestational diabetes mellitus (GDM) is the first manifestation of glucose intolerance during pregnancy and causes great damage to the health of both mothers and infants [1]. As dietary patterns evolve and standards of living improve, the incidence of GDM is on the rise. GDM may result in harm to maternal tissues, increased risk of postpartum infections, obesity, and cardiovascular issues for pregnant women, while also posing risks of harmful conditions for the fetus, such as respiratory distress, macrosomia, and hypoglycemia [2]. Emerging evidence indicates that GDM patients have a higher chance of experiencing adverse outcomes, with this risk rising progressively as blood glucose levels increase [3, 4]. Therefore, early prediction and intervention for GDM are crucial to reducing poor maternal and infant outcomes and even fetal death.
MicroRNAs (miRNAs) are non-coding single-stranded RNA molecules that play critical roles in various physiological processes [5]. Scholars working on pregnancy-related diseases have reported that miR-30b-5p influences the development of preeclampsia via trophoblast cells [6]. Abnormal expression of miR-411 and miR-376c is linked to the progression of gestational hypertension [7]. Another study reported that miR-221 participates in GDM by targeting P21-activated protein kinase 1 (PAK1) [8]. Interestingly, Mitra et al. [9] proposed miRNAs as potential markers for GDM and adverse pregnancy outcomes. Initially, miR-204-5p was reported to be dysregulated in melanoma and related to disease progression [10]. Furthermore, miR-204-5p is considered a therapeutic target for diabetic retinopathy [11]. In a recent study, miR-204-5p was demonstrated to be downregulated in patients with diabetic nephropathy and to regulate the Keap1/Nrf2 pathway to alleviate injury in diabetic nephropathy [12]. Moreover, low expression of miR-204-5p has been implicated in diabetic cataracts, breast cancer, and pregnancy-induced hypertension [13, 14, 15]. However, the role of miR-204-5p in GDM has not been fully elucidated, particularly in the context of predicting poor maternal and infant outcomes in patients with GDM on the basis of miR-204-5p expression.
Therefore, the present study hypothesized that miR-204-5p is dysregulated in GDM patients and may be associated with adverse maternal and infant outcomes in GDM patients. Therefore, we determined the expression of miR-204-5p in the serum of GDM patients and analyzed its predictive potential in GDM. Additionally, the possibility of establishing miR-204-5p as a predictor of the risk of GDM was explored by evaluating the ability of miR-204-5p to predict poor maternal and infant outcomes.
The study was conducted as a prospective study in accordance with the principles of the Declaration of Helsinki. All protocols of this investigation were approved by the Ethics Committee of Wuxi No.2 People’s Hospital (approval number 2022-127). All participants received thorough information about the study and signed written consent to participate.
A sample of 107 pregnant women who underwent the documentation and diagnosis of GDM in the obstetrics department of Wuxi No.2 People’s Hospital between January 2023 and December 2023 were enrolled in the present study as the GDM group. Moreover, 92 healthy pregnant women were included in the study as the control group. All pregnant women who formed the GDM group fulfilled the following criteria: (1) age between 20 and 40 years, with singleton pregnancies. (2) GDM patients were diagnosed at 24 weeks of gestation by an oral glucose tolerance test (OGTT) and an obstetrician, and the patients met the requirements of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) [16]. (3) Prenatal examination and delivery at our hospital and cooperation during treatment. The following pregnant women were excluded from the study: (1) had a history of diabetes prior to pregnancy. (2) Other pregnancy complications, such as gestational hypertension. (3) Cardiac and hepatic insufficiency or the presence of a malignant tumor. In addition, all healthy pregnant women underwent antenatal examinations, and the maternal and infant data were normal, with no other complications or history, such as diabetes or sexual dysfunction.
The pregnancy outcomes of GDM patients were evaluated according to the definition of poor maternal and infant outcomes in the IADPSG. The poor outcome group included pregnant women with postpartum hemorrhage and infection, premature birth in newborns, respiratory distress syndrome, and macrosomia.
Blood samples were drawn from all participants fasted for 12 hours. Each collected sample was centrifuged at 3000 r/min for 10 min, and the resulting supernatant was aspirated and stored for later use.
The serum samples were subsequently evaluated for fasting blood glucose (FBG), 2-hour postprandial blood glucose (2 h PBG), glycosylated hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels using an automated biochemical analyzer (Beckman Coulter Co., Brea, CA, USA). The intra- and inter-assay coefficients of variation for FBG were 9.4% and 11.9%, 2 h PBG were 8.5% and 10.6%, HbAlc were 5.5% and 6.0%, TG were 11.9% and 13.4%, TC were 9.8% and 10.6%, LDL-C were 10.7% and 11.2%, HDL-C were 11.9% and 13.0%, and the range between 1–25 µL.
Total RNA was extracted from each serum sample using the TRIZOL kit (Solebol, Beijing, China) and then evaluated for RNA integrity and purity. Next, the corresponding cDNA was obtained from the extracted RNA using a quantitative reverse transcription kit (Toyobo, Osaka, Japan). The cDNA was subsequently mixed with the primers and relevant reagents to prepare the reverse transcription quantitative polymerase chain reaction (RT-qPCR) mixtures, and PCR was performed according to the instructions provided with the miScript SYBR® Green qPCR Kit (Qiagen, Hilden, Germany). The reaction system was configured and placed inside a fluorescence quantitative PCR instrument (Bio-Rad, Hercules, CA, USA), and each sample was evaluated in triplicate. miR-204-5p expression was calculated using the 2-ΔΔCt method, and U6 was used as the internal reference. The sequences for miR-204-5p were (forward) 5′-GCCGAGUUCCCUUUGUCAUC-3′, and (reverse) 5′-CTCAACTGGTGTCGTGGA-3′. The sequences for U6 were (forward) 5′-CTCGCTTCGGCAGCACA-3′ and (reverse) 5′-AACGCTTCACGAATTTGCGT-3′.
SPSS 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad 9.0 (Dotmatics, Boston, MA,
USA) were applied to process and analyze all the data. The measured data are
expressed as mean
No statistically significant differences were noted between the 107 patients
with GDM in the GDM group and the 92 healthy controls in terms of age, body mass
index (BMI), gestation, or number of gestational births (p
RT-qPCR assays revealed that the serum miR-204-5p level was lower in the GDM
group than in the control group (p
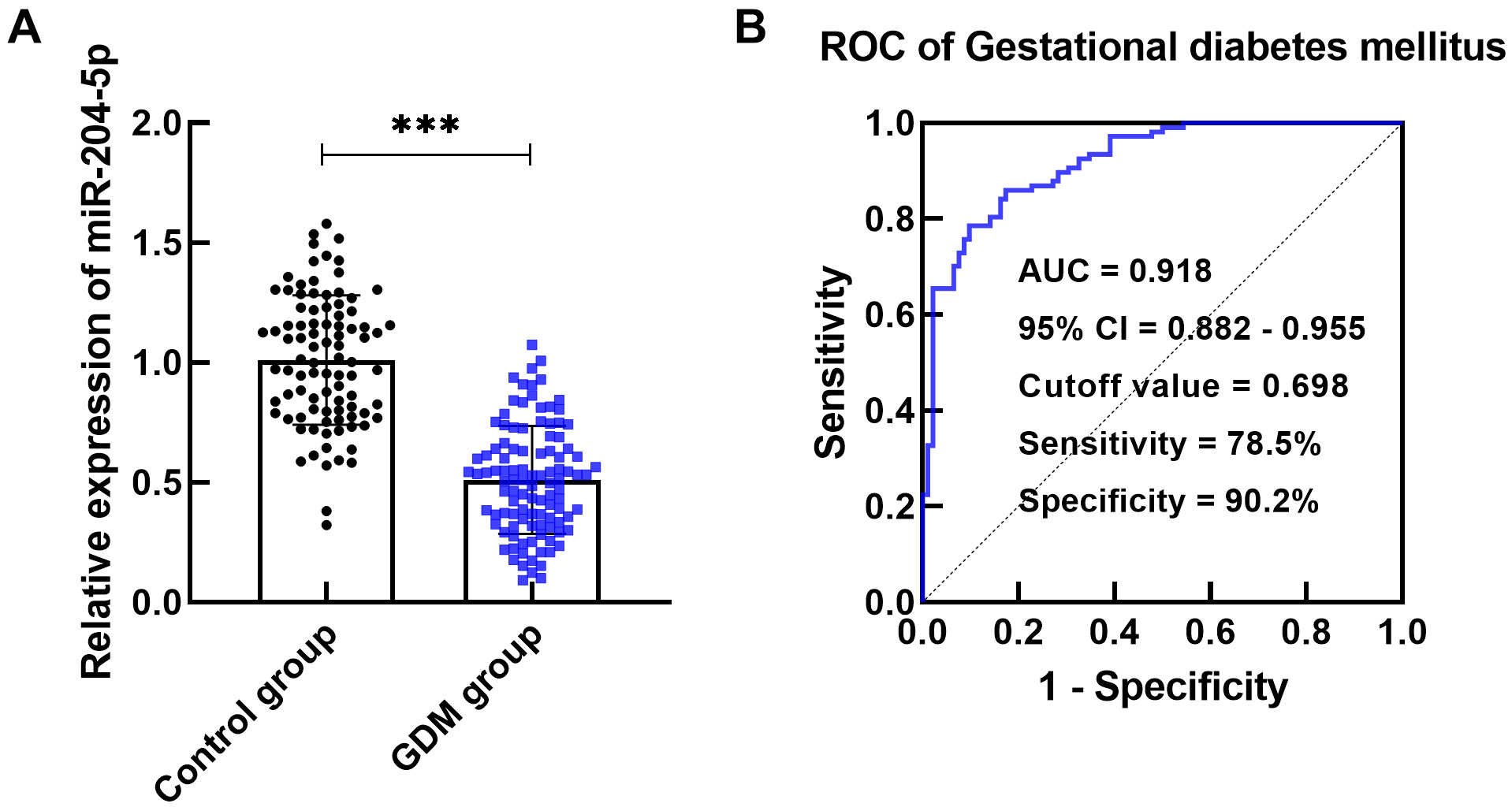 Fig. 1.
Fig. 1.
miR-204-5p expression in gestational diabetes mellitus (GDM)
patients. (A) miR-204-5p expression was decreased in the GDM group compared to
the control group (***p
Serum miR-204-5p expression was significantly reduced in the group of GDM
patients with poor outcomes compared to the good outcomes (p
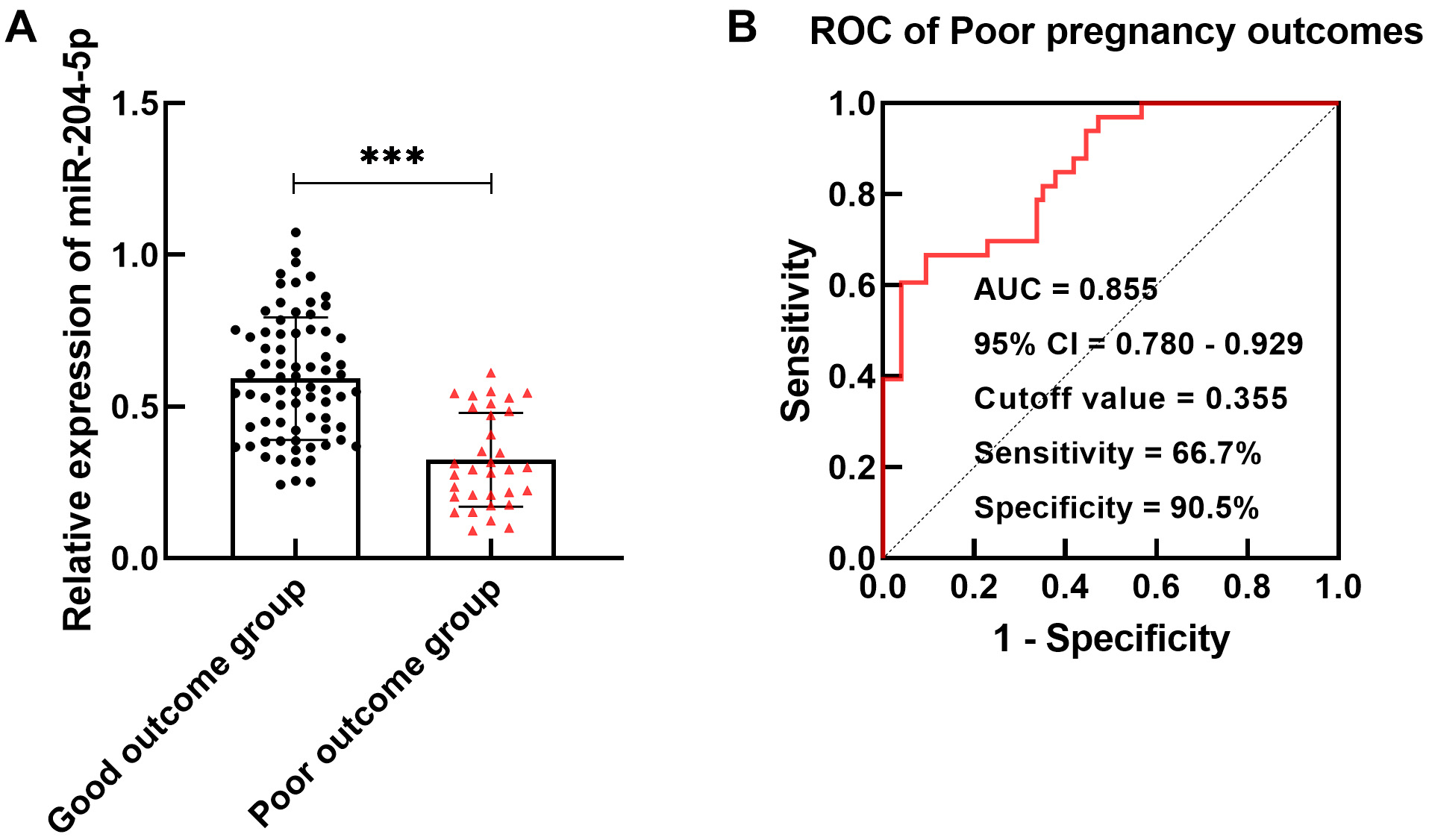 Fig. 2.
Fig. 2.
miR-204-5p in GDM patients with poor maternal and infant
outcomes. (A) miR-204-5p content was lower in the poor outcome group
(***p
The levels of FBG, 2 h PBG,
HbA1c, TG and TC were obviously greater in the poor outcome group than in the
good outcome group, and the difference between the two groups was statistically
significant (p
| Variables | OR | 95% CI | p |
| miR-204-5p ( |
0.151 | 0.043–0.526 | 0.003 |
| FBG ( |
0.171 | 0.051–0.573 | 0.004 |
| 2 h PBG ( |
0.199 | 0.061–0.648 | 0.007 |
| HbA1c ( |
0.179 | 0.051–0.628 | 0.007 |
| TG ( |
0.640 | 0.202–2.029 | 0.449 |
| TC ( |
0.427 | 0.140–1.297 | 0.133 |
| LDL-C ( |
0.672 | 0.219–2.062 | 0.487 |
| HDL-C ( |
2.185 | 0.673–7.091 | 0.193 |
Noted: FBG, fasting blood glucose; 2 h PBG, 2-hour postprandial blood glucose; HbA1c, glycated hemoglobin A1c; TG, triglycerides; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; OR, odd ratio.
A pregnant woman’s body demands higher glucose levels and exhibits a surge in the production of progesterone, which increases the risk of GDM if insulin resistance occurs due to insufficient insulin secretion [3, 17]. Moreover, an unhealthy lifestyle, lack of physical activity, and family genetics can further contribute to this risk of GDM. Therefore, the treatment of GDM should focus on the safety of both the mother and child and minimizing complications. Clinical intervention often includes reasonable diet rearrangements and the use of hypoglycemic drugs [18]. The relevant literature reports that regular prenatal examinations and blood glucose monitoring can effectively reduce the incidence of GDM and the rate of poor maternal and infant outcomes [19]. Therefore, exploring sensitive biomarkers for the occurrence of GDM and poor maternal and infant outcomes is of practical importance.
miRNAs in GDM patients function as metabolic and developmental regulators and may be involved in GDM pathogenesis [20]. For instance, Ye et al. [21] reported that abnormal levels of miRNAs mediate insulin expression, which may serve as a predictor of GDM. Juchnicka et al. [22] also suggested that miRNAs modulate the process of GDM by influencing protein formation and pathways. A study confirmed that miR-29b is poorly expressed in the placenta of GDM patients and is involved in the pathogenesis of GDM through the regulation of the biological functions of trophoblast cells [23]. Reduced levels of miR-96-5p were reported in the placental and plasma samples of GDM patients, indicating the value of this miRNA as a diagnostic factor [24]. The present study confirmed through PCR detection that miR-204-5p exhibited low expression in GDM patients and exhibited high sensitivity and specificity in the early identification of GDM patients. In the current clinical diagnosis of GDM, FBG, 2 h PBG, HbA1c, TG, and TC are often used as auxiliary detection indicators to screen GDM patients. Since physiological changes occur during pregnancy, those indicators may be expressed abnormally during pregnancy [25]. FBG, 2 h PBG, HbA1c, TG, and TC levels were elevated in GDM patients compared with healthy control pregnant women in this study. In addition, miR-204-5p expression was clearly correlated with the biochemical indices of GDM patients, suggesting that reduced miR-204-5p levels are a risk factor for GDM in pregnant women.
Statistical analysis of the maternal and infant outcomes of GDM patients revealed downregulation of miR-204-5p in the poor outcome group. Silencing miR-204-5p also had a certain predictive effect on poor maternal and infant outcomes. Evidence suggests that altered miR-204-5p expression may be associated with atypical fetal development and poor outcomes [26]. Moreover, miR-204 may mediate the development and treatment of preeclampsia by affecting trophoblast cell activity [27]. Accordingly, it was inferred that pregnant women with decreased miR-204-5p levels have a greater probability of developing GDM and poor maternal and infant outcomes.
However, this inference must be accepted only after considering certain limitations of the present study. The first limitation is that the study mainly discusses the clinical information of the included GDM patients without reporting any cellular level findings. However, the specific underlying molecular mechanisms have yet to be explored through further research. Additionally, due to considering the time and cost constraints, only a limited number of patients were included, which could limit the comprehensiveness of the findings.
In summary, miR-204-5p expression was decreased in GDM patients compared to healthy pregnant women, and miR-204-5p expression in GDM patients with poor maternal and infant outcomes was lower than that in GDM patients with normal pregnancies. Moreover, miR-204-5p is correlated with the early diagnosis of GDM and has high predictive value for poor maternal and infant outcomes in GDM, providing a novel marker for GDM patient treatment to mitigate the damage caused to maternal and infant health due to this condition.
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Conceptualization, YS and XD; data curation, YS and XD; formal analysis, YS and XD; funding acquisition, XD; investigation, YS; methodology, YS; project administration, XD; resources, YS; software, YS; supervision, XD; validation, YS; visualization, YS and XD; roles/writing — original draft, YS; writing — review & editing, XD. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was performed in line with the principles of the Declaration of Helsinki. All protocols of this investigation were approved by the Ethics Committee of Wuxi No.2 People’s Hospital (approval number 2022-127). The participants’ right to be informed about the study was ensured and written informed consent was obtained from all individual participants included in the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.ceog5110230.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


