1 School of Nursing and Public Health, University of KwaZulu-Natal, 4001 Durban, KwaZulu-Natal, South Africa
2 School of Health Systems and Public Health, University of Pretoria, 0002 Pretoria, Gauteng, South Africa
3 Clinical Department, Medical Centre Oshakati, 15001 Oshakati, Oshana, Namibia
4 Department of Obstetrics and Gynecology, University of Zimbabwe, 0002 Harare, Zimbabwe
Abstract
Maternal mortality remains a major challenge in sub-Saharan Africa (SSA), accounting for 70% of world's maternal deaths. Hemorrhage is the leading direct cause of maternal mortality worldwide, with postpartum hemorrhage (PPH) responsible for more than two-thirds of hemorrhage-related deaths. This systematic review and meta-analysis integrated data from studies conducted in SSA to provide an accurate estimation of the prevalence of PPH and to identify regional risk variables.
We conducted a search across multiple databases for peer-reviewed articles published between 2013 and 2023. This study included quantitative research employing cross-sectional, case-control, or cohort designs, regardless of sample size, and published in English. This review excluded literature reviews, meta-syntheses, qualitative studies, editorials, systematic reviews, and meta-analyses. We used MetaXL to estimate the pooled magnitude of PPH in SSA, and employed the Q test, I2 statistic, and funnel plots to assess statistical heterogeneity. Furthermore, we used MetaXL to perform subgroup and sensitivity analyses. Finally, we used IBM SPSS Statistics for Windows (Version 29.0.2.0 Armonk, NY, USA), to estimate the pooled effect size of the factors associated with PPH.
26 methodologically rigorous articles were included in this review and meta-analysis. The pooled magnitude of PPH in SSA was 8.6%. The studies exhibited significant heterogeneity. Individual factors associated with PPH include the woman's age (crude odds ratio [OR] = 4.37, 95% confidence interval [95% CI] = 3.03–6.29), place of residence (OR = 2.06, 95% CI = 1.51–2.82), and parity (OR = 3.13, 95% CI = 1.38–7.11). Antenatal factors include antenatal care (ANC) attendance (OR = 3.43, 95% CI = 1.12–10.05), antepartum hemorrhage (APH) (OR = 6.83, 95% CI = 3.64–12.80), and twin pregnancy (OR = 8.25, 95% CI = 3.80–17.92). Intrapartum factors include induction of labor (OR = 4.39, 95% CI = 2.01–9.61), and mode of delivery (OR = 2.61, 95% CI = 1.80–3.78). Postpartum factors include previous history of PPH (OR = 10.86, 95% CI = 3.71–31.84), and oxytocin use after delivery (OR = 0.17, 95% CI = 0.09–0.32).
The pooled magnitude of PPH in SSA in this study was considerably higher than that in other regions. Therefore, there is a need to strengthen strategies aimed at improving ANC attendance in SSA to ultimately reduce PPH. Additionally, close monitoring of women with risk factors for PPH and the careful use of oxytocin for labor induction are strategies that can also be used to reduce PPH in SSA.
Keywords
- postpartum hemorrhage
- magnitude
- risk factors
- sub-Saharan Africa
- systematic review
- meta-analysis
Maternal mortality remains a major public health challenge, especially in sub-Saharan Africa (SSA). In 2020, SSA accounted for 70% of global maternal deaths [1]. The region’s maternal mortality ratio (MMR) remains above 500 per 100,000 live births, with a lifetime risk of maternal death of 1 in 41, compared to 1 in 11,000 in Western Europe [2]. The causes of maternal mortality vary worldwide. One systematic review found that hemorrhage accounts for 27% of maternal deaths worldwide. The second most prevalent direct cause is hypertension (14%), followed by sepsis (10%), and abortion (7.9%). Embolism and other direct causes contribute to 13% of global maternal deaths [3]. While the specific causes of maternal death vary by region, hemorrhage remains the predominant factor in SSA. In a previous systematic review by Say et al. [3], postpartum hemorrhage (PPH) was identified as the cause of more than two-thirds of reported hemorrhage deaths. The prevalence of PPH varies among different populations. One systematic review by Feduniw et al. [4] reported that the prevalence of PPH in SSA is 3.8%, compared to 2.7% in Asia and 2% in the United States of America (USA).
Diagnosis of PPH is based on blood loss exceeding 500 mL after vaginal delivery or 1000 mL following a cesarean section within 24 hours. Additionally, PPH can be diagnosed based on blood loss causing hypovolemia, a 10% drop in hematocrit, or the need for blood transfusion, regardless of the delivery mode [5]. PPH can be categorized as primary, occurring within 24 hours of delivery, or secondary, occurring between 24 hours and six weeks postpartum [6]. Typical causes of PPH include uterine atony, retained placenta, cervical and vaginal lacerations, uterine rupture, uterine inversion, and coagulation disorders [7]. Although many cases of PPH lack identifiable risk factors, certain obstetric factors are associated with an increased risk, including grand multiparity, prolonged labor, inadequate antenatal care (ANC) attendance, and a history of prior PPH [8].
Women who experience PPH may suffer from both short-term and long-term health effects. Immediate effects of PPH include acute organ failure, hypovolemic shock, and maternal mortality. Due to hypovolemic shock, women with severe PPH are at increased risk of developing acute heart failure and myocardial ischemia [9]. Long-term effects may include persistent fatigue, psychological disorders such as posttraumatic stress disorder (PTSD), and an increased risk of cardiovascular disease later in life for women who require blood transfusions [10, 11].
The United Nations (UN) aims to reduce the global MMR to fewer than 70 per 100,000 live births by 2030 [12]. To achieve this goal in SSA, it is crucial to effectively address PPH, the leading cause of maternal death in the region. This systematic review and meta-analysis aimed to combine data from several studies conducted in SSA to accurately quantify the occurrence of PPH and estimate the risk factors associated with PPH in the region. Understanding the prevalence and risk factors of PPH in SSA is crucial for developing targeted interventions to improve maternal health outcomes.
This systematic review and meta-analysis were registered with the International Prospective Register of Systematic Reviews (PROSPERO) (Registration Number: CRD42024558301).
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) [13], the Centre for Reviews and Dissemination (CRD) guidelines for systematic reviews in healthcare [14], and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [15].
The research questions guiding this study were:
• What is the magnitude of PPH in SSA?
• What are the risk factors for PPH in SSA?
The study’s eligibility was established using the problem-interest-context (PICo) framework. PPH was identified as the problem, postpartum women as the interest, and SSA as the context.
Studies were included if they reported the prevalence of PPH in SSA, identified the risk factors for PPH in SSA along with corresponding risk estimates (odds ratios (OR) or relative risks), and were original quantitative studies of a cross-sectional, case-control, or cohort design, irrespective of sample size. The studies were required to be peer-reviewed, published in health-related journals in English, and dated between 1 January 2013 and 31 December 2023. We selected studies published during this period to ensure that the results are relatively recent and applicable to the current context. Excluded from this review were literature reviews, qualitative studies, editorials, systematic reviews, meta-analyses, meta-syntheses, and studies published before 2013.
The outcome variable in this study was PPH. Visual estimation by a qualified health provider is the most common way to diagnose PPH and quantify blood loss. However, this method has limitations, as it can underestimate blood loss, particularly at higher volumes [16]. More objective methods are available, such as gravimetric blood collection using a plastic collector bag or tray, which measures all expelled blood during the third stage of labor. Once bleeding ceases, the vessel and its contents can be weighed, or if the plastic bag is calibrated, the blood loss can be measured directly [17]. While advanced methods like hemoglobin concentration in venous blood sampling, spectrophotometry, dye dilution techniques, contrast-enhanced ultrasound, hematocrit levels, postnatal packed cell volume levels, and nuclear medicine imaging exist, they are less readily available in routine clinical practice [18]. In this study, we included all studies that measured the prevalence of PPH, irrespective of the method used to measure the blood loss.
A comprehensive electronic search was conducted across several databases and websites for peer-reviewed articles published between 1 January 2013, and 31 December 2023. These databases included Google Scholar, EMBASE, ScienceDirect, CINAHL, MEDLINE, Africa Journals Online (AJOL), SCOPUS, and PubMed. Grey literature was also searched from websites of organizations such as the United Nations International Children’s Emergency Fund (UNICEF), the World Health Organization (WHO), and the health ministries of countries in SSA. All databases and websites were searched from 1 to 10 June 2024. The search strategy for PubMed is detailed in Supplementary Table 1.
Keywords and search terms used to retrieve the relevant articles included ‘PPH’, ‘determinants’, ‘sub-Saharan Africa’, ‘risk factors’, ‘postpartum women’, ‘prevalence’, ‘incidence’, ‘magnitude’, ‘correlations’, and all countries in SSA. Search terms were combined using Boolean operators.
All articles with relevant titles were transferred to Covidence (https://www.covidence.org/), a systematic review tool.
Following duplicate removal, two independent reviewers (EM and PM) screened the abstracts of the remaining articles. Full texts of eligible articles were then retrieved and reviewed by two reviewers (EM and GM). Any disagreements were resolved through discussion until consensus was reached. In cases where consensus remained elusive, a third reviewer (TD) moderated the discussion to achieve a final decision. Additionally, the lead reviewer (EM) performed a reference list search of all included articles to identify potentially relevant studies that were not captured in the initial search.
The Newcastle-Ottawa quality assessment scale [19] was used to evaluate the methodological quality of the included studies. This scale assesses three key domains: the selection of study participants, the comparability of groups (for case-control or cohort studies), and the quality of outcome data and statistical analysis reported in the original articles. For case-control studies and cohort studies, the quality of comparability can receive a maximum of two stars, the quality of outcomes can receive a maximum of three stars, and the quality of outcomes and statistical analysis can receive a maximum of four stars. For cross-sectional studies, the maximum ratings are one star for comparability, three stars for outcomes, and five stars for participant selection. Only studies classified as low-risk, with a rating of seven or more stars, were included in the meta-analysis. Two reviewers (EM and TD) independently evaluated and compared the included studies. Any discrepancies were resolved through discussion with two additional reviewers (PM and GM) until a consensus was reached.
The reviewers developed and pilot-tested a data extraction form. Two reviewers (EM and GM) extracted data from the included articles and subsequently compared their findings. The following information was extracted from each study: the first author, the year of publication, the country where the study was conducted, the study design, the method used for diagnosing PPH, the sample size, the sampling method, the number of women who experienced PPH, the risk factors for PPH, and the frequency of PPH.
Data were exported to MetaXL for analysis to quantify the pooled prevalence of PPH in SSA. A random-effects meta-analysis model was employed to account for potential heterogeneity between studies. The Q test and I2 statistic were used to determine the statistical heterogeneity of the studies. I2 cut-off points of 25%, 50%, and 75% were used to classify heterogeneity as low, medium, and high, respectively [20]. Subgroup analyses were conducted to explore potential variations in the prevalence of PPH based on the year of publication, sample size, and the method used for diagnosing PPH. Publication bias was evaluated using funnel plots. Sensitivity analysis was performed to determine the impact of any single study on the overall prevalence of PPH. Additionally, data were exported to IBM SPSS Statistics for Windows (Version 29.0.2.0 Armonk, NY, USA), to determine both the pooled effect size of factors associated with PPH and the effect size of each factor within each study. The pooled effect sizes were estimated using ORs. Only risk factor variables that were statistically significant in at least two of the included studies were included in the meta-analysis. Non-dichotomous risk factor variables were reclassified as dichotomous variables before calculating ORs. Forest plots were used to visually display pooled estimates along with their 95% confidence intervals (95% CIs). A p-value of less than 0.05 was considered statistically significant for all statistical tests.
We retrieved 1240 articles from all the databases and websites searched. After removing duplicates (n = 395), 845 articles remained. Of these, 815 were excluded due to being published before 2013, being qualitative studies, or being systematic reviews and meta-analyses. Following the eligibility assessment of the remaining 30 articles, 4 were excluded, as illustrated in Fig. 1. 2 excluded articles [21, 22] focused solely on the detection and management of PPH, 1 [23] addressed PPH mortality, and 1 [24] lacked data on the prevalence of PPH or risk estimates.
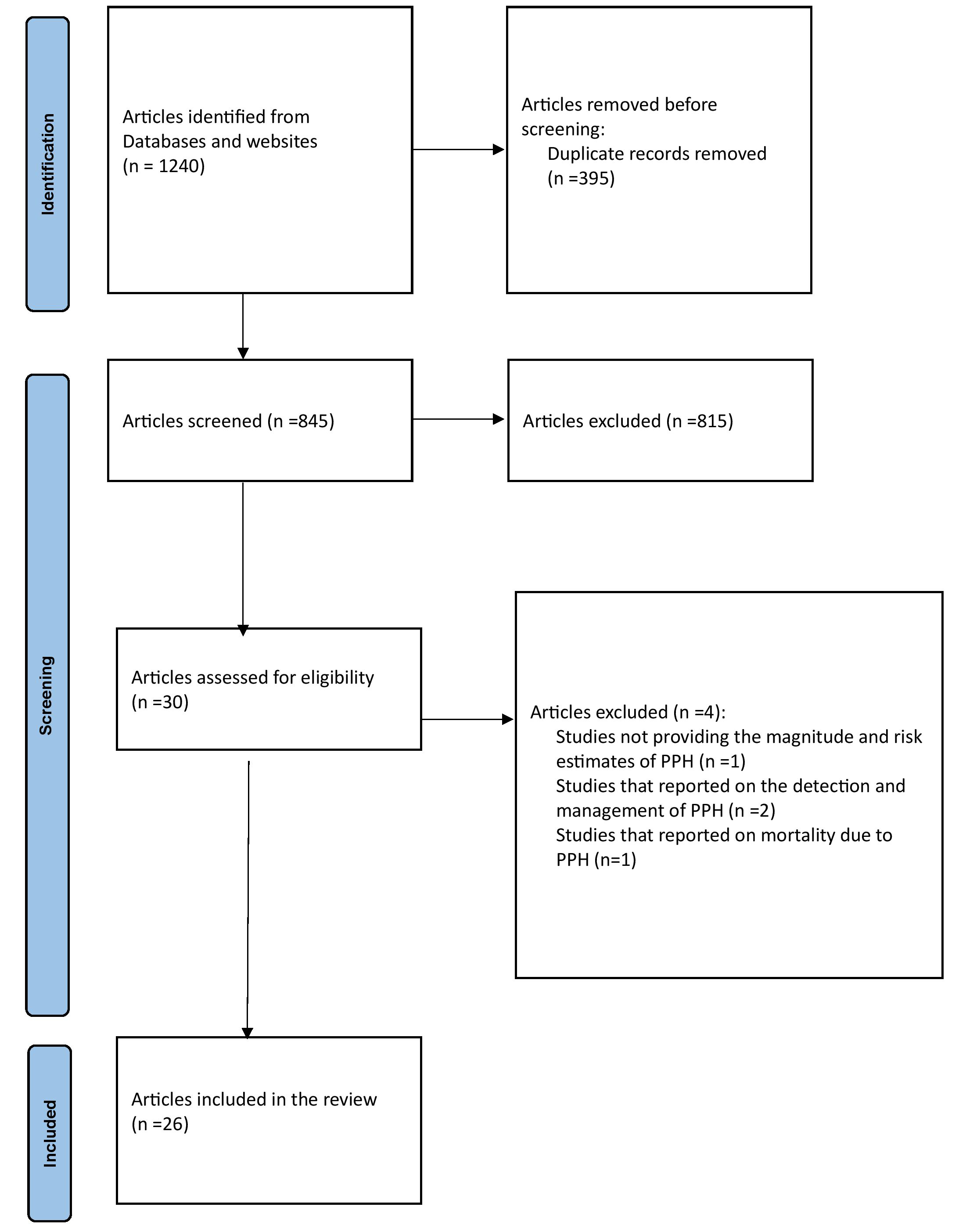 Fig. 1.
Fig. 1.
PRISMA-P flowchart. PPH, postpartum hemorrhage; PRISMA-P, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols.
The reviewers agreed that all the studies included were of high quality, as each received seven stars or more. However, 5 of the studies [25, 26, 27, 28, 29] did not receive a star in the comparability domain due to the absence of multivariate analysis or insufficient explanation of how confounding bias was addressed. Additional details are presented in Supplementary Table 2.
Of the 26 studies that were included in this systematic review and meta-analysis, 10 were conducted in Ethiopia [30, 31, 32, 33, 34, 35, 36, 37, 38, 39], 4 in Nigeria [25, 26, 27, 40], 3 in Cameroon [41, 42, 43], 2 in Mozambique [44, 45], and the remaining in Rwanda [46], Gabon [47], Zimbabwe [48], South Sudan [49], Ghana [28], Kenya [29], and Uganda [50]. Of the included studies, 10 [25, 29, 30, 31, 32, 33, 38, 39, 41, 43] employed a cross-sectional study design, 6 [34, 37, 40, 42, 46, 49] utilized a case-control design, 5 [35, 36, 45, 48, 50] used a cohort design, 4 [26, 27, 44, 47] conducted a retrospective review, and 1 [28] implemented a quasi-experimental interventional study design. Of the included studies, 10 [25, 26, 27, 29, 34, 40, 45, 47, 48, 50] used all eligible participants, 5 studies [32, 33, 35, 38, 49] used a systematic random sampling method, 4 [36, 37, 39, 41] used consecutive sampling, 3 [28, 30, 31] used simple random sampling method, 2 [43, 44] used convenience sampling, and 2 [42, 46] used all cases of PPH but selected controls using a random sampling method. The included studies’ sample sizes ranged from 132 [49] to 42,728 [47]. The reported prevalence of PPH ranged from 1.33% [42] to 33.3% [37, 49]. For further details, refer to Table 1 (Ref. [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50]).
| First author, publication year | Reference | Country where study was conducted | Study design | Sampling method | Method used for diagnosing PPH | Sample size | Number of PPH cases | Prevalence of PPH (%) |
| Green K., 2015 | [25] | Nigeria | Cross-sectional | All deliveries during study period included | Not specified | 3694 | 178 | 4.28 |
| Allagoa DO., 2021 | [26] | Nigeria | Retrospective review | All deliveries during study period included | Not specified | 4571 | 66 | 1.4 |
| Onyema OA., 2015 | [27] | Nigeria | Retrospective review | All deliveries during study period included | The need for blood transfusion | 4889 | 166 | 3.4 |
| Valdes V., 2018 | [28] | Ghana | Quasi-experimental interventional study | Simple random | Self-reported by the postpartum women | 2136 | 95 | 4.4 |
| Clarke-Deelder E., 2023 | [29] | Kenya | Cross-sectional | All eligible women during study | VEBL | 907 | 75 | 8.3 |
| Amanuel T., 2021 | [30] | Ethiopia | Cross-sectional | Simple random | Not specified | 298 | 28 | 9.4 |
| Mesfin S., 2021 | [31] | Ethiopia | Cross-sectional | Simple random | Clinical signs and symptoms and a 10% decline in hematocrit level from baseline | 642 | 83 | 12.9 |
| Habitamu D., 2019 | [32] | Ethiopia | Cross-sectional | Systematic random | Clinical signs and symptoms | 144 | 11 | 7.6 |
| Zenebe GA., 2023 | [33] | Ethiopia | Cross-sectional | Systematic random | VEBL | 577 | 24 | 4.2 |
| Gebretsadik A., 2021 | [34] | Ethiopia | Case-control | All deliveries during study period included | Not specified | 8506 | 221 | 2.6 |
| Tiruneh B., 2022 | [35] | Ethiopia | Retrospective cohort | Systematic random | VEBL | 1060 | 94 | 8.9 |
| Zewdu D., 2023 | [36] | Ethiopia | Retrospective cohort | Consecutive | Hemoglobin difference of |
728 | 25 | 3.4 |
| Muluye G., 2023 | [37] | Ethiopia | Case-control | Consecutive | Not specified | 318 | 106 | 33.3 |
| Dagne AH., 2022 | [38] | Ethiopia | Cross-sectional | Systematic random | VEBL | 493 | 67 | 13.6 |
| Kebede BA., 2019 | [39] | Ethiopia | Cross-sectional | Consecutive | Clinical signs and symptoms | 422 | 70 | 16.6 |
| Lamina MA., 2015 | [40] | Nigeria | Retrospective case-control study | All deliveries during study period included | Not specified | 5929 | 272 | 25.6 |
| Takang W., 2022 | [41] | Cameroon | Cross-sectional | Consecutive non-probability | VEBL and direct collection of blood | 197 | 14 | 7.1 |
| Nana TN., 2021 | [42] | Cameroon | Descriptive and case-control | Random selection of controls | Clinical signs and symptoms | 12,240 | 163 | 1.3 |
| Halle-Ekane, G.E., 2016 | [43] | Cameroon | Cross-sectional | Convenience sampling | VEBL and direct collection of blood | 550 | 130 | 23.6 |
| Lancaster L., 2020 | [44] | Mozambique | Retrospective review | Convenience sampling | Clinical signs and symptoms, if an intervention for hemorrhage was performed, a diagnosis of placenta abruptio, and the need for transfusion | 495 | 59 | 12.0 |
| Glenzer MM., 2023 | [45] | Mozambique | Prospective cohort | All deliveries during study period included | Clinical signs and symptoms, if an intervention for hemorrhage was performed, a diagnosis of placenta abruptio, and the need for transfusion | 8799 | 1276 | 14.5 |
| Bazirete O., 2022 | [46] | Rwanda | Observational case-control study | Simple random sampling for controls with all cases selected | VEBL and clinical signs and symptoms | 430 | 108 | 25.1 |
| Ambounda NL., 2021 | [47] | Gabon | Analytical retrospective study | All deliveries during study period included | VEBL, clinical signs and symptoms, the need for transfusion, and hemodynamic decompensation | 42,728 | 671 | 1.6 |
| Ngwenya S., 2016 | [48] | Zimbabwe | Retrospective descriptive cohort | All deliveries during study period included | Not specified | 4567 | 74 | 1.6 |
| Ujjiga T., 2014 | [49] | South Sudan | Case-control | Systematic random for both cases and controls | Not specified | 132 | 44 | 33.3 |
| Ononge, S., 2016 | [50] | Uganda | Prospective cohort | All eligible women during study | Direct collection of blood | 1188 | 107 | 9.0 |
| TOTAL | 106,640 | 4227 | 8.6 (Weighted average) |
PPH, postpartum hemorrhage; VEBL, visual estimation of blood loss.
To determine the prevalence of PPH, a total of 106,640 participants were
included. Among all the participants, 4227 experienced PPH. The pooled prevalence
of PPH in SSA was 8.6% (95% CI = 6.4–11.0%), I2 = 99%,
p
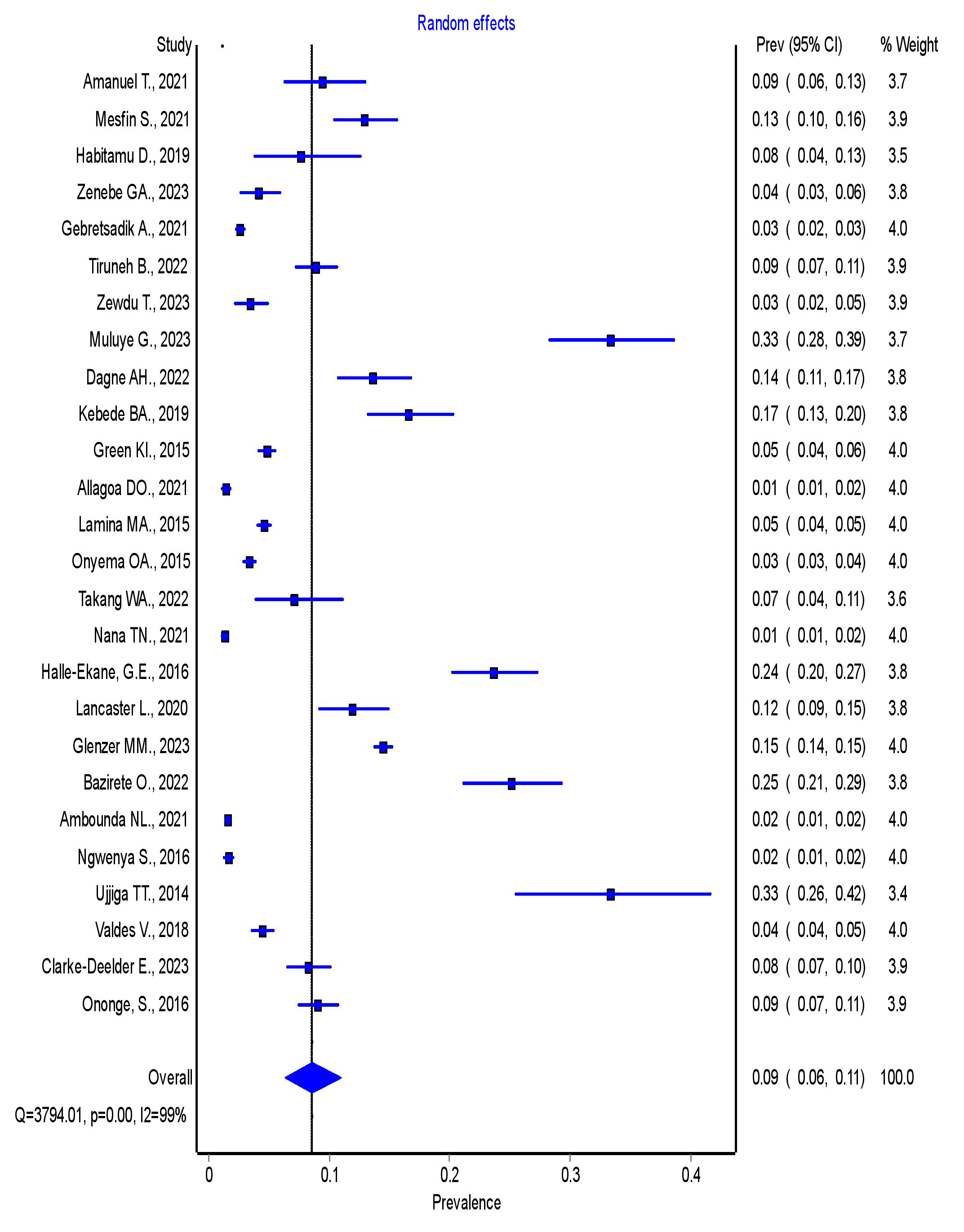 Fig. 2.
Fig. 2.
Forest plot showing the overall pooled prevalence of PPH in SSA. PPH, postpartum hemorrhage; SSA, sub-Saharan Africa; 95% CI, 95% confidence interval.
To determine the sources of heterogeneity in the pooled prevalence of PPH, we performed subgroup analyses based on publication year, sample size, and the specification of the diagnostic method used for PPH.
3.4.2.1 Subgroup Analysis by Publication Year
The subgroup analysis by publication year revealed that the pooled prevalence of PPH was 9% (95% CI = 5–13%) for studies published in 2013–2017, and 8% (95% CI = 6–12%) for studies published in 2018–2023. There was no statistically significant difference in the pooled prevalence of PPH between studies published from 2013–2017 and those published from 2018–2023. Further details are presented in Fig. 3.
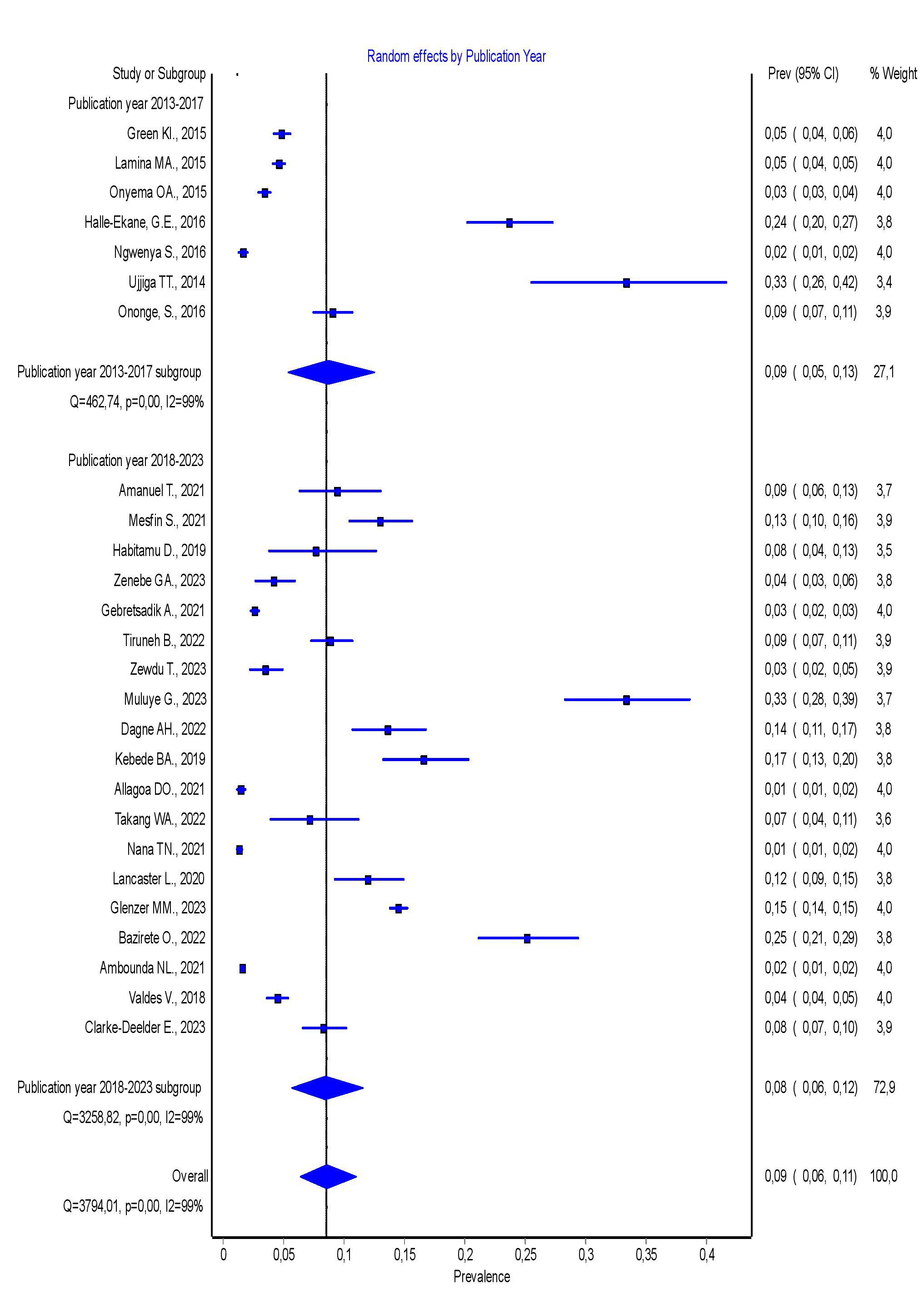 Fig. 3.
Fig. 3.
Forest plot showing the pooled prevalence of PPH in SSA by publication year. PPH, postpartum hemorrhage; SSA, sub-Saharan Africa; 95% CI, 95% confidence interval.
3.4.2.2 Subgroup Analysis by Sample Size
The subgroup analysis by sample size revealed that the pooled prevalence of PPH was 14% (95% CI = 9–19%) for studies with a sample size of less than 1000, and 4% (95% CI = 2–6%) for studies with a sample size greater than 1000. These results show that studies with a sample size greater than 1000 had a statistically significantly lower pooled magnitude of PPH compared to studies with a sample size which was less than 1000. Additional details are presented in Fig. 4.
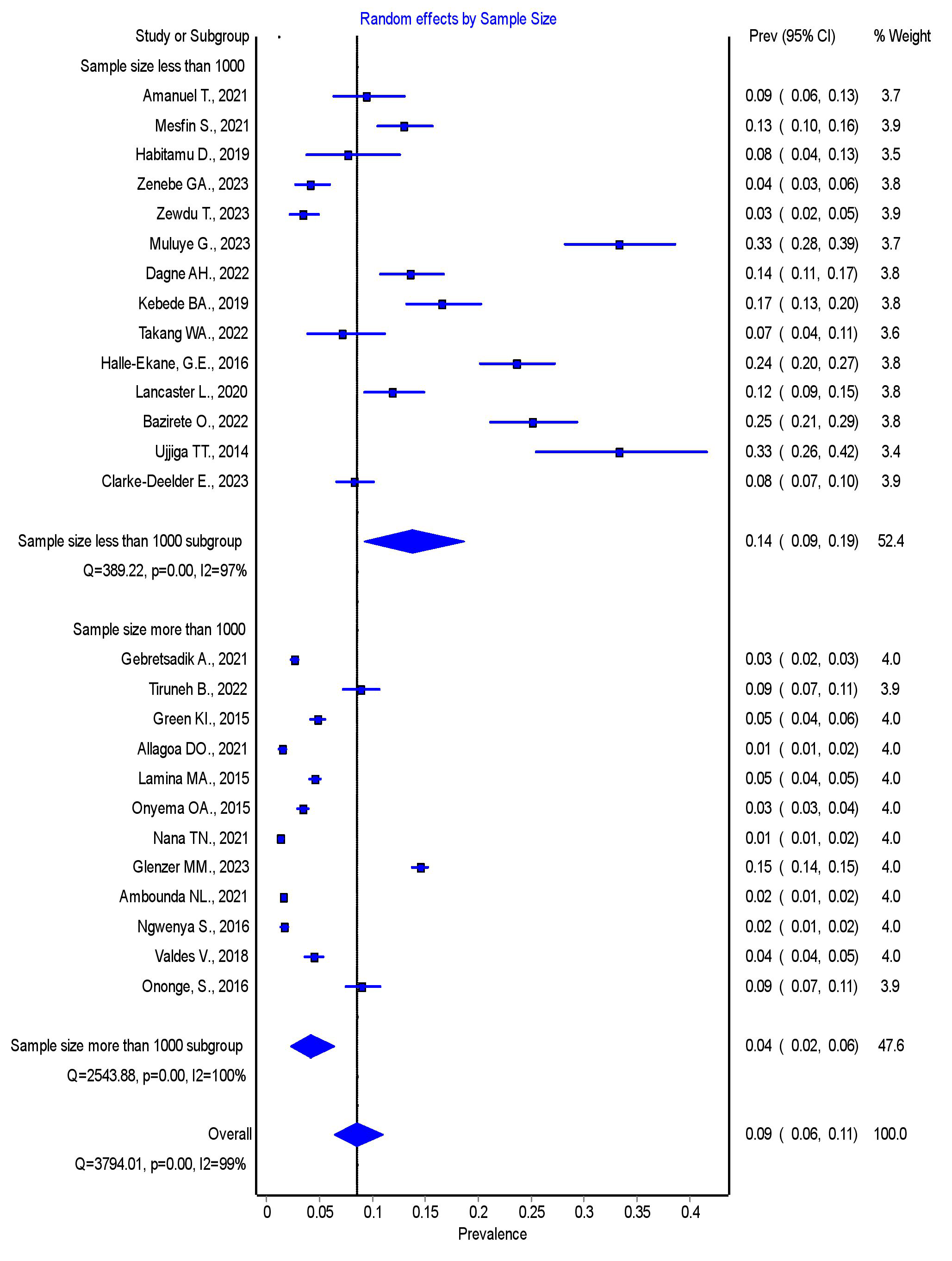 Fig. 4.
Fig. 4.
Forest plot showing the pooled prevalence of PPH in SSA by sample size. PPH, postpartum hemorrhage; SSA, sub-Saharan Africa; 95% CI, 95% confidence interval.
3.4.2.3 Subgroup Analysis by Specification of Method Used for Diagnosing PPH
The subgroup analysis by the method used for diagnosing PPH revealed that the pooled prevalence was 8% (95% CI = 5–11%) for studies with an unspecified diagnostic method, and 9% (95% CI = 6–12%) for studies with a specified method. There was no statistically significant difference in the pooled prevalence of PPH between studies with an unspecified diagnostic method and those with a specified method. Further details are presented in Fig. 5.
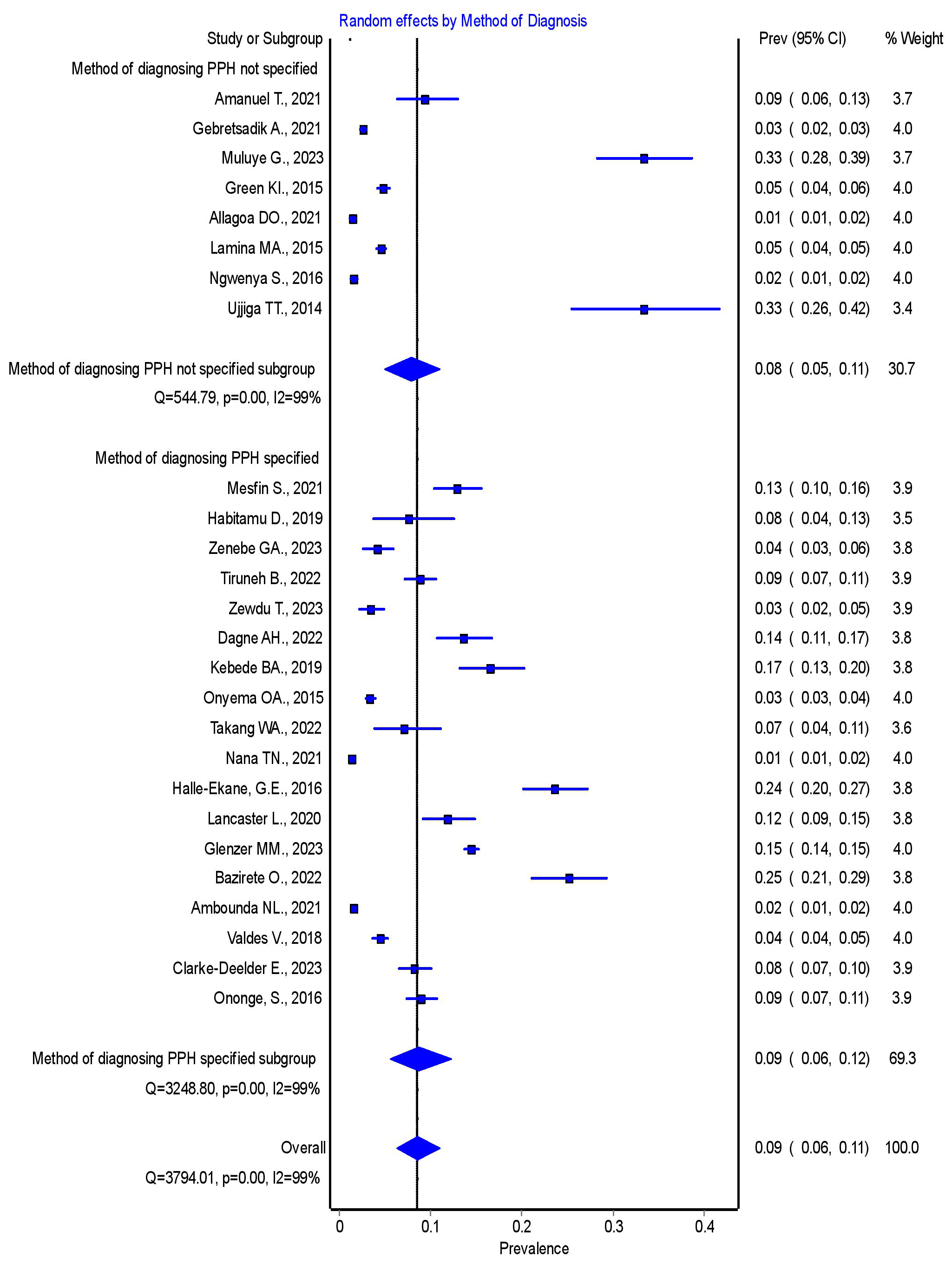 Fig. 5.
Fig. 5.
Forest plot showing the pooled prevalence of PPH in SSA by the specification of the diagnostic method used. PPH, postpartum hemorrhage; SSA, sub-Saharan Africa; 95% CI, 95% confidence interval.
The funnel plot had a distribution that was not symmetrical, which indicates a publication bias. The funnel plot is presented in Fig. 6.
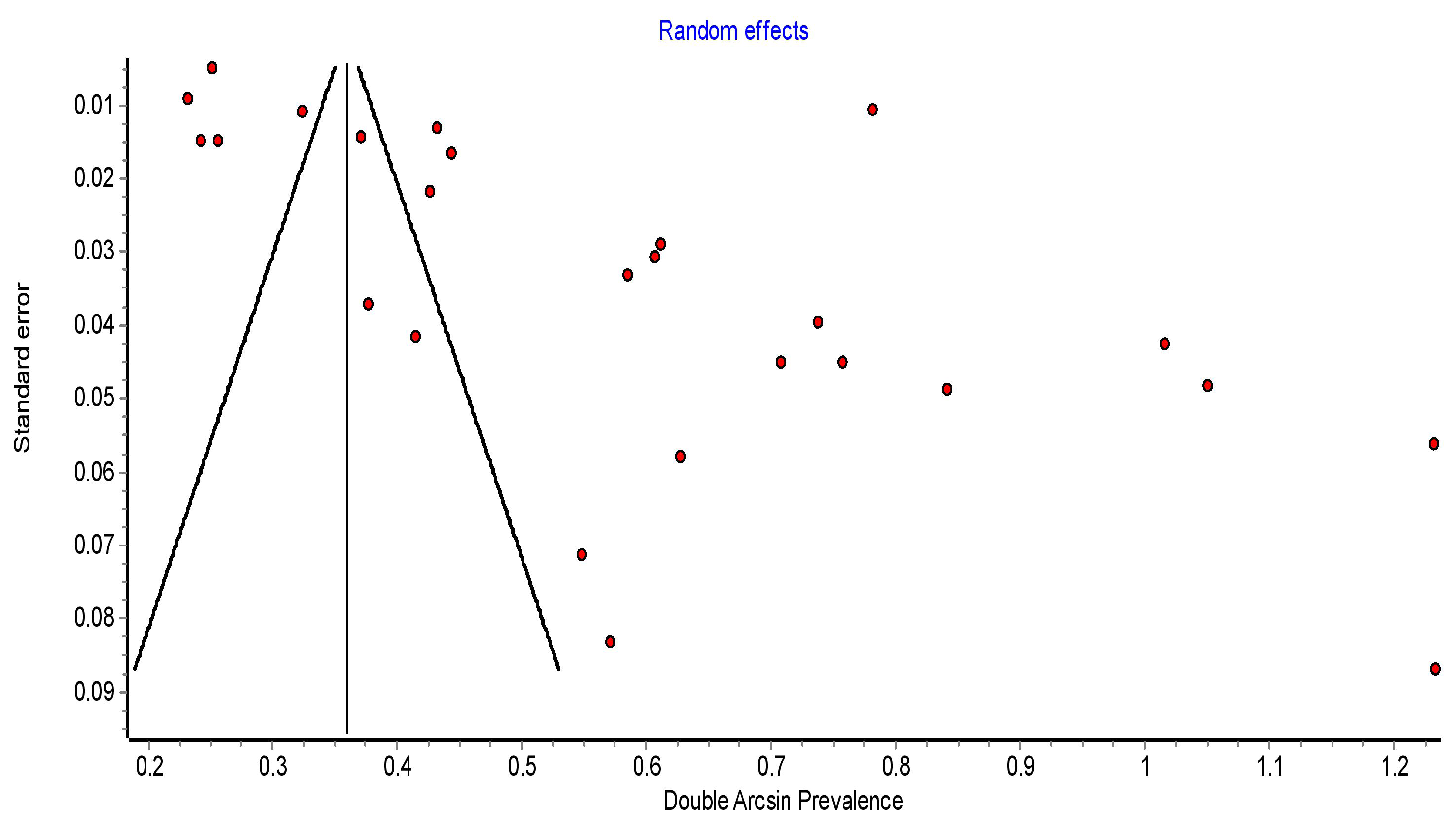 Fig. 6.
Fig. 6.
Funnel plot showing symmetrical distribution of articles on PPH in SSA. PPH, postpartum hemorrhage; SSA, sub-Saharan Africa.
We conducted a sensitivity analysis by systematically excluding one study at a time to estimate the effect of each individual study on the overall pooled prevalence of PPH in SSA. The findings of sensitivity analysis indicated that no single study had a significant effect on the overall pooled prevalence of PPH in SSA. Further details are provided in Supplementary Table 3.
This systematic review and meta-analysis revealed several factors associated with PPH. These factors were categorized into individual, antepartum, intrapartum, and postpartum factors. The individual factors identified include the woman’s age [30, 31, 32, 34, 35, 36, 38, 39, 46], place of residence [31, 34], parity [32, 34, 40, 43], gravidity [31, 32, 43], and history of abortion [35, 43]. Antenatal factors include ANC attendance [30, 31, 32, 34, 35, 36, 37, 38], antepartum hemorrhage (APH) [33, 34, 35, 36, 46], twin pregnancy [33, 46], and anemia [39, 46]. Intrapartum factors identified include induction of labor [35, 37, 38, 41], mode of delivery [30, 35, 37, 39], and the occurrence of stillbirth or intrauterine fetal death (IUFD) [35, 36, 46]. Postpartum factors associated with PPH include previous history of PPH [30, 32, 34, 39, 43], history of uterine atony [30, 33], and the use of oxytocin after delivery [37, 49]. More details on these associations are presented in Table 2 (Ref. [30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 43, 46, 49]) and Supplementary Tables 4,5.
| Variables | Number of studies | References of included studies | OR (95% CI) | Heterogeneity of studies | Homogeneity test |
| I2 (%) | p-value | ||||
| Age (years) | 9 | [30, 31, 32, 34, 35, 36, 38, 39, 46] | 4.37 (3.03–6.29) | 64.3 | p |
| ANC attendance | 7 | [30, 32, 34, 35, 36, 37, 38] | 3.43 (1.12–10.5) | 94.1 | p |
| Previous history of PPH | 5 | [30, 32, 34, 39, 43] | 10.86 (3.71–31.84) | 79.9 | p |
| Mode of delivery | 4 | [30, 35, 37, 39] | 2.61 (1.80–3.78) | 38.6 | p = 0.18 |
| Residence | 2 | [31, 34] | 2.06 (1.51–2.82) | 0.0 | p = 0.33 |
| History of uterine atony | 2 | [30, 33] | 11.10 (3.00–41.06) | 71.5 | p = 0.06 |
| Gravidity | 2 | [31, 43] | 4.46 (2.44–8.14) | 16.6 | p = 0.27 |
| Parity | 2 | [34, 40] | 3.13 (1.38–7.11) | 88.9 | p |
| APH | 5 | [33, 34, 35, 36, 46] | 6.83 (3.64–12.80) | 63.9 | p |
| Twin pregnancy | 2 | [33, 46] | 8.25 (3.80–17.92) | 0.0 | p = 0.62 |
| Anemia during pregnancy | 2 | [39, 46] | 5.60 (3.71–8.47) | 0.0 | p = 0.42 |
| Stillbirth or IUFD | 3 | [35, 36, 46] | 4.39 (2.01–9.61) | 62 | p = 0.06 |
| Oxytocin use after delivery | 2 | [37, 49] | 0.17 (0.09–0.32) | 0.0 | p = 0.95 |
| Induction of labor | 4 | [35, 37, 38, 41] | 6.76 (1.56–29.29) | 94.1 | p |
| History of abortion | 2 | [35, 43] | 3.88 (1.65–9.14) | 65.6 | p = 0.09 |
PPH, postpartum hemorrhage; SSA, sub-Saharan Africa; ANC, antenatal care; OR, odds ratio; 95% CI, 95% confidence interval; APH, antepartum hemorrhage; IUFD, intrauterine fetal death.
This systematic review and meta-analysis found a statistically significant
association between the woman’s age and PPH across 9 studies
[30, 31, 32, 34, 35, 36, 38, 39, 46]. A total of 4750 participants were included in the study
to determine the association between women’s age and PPH (Supplementary
Table 4). The pooled OR revealed that women aged 35 years and older were
approximately four times more likely to experience PPH compared to those younger
than 35 years of age, with a crude OR of 4.37 (95% CI = 3.03–6.29),
I2 = 64.3%, p
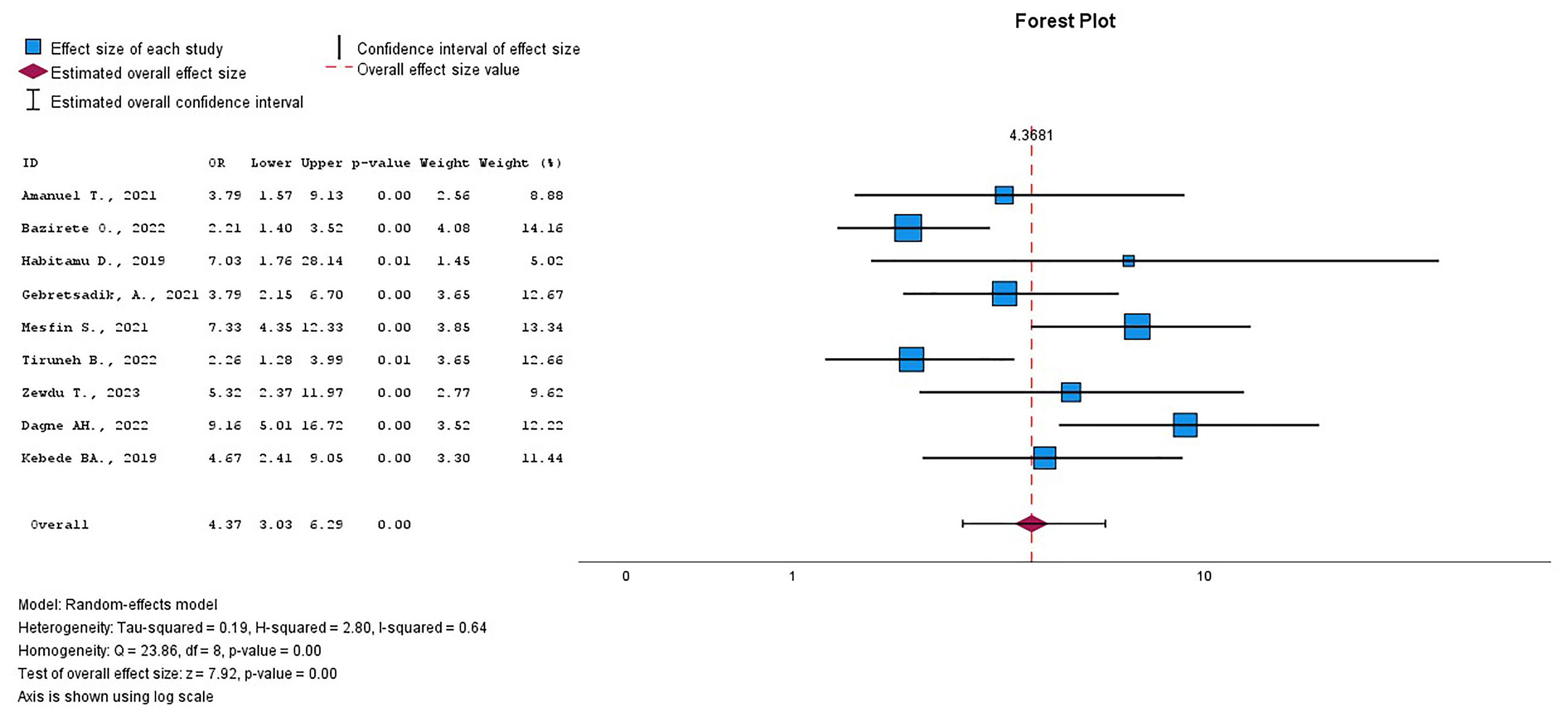 Fig. 7.
Fig. 7.
The pooled OR of the association between age and PPH. OR, odds ratio; PPH, postpartum hemorrhage.
This systematic review and meta-analysis revealed that ANC attendance was
statistically significantly associated with PPH among the 7 included studies
[30, 32, 34, 35, 36, 37, 38]. This study included 4289 women to investigate the relationship
between ANC attendance and PPH (Supplementary Table 4). Women who did
not attend ANC had a threefold higher risk of PPH compared to those who did, with
an OR of 3.43 (95% CI = 1.12–10.05), I2 = 94.1%, p
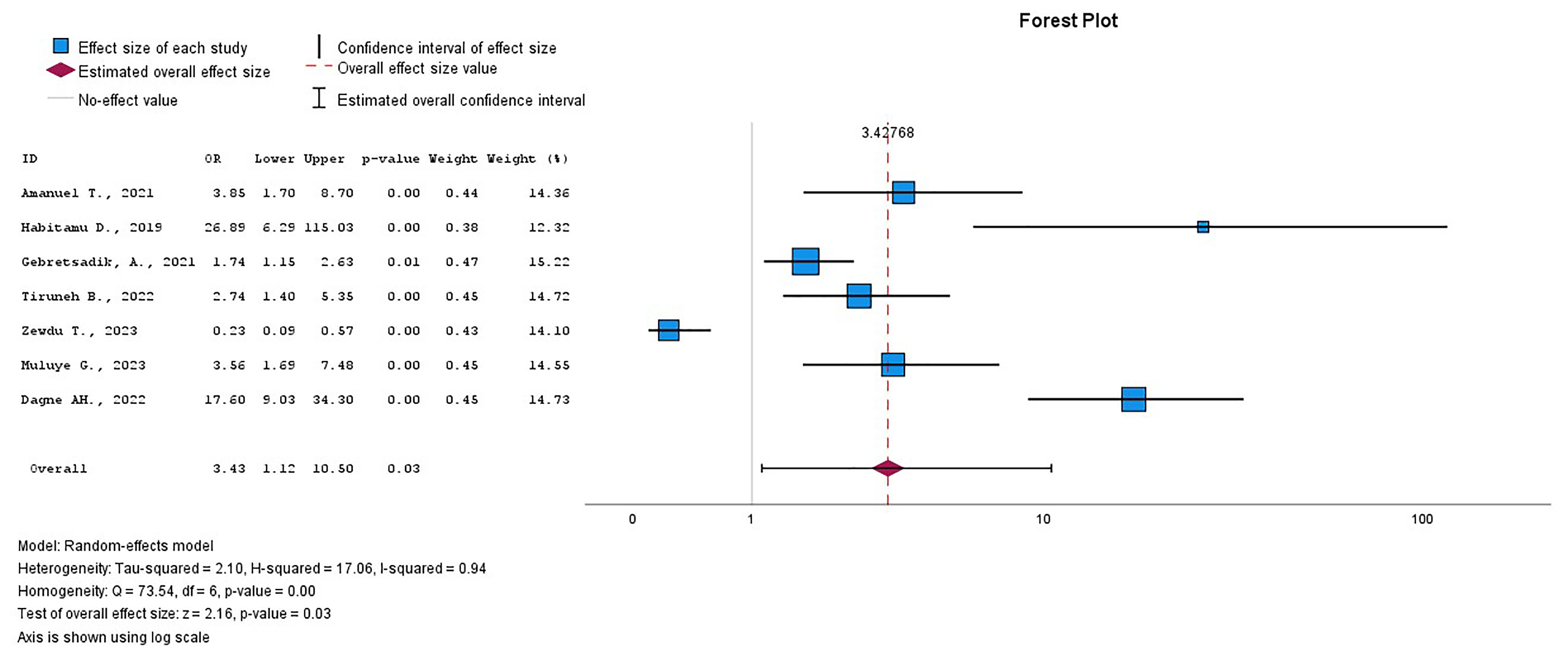 Fig. 8.
Fig. 8.
The pooled OR of the association between ANC attendance and PPH. OR, odds ratio; ANC, antenatal care; PPH, postpartum hemorrhage.
This review found an association between the mother’s delivery mode and PPH. PPH
was three times more common in women who had a cesarean section or instrumental
birth, with an OR of 2.61 (95% CI = 1.80–3.78), I2 = 38.6%,
p = 0.18. Having a stillbirth/ IUFD or induction of labor was
statistically significantly associated with PPH. Women who experienced
stillbirth/IUFD or labor induction had a four- or seven-fold higher risk of PPH,
with an OR of 4.39 (95% CI = 2.01–9.61), I2 = 62%,
p = 0.06, and with an OR of 6.76 (95% CI = 1.56–29.29),
I2 = 94.1%, p
Previous history of PPH and uterine atony during the current delivery were
statistically significantly associated with PPH. Women who had a previous history
of PPH or uterine atony after the current delivery both had about an eleven times
higher likelihood of experiencing PPH compared to those who did not have either,
with an OR of 10.86 (95% CI = 3.71–31.84), I2 = 79.9%,
p
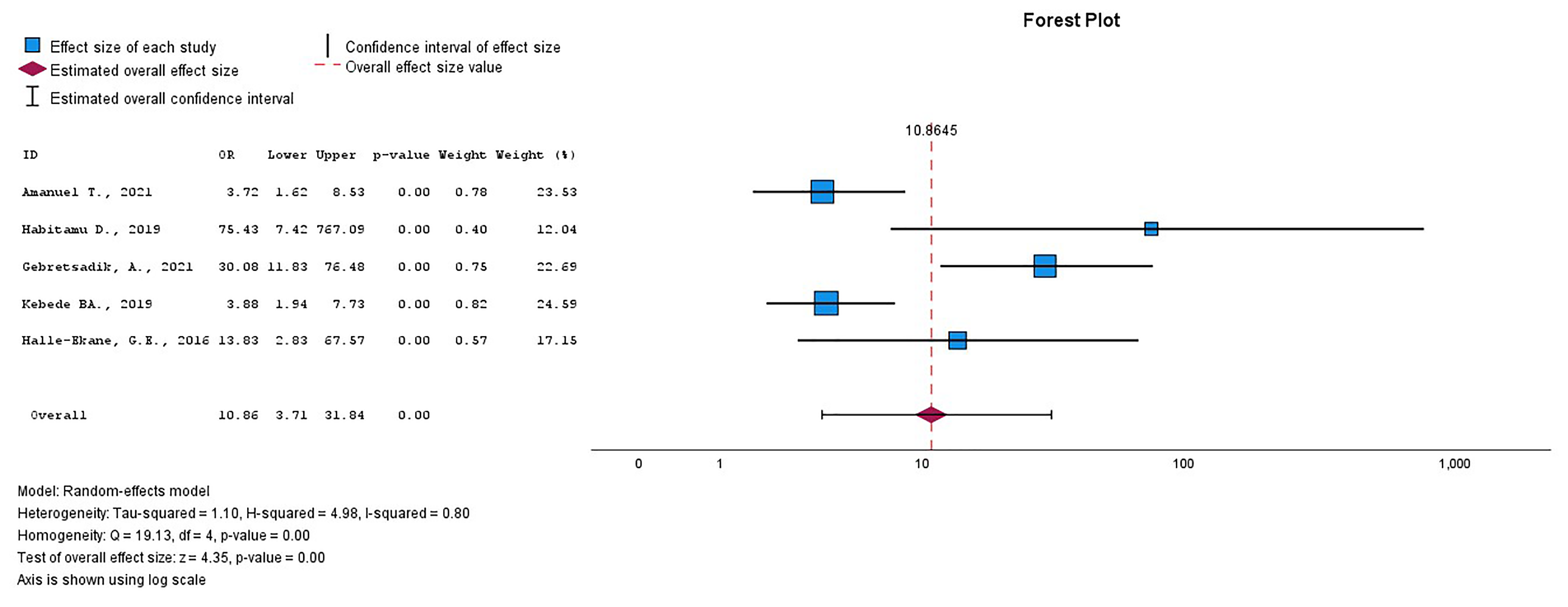 Fig. 9.
Fig. 9.
The pooled OR of the association between previous history of PPH and the current PPH. OR, odds ratio; PPH, postpartum hemorrhage.
PPH is one of the leading causes of maternal deaths worldwide. Although its prevalence varies across different regions, SSA experiences the highest magnitude [3, 4]. Reducing the incidence of PPH will consequently result in a reduction in maternal deaths. However, to effectively reduce the incidence of PPH, targeted interventions based on current knowledge of the issue should be developed.
According to this systematic review and meta-analysis, the pooled incidence of PPH in SSA was 8.6%. This result is comparable to that reported in a previous systematic review and meta-analysis conducted for Ethiopia, which found an incidence of 8.4 [8]. This similarity may be attributed to Ethiopia having a larger number of studies included in the current review. The high prevalence observed in our study might be due to the inclusion a greater number of studies from referral hospitals, which often handle more complex cases and, therefore, report higher rates of PPH compared to lower-level facilities. Additionally, the utilization of postnatal care by women in SSA may be influenced by a variety of factors, including sociodemographic characteristics and aspects of the health system, such as accessibility to healthcare facilities and the quality of care provided [51]. The magnitude of PPH in this review was also higher than that reported in other studies, which found rates of 2% in the USA [4], and 8% in Asia [52]. The differences in reported rates may be attributed to the varied healthcare systems in which the studies were conducted. A shortage of skilled birth attendants, inadequate access to medications for controlling bleeding, and insufficient equipment in SSA may contribute to the higher PPH rates [53].
The current review identified several risk factors for PPH. Women over 35 years of age had a greater risk of PPH compared to those under 35 years of age. This finding is in contrast to that of Tolossa et al.’s [54] systematic review and meta-analysis, which found no statistically significant relationship between age and PPH in Ethiopia. However, our finding is consistent with the increased likelihood of several pregnancies among older women, which can lead to uterine atony. According to this review, multiparity, multiple pregnancies, anemia, a history of PPH, and labor induction all increase the risk of PPH. The finding that anemia increases the risk of PPH is consistent with the results of a prospective cohort study conducted in Pakistan, Nigeria, Tanzania, and Zambia [55]. The conclusion of this review, which indicates an association between labor induction and PPH, is consistent with findings from a Swedish study that reported an increased risk of PPH associated with labor induction [56]. Furthermore, the finding of the current study that multiparity increases the risk of PPH aligns with the findings of a systematic review and meta-analysis conducted in Ethiopia [8]. A population-based cohort study from Sweden further supports the association between a history of PPH and an increased risk of PPH in subsequent deliveries [57]. Additionally, a study conducted in Norway found that multiple pregnancies are associated with a higher likelihood of PPH [58]. These risk factors all contribute to weakened uterine contractions, which are essential for regulating placental blood flow and preventing PPH [59]. A previous history of PPH may also increase the risk of PPH due to factors such as undiagnosed coagulation disorders, underlying genetic susceptibility, and myometrial exhaustion [57]. Induction of labor can lead to uterine hyperstimulation, which increases the risk of atony and subsequent PPH [60].
This review also revealed that rural women are more likely to experience PPH compared to urban women, reflecting similar findings from an Egyptian study [61]. This disparity may be attributed to the fact that rural women are more likely to deliver at home without access to oxytocin, a medication that promotes uterine contraction and helps reduce postpartum bleeding [62, 63]. This review revealed that women who undergo assisted delivery using instruments like forceps or who have a cesarean section are at a higher risk of experiencing PPH. This finding is consistent with a scoping review conducted in low-to-middle-income countries, which identified an increased risk of PPH associated with cesasrean sections [24]. This finding may be explained by the fact that many indications for cesarean section - such as APH, multiple pregnancies, and a history of previous cesarean section - are themselves associated with an increased risk of PPH [64]. The current review revealed that women with IUFD are at a higher risk of experiencing PPH. Accordingly, a study conducted in Brazil also revealed that IUFD increased the risk of PPH [65]. This finding may be a result of disseminated intravascular coagulation that develops due to IUFD [66]. Finally, the current review revealed that women who did not attend ANC were more likely to experience PPH. This finding may be attributed to the fact that women who do not attend ANC are less likely to have antenatal conditions that increase the risk of PPH diagnosed and managed. Furthermore, women who do not attend ANC are also more likely to deliver at home, which can increase the risk of vaginal and cervical tears that may contribute to PPH.
Based on the results of the current study, we propose several recommendations. One key recommendation is to implement strategies to enhance ANC utilization among women in SSA. The strategies may include ensuring that healthcare facilities are accessible and located near communities, especially in rural areas, utilizing community health workers, removing user fees, and providing targeted health education [67]. Increased ANC attendance can improve the identification and management of pre-existing conditions that elevate PPH risk. Additionally, attending ANC promotes facility-based deliveries, which can help reduce complications during childbirth. A study in Ethiopia found that complete adherence to ANC visits reduced the risk of PPH by over 80% [68]. To reduce the association between induction of labor and PPH, we recommend administering lower doses of oxytocin for short durations. This approach may help prevent oxytocin receptor desensitization, which can lead to uterine atony [69]. We recommend closer monitoring of older women and multiparous women due to their increased risk of PPH.
We also recommend leveraging artificial intelligence (AI) to predict PPH by analysing routine clinical data. A study conducted in Kenya revealed that routine clinical and demographic data can be used to identify women at high risk of developing PPH [70]. Machine learning algorithms can use various factors to predict PPH, including a woman’s pre-pregnancy weight, body mass index, fetal macrosomia, admission hematocrit, pregnancy-induced hypertension, and the length of second-stage labor [71]. A study conducted in Iran revealed that PPH can be predicted using both traditional statistical analysis and a machine learning model. The study found that extreme gradient boosting (XGBoost) was the best model for predicting PPH [72]. Incorporating predictive models into clinical practice will allow healthcare professionals to tailor and stratify the care of women at high risk of PPH.
A key strength of this review is its adherence to established guidelines for systematic reviews and meta-analyses, which ensures the reproducibility and reliability of the results. Additionally, most of the included studies had large sample sizes, which potentially improved the precision of effect estimates. However, the limitation of this review is that most of the included studies were from only a few countries in SSA, which may limit the generalizability of the findings to the entire region. Second, the review identified significant heterogeneity and publication bias within the included studies.
PPH is the leading cause of maternal death due to hemorrhage worldwide. Beyond the immediate risk of death, PPH can also lead to various long-term consequences. The magnitude of PPH in SSA is considerably higher compared to other regions. Although effective strategies for managing PPH are well-established, risk factors may not always be readily identifiable in individual cases. This systematic review identified several risk factors for PPH, categorized into individual, antepartum, intrapartum, and postpartum factors. To reduce PPH in SSA, we recommend increasing ANC attendance, close monitoring of high-risk women, and using oxytocin carefully during labor induction.
The data that support the findings of this manuscript can be provided on request from the first author.
EM designed the research study. EM, TD, PM and GM performed the research. AR was involved in supervision, data analysis, and interpretation of data. EM analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We acknowledge Sphamandla Josias Nkambule for critically reviewing and editing the manuscript before submission. We also thank all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.ceog5110229.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.









