1 National Clinical Research Center for Obstetric & Gynecologic Diseases, Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 100730 Beijing, China
2 Department of Gynecology and Obstetrics, Key Laboratory of Obstetrics and Gynecologic and Pediatric Diseases and Birth Defects of the Ministry of Education, West China Second University Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
†These authors contributed equally.
Abstract
Background: Primary dysmenorrhea (PD) afflicts many childbearing-age
women, with a high prevalence ranging from 17% to 90%. The Dingkun pill (DKP),
a traditional Chinese medicine formula, has been prescribed for managing
menstrual disorders empirically in clinical practice for a long time, but there
are very few high-quality studies supporting this practice. Therefore, this trial
aimed to assess the efficacy and safety of DKP in patients with PD.
Methods: Our study was a multicenter, prospective, randomized,
double-blind, placebo-controlled study. DKP or placebo was prescribed to
participants from the 5th to 14th day of each menstrual cycle for 12 weeks.
Changes in pain intensity were measured by a visual analog scale (VAS) and were
compared between groups using repeated measures analysis. The pain mediators and
sex hormones were also assessed before and after the treatment, and their
intergroup changes from the baseline were analysed by student t-test.
The hemodynamic indices and safety profile of DKP were also investigated.
Results: A total of 156 women were recruited and randomly allocated to
receive either DKP or placebo, of whom 142 (73 in DKP and 69 in sham control)
completed the study. A more distinctive reduction in VAS scores was observed in
the DKP group, compared with placebo (–2.68
Keywords
- primary dysmenorrhea
- Dingkun pill
- traditional Chinese medicine
- alternative therapy
- pain management
Dysmenorrhea, the painful menstruation of uterine origin, is the most common complaint in adolescent girls and women of reproductive age during their gynecological visits [1]. Primary dysmenorrhea (PD) is characterized by a spasmodic, cramping sensation at the lower abdomen without any identifiable pelvic pathology [2, 3]. Typically, painful sensation usually occurs after a few months when ovulatory cycles are attained and are accompanied by systemic symptoms such as fatigue, nausea, vomiting, diarrhea, and sleep disturbance [3]. The prevalence rate varies based on different assessment methods. Generally, PD has been estimated to affect 50% to 95% of females around the world [4, 5]. As the leading cause of absenteeism in school, PD is associated with loss of productivity at work; menstrual cramp also increases the risk of developing depressive symptoms and chronic pain disorders [6, 7]. Given the high prevalence and negative consequences, PD has drastically affected the social relationships of young females by interfering with their daily activities and has become a public health concern [8].
The pathomechanism of PD remains complex and has not been fully elucidated. The over-production of uterine prostaglandins (PG) appears to be the main cause of the hypercontractility of smooth muscles in vessels and uterine myometrium [9]. Based on the PG-dominated etiology, non-steroid anti-inflammatory drugs have been prescribed as the first-line treatment, followed by combined oral contraceptives [2]. However, both pharmacological agents are not suitable for long-term administration and have adverse effects on the digestive tract and central nervous system [10]. Additionally, conventional use of hormone contraceptives is associated with an increased risk of venous thromboembolism [11]. These concerns show an urgent need for alternative approaches to managing menstrual pain.
There’s mounting evidence supporting the application of complementary and alternative medicine in the pain management field as it contains a wide range of medical practices that have been greatly developed and evolved in recent years. A systematic review found the pulsed electromagnetic fields had a significant inhibitory effect on the pain scores in patients with osteoarthritis when compared with the sham group (standardized mean differences [SMD] = 0.71, 95% confidence interval [CI]: 0.08–1.34, p = 0.03) [12]. Moreover, the association between acupuncture analgesia and the endogenous opioid system has been corroborated by many studies [13]. Results from a randomized trial also showed rubbing oil could provide additional benefits to people suffering from low back pain [14]. In China, it has been reported that over half of the patients with painful periods use traditional Chinese medicine (TCM) to treat menstruation-associated discomforts and TCM shows significant efficacy [15]. Based on the theory of conventional Chinese medicine, PD is the clinical manifestation of Qi stagnation and blood stasis syndrome [16]. TCM acts on pain perception in a holistic way with multiple components and targets, in contrast to western painkillers with a single targeted pathway [17]. The Dingkun pill (DKP), one of the well-known TCM, was developed during the Qing dynasty (A.D. 1730) and has been used as a treatment strategy for various gynecological diseases over the past three centuries in East Asia [18]. DKP, comprising thirty herbs and animal-orient ingredients, has been used in the clinical setting for its Qi-nourishing and blood-activating features [19]. In the Chinese literature, there is strong evidence that DKP has an analgesic effect on dysmenorrhea [18, 20]. However, most of them are empirical reports and do not strictly comply with the standard of the CONSORT statement, which makes it difficult to support the conclusion that DKP is therapeutic in dysmenorrhea. Here, we hypothesized that DKP could alleviate PD compared with placebo and aimed to investigate the clinical efficacy, safety, and mechanism of action of DKP in PD using high-level evidence.
This was a prospective, multicenter, randomized controlled trial to evaluate the safety and efficacy of DKP on PD management, and the study was designed in a double-blinded, placebo-controlled manner to minimize the potential placebo effect on pain management. The protocol of this study was evaluated and authorized by the Institutional Review Board of eight participating hospitals (No.ZS-1913), and the trial was registered on ClinicalTrials.gov with identify the number (NCT03953716). All patients recruited were fully informed, and the informed consent was signed by each participant.
Patients diagnosed with PD aged between 18 to 35 years with regular menstrual cycles were eligible for the recruitment. The exclusion criteria were described following: (1) had been diagnosed with pelvic pathologies related to secondary dysmenorrhea including but not limited to endometriosis, previous pelvic inflammatory disease, uterine fibromatosis, adenomyosis, intrauterine devices, and ovarian cysts; (2) administration of any hormonal or analgesic agents within 12 weeks before enrollment; (3) diagnosed with concurrent basic diseases including neuropathologic pain, anemia, immunodeficiency or liver and kidney malfunction; (4) addiction for drugs, alcohol, and cigarette.
We performed a t-sample t-test power analysis via PASS software
(version:15.0.13 NCSS LLC, Kaysville, UT, USA), the analysis showed that with a
sample population of 132 women, the analysis would be able to detect a 2-point
pain reduction weighed by the visual analog scale (
Generally, the DKP (Shanxi Guangyuyuan Traditional Chinese Medicine Co., Ltd., Taiyuan, Shanxi, China) is a solid pellet developed by the water-honeyed protocol. The placebo pills, provided by Guangyuyuan Co., Ltd., were made of 45% caramel and 55% starch with identical appearance, smell and taste to the DKP. Both participants and investigators were blinded to the allocation and treatment. To ensure the blinding process, both the placebo and DKP were encapsulated in identical bottles and boxes. The randomization list was kept by an independent investigator who didn’t participate in any process of intervention assignment, data collection, or analysis.
Eligible patients were randomly allocated to receive either DKP or placebo for 12 weeks in a 1:1 ratio, according to a computerized random number table which was generated by SAS (version 9.2, Cary, NC, USA). The daily doses of both pills were filled into small plastic bottles, each of them containing 7 grams (g) of interventional medicine. Participants were instructed to orally take 3.5 g of DKP or placebo twice daily from the 5th to 14th day of the menstrual cycle for 12 weeks. Adherence was assessed by bottle counts at each visit. The usage of rescue painkillers (Ibuprofen, batch number: 3200600, Sino-GlaxoSmithKline, Tianjin, China) was allowed when unbearable pain occurred. The specific dosage and time of painkillers that participants requested were recorded in the case report form.
The pain intensity of dysmenorrhea was described using a 10-point visual analog scale (VAS) ranging from 0 points (no pain) to 10 points (worst). The measure was taken on the first or second day of menstruation at baseline and every 4 weeks for 3 continuous cycles as the primary outcome. Additionally, based on the VAS rating, pain intensity was further stratified into mild pain (1–3 scores), moderate pain (4–6 scores), and severe pain (7–10 scores).
As the secondary endpoints, the levels of serum beta-endorphin, prostaglandin
F2alpha (PGF2
We conducted sonographic assessments at 10:00–12:00 AM on the 7th to 12th day of the menstrual cycle in all the patients. The color Doppler ultrasound used in the study was Logiq 500 (General Electric, Boston, MA, USA) with a 5 MHz transvaginal probe. All the scanning techniques were performed by the same examiner in each participating center, who was instructed with standardized procedures for ultrasonographic measurements before the recruitment. At first, the conventional ultrasound was used to eliminate the pathology in the uterine and adnexa. Then, using the color Doppler ultrasound imaging, the lateral level of the uterine cervico-coopereal was visualized in a longitudinal plane. The values of resistance index (RI = maximal systolic flow – minimal diastolic flow/maximal systolic flow), pulsatility index (PI = maximal systolic flow – minimal diastolic flow/mean flow), and the ratio of flow velocity between peak-systolic and end-diastolic (S/D) in the bilateral uterine ascending arteries were calculated. All measurements were performed at the study entry and after the treatment.
Data from patients who completed the full treatment course stuck to the
instruction and finished follow-ups in time were included for final analysis. and
the results were demonstrated as mean
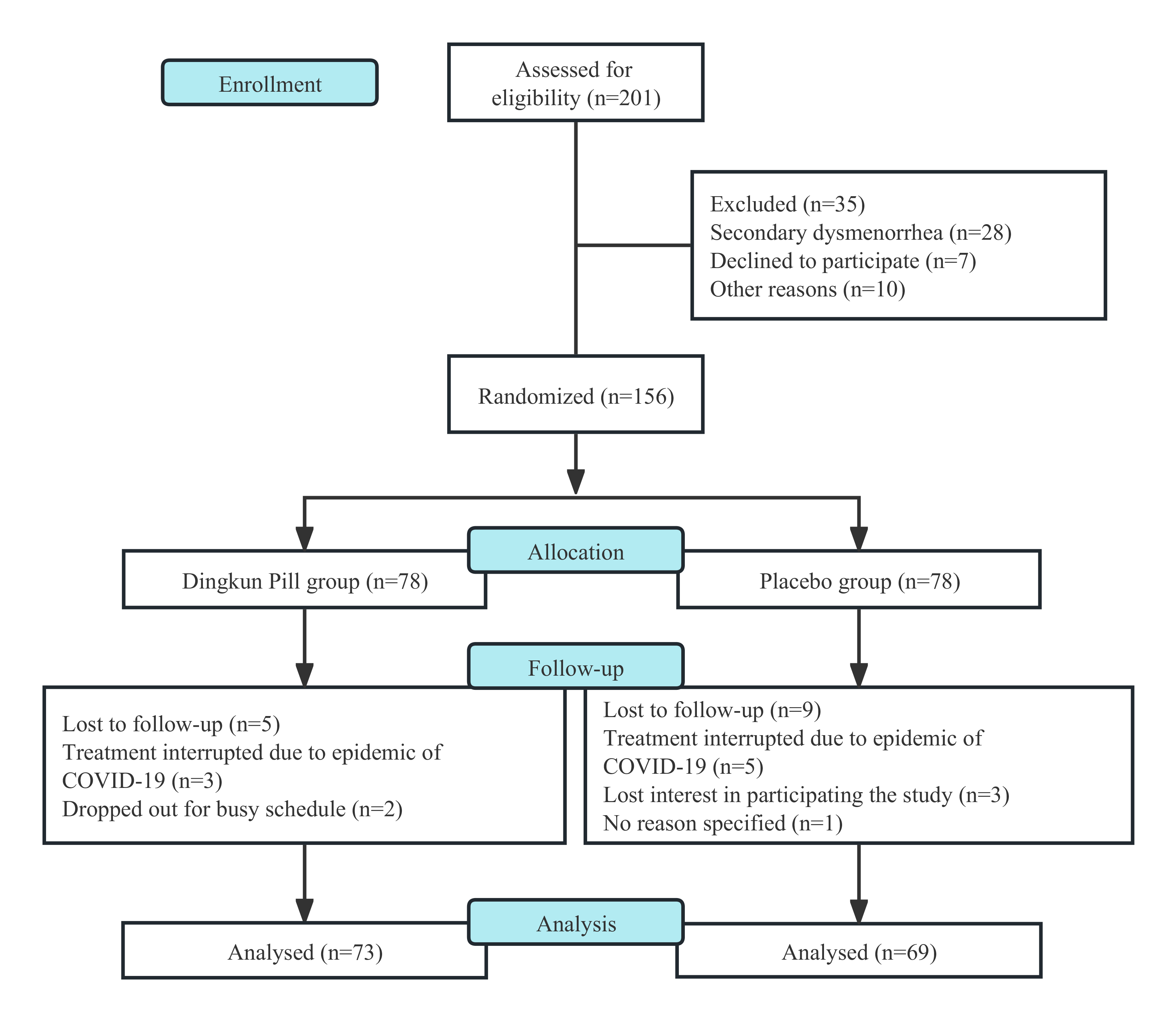
In this research, 201 patients at the base were screened for eligibility, in which 156 of them were eligible to participate (Fig. 1). With full consent, they were randomly assigned to either the DKP group (n = 78) or the placebo group (n = 78). During the treatment course, 14 (9.0%) of them failed and were lost to follow-up, and 142 participants completed the 12-week study and subsequent follow-up (73 in DKP and 69 in placebo). Data from these patients were included in final analyses, and the well-matched demographic characteristics at study entry were listed in Table 1.
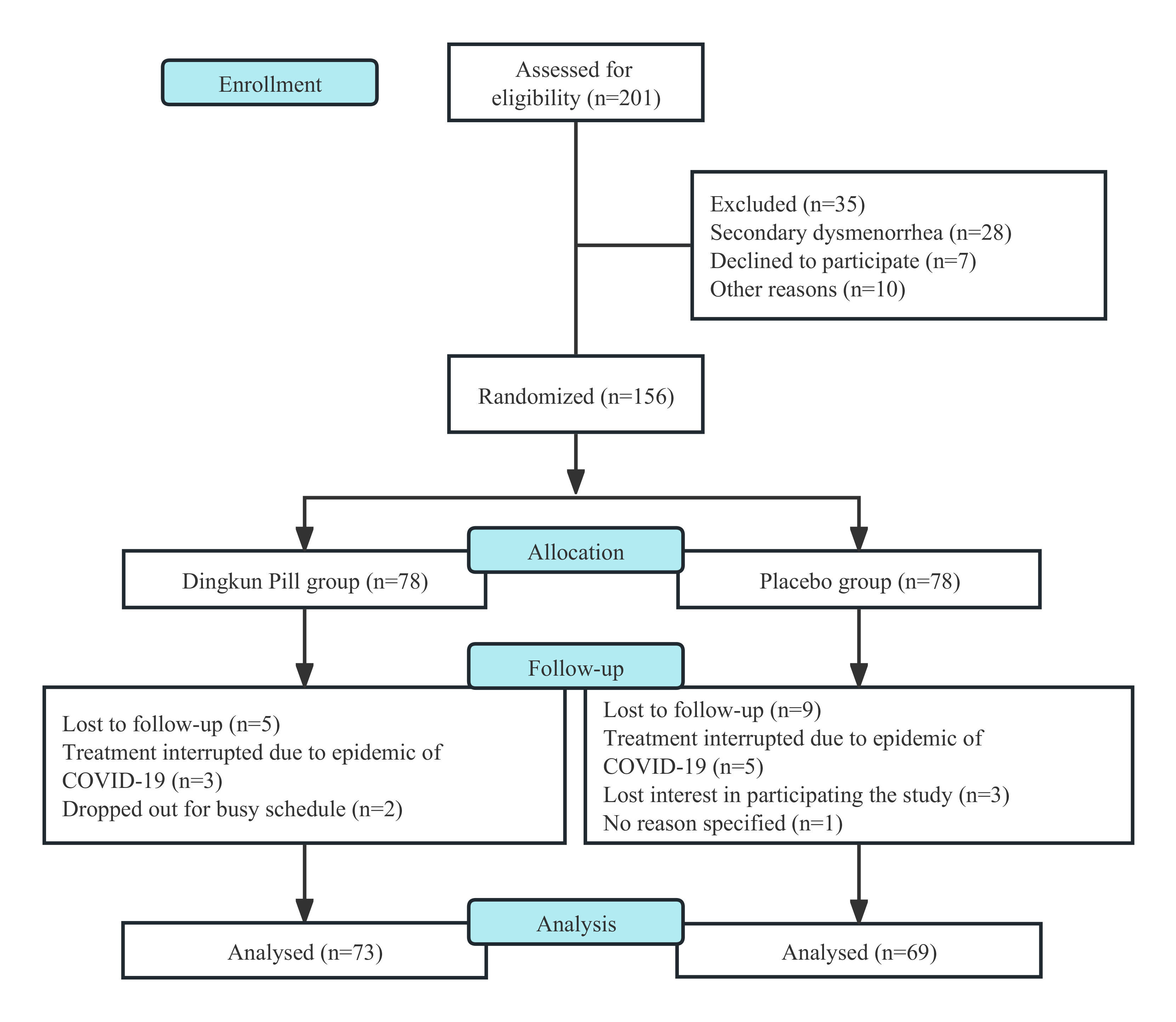 Fig. 1.
Fig. 1.The process of study from screening to completion during the 12 weeks.
| Characteristics | DKP group | Placebo group | p value | |
|---|---|---|---|---|
| (n = 73) | (n = 69) | |||
| Age, yr | 24.86 |
25.58 |
0.292 | |
| Height, cm | 162.73 |
161.31 |
0.141 | |
| Weight, kg | 53.15 |
53.54 |
0.816 | |
| BMI, kg/m |
20.05 |
20.49 |
0.415 | |
| Age at menarche, yr | 12.93 |
13.06 |
0.539 | |
| Menstrual cycle, days | 29.47 |
29.65 |
0.742 | |
| Proportion of patients | 0.985 | |||
| Mild patients | 20 (27.4) | 18 (24.6) | ||
| Moderate patients | 27 (37.0) | 26 (40.0) | ||
| Severe patients | 26 (35.6) | 25 (35.4) | ||
| VAS scores | ||||
| All patients | 5.19 |
5.35 |
0.813 | |
| Patients with mild pain | 2.65 |
2.5 |
0.425 | |
| Patients with moderate pain | 4.33 |
4.38 |
0.775 | |
| Patients with severe pain | 8.04 |
8.43 |
0.359 | |
| Beta-endorphin, pg/mL | 28.45 |
28.81 |
0.756 | |
| PGF2 |
44.71 |
45.28 |
0.770 | |
| Oxytocin, pg/mL | 219.13 |
219.22 |
0.991 | |
| Vasopressin, pg/mL | 1627.37 |
1602.42 |
0.734 | |
Data are presented as mean
Abbreviations: DKP, Dingkun pill; BMI, body mass index; VAS, visual analog
scale; PGF2
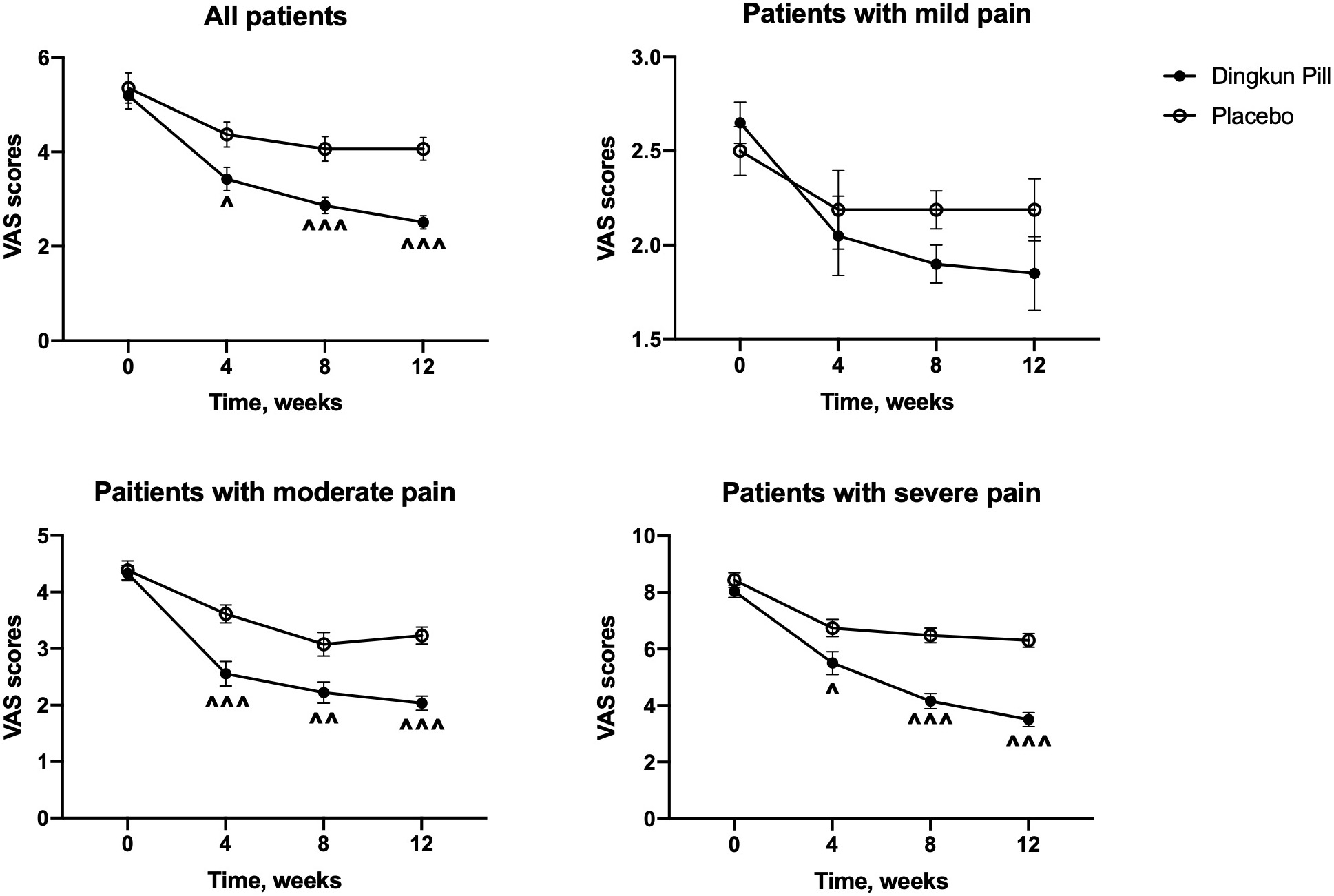
The absolute changes in VAS scores of all participants were calculated and
demonstrated in Fig. 2. A significant reduction of VAS score was found in the DKP
group, compared to the placebo group (p
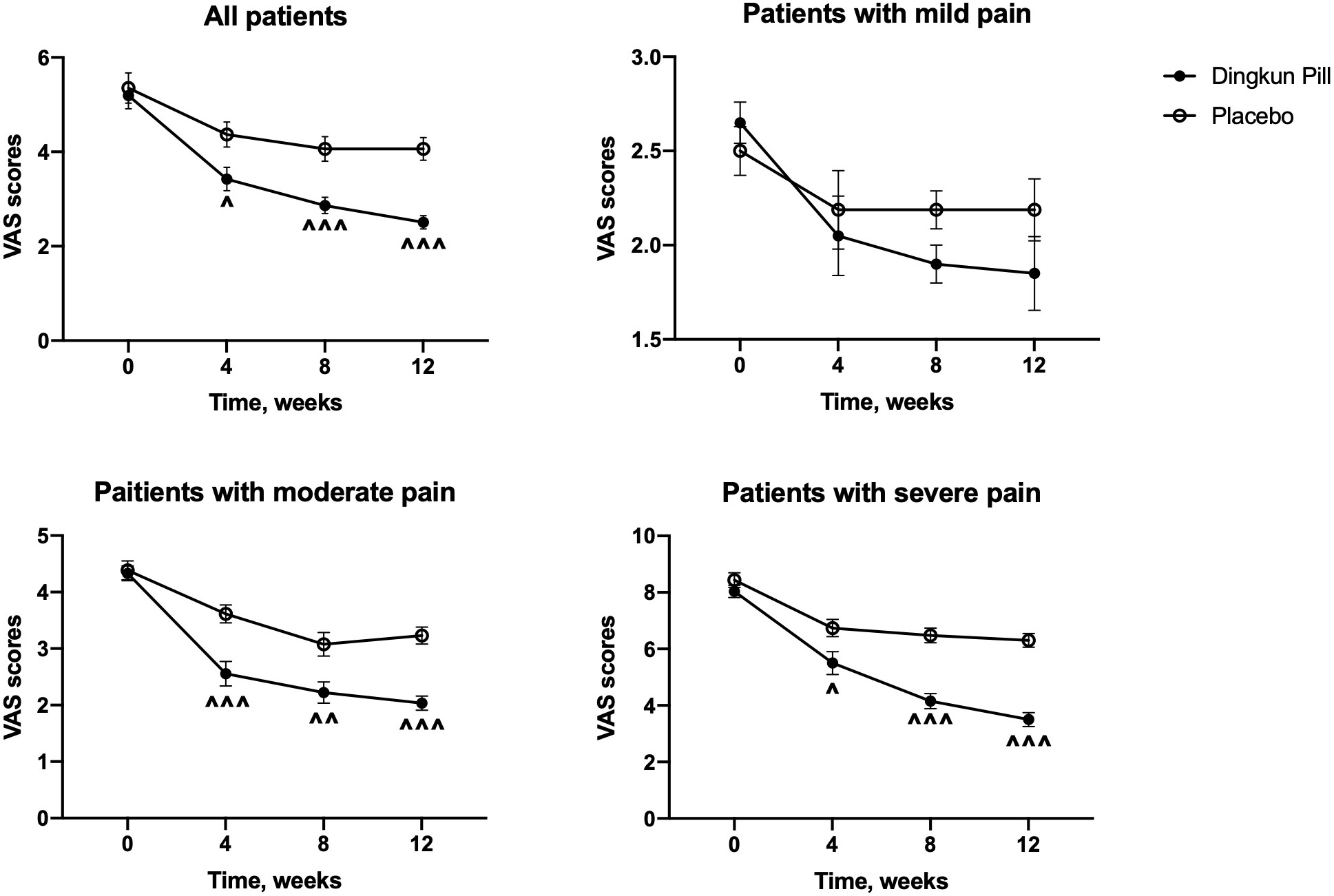 Fig. 2.
Fig. 2.Changes in mean scores of visual analogue scale over time in
patients and patients with different degrees of dysmenorrhea treated with Dingkun
Pill and placebo. Data are expressed as mean
Additionally, rescue painkillers were used in eleven cases (15.9%) in the placebo group and four cases (5.4%) in the DKP group. In the second month of the treatment, the number of patients taking additional analgesics decreased to seven (10.1%) in the placebo group and two (2.7%) in the DKP group. Four participants (5.8%) in the placebo group continued to take painkillers at the 12th week.
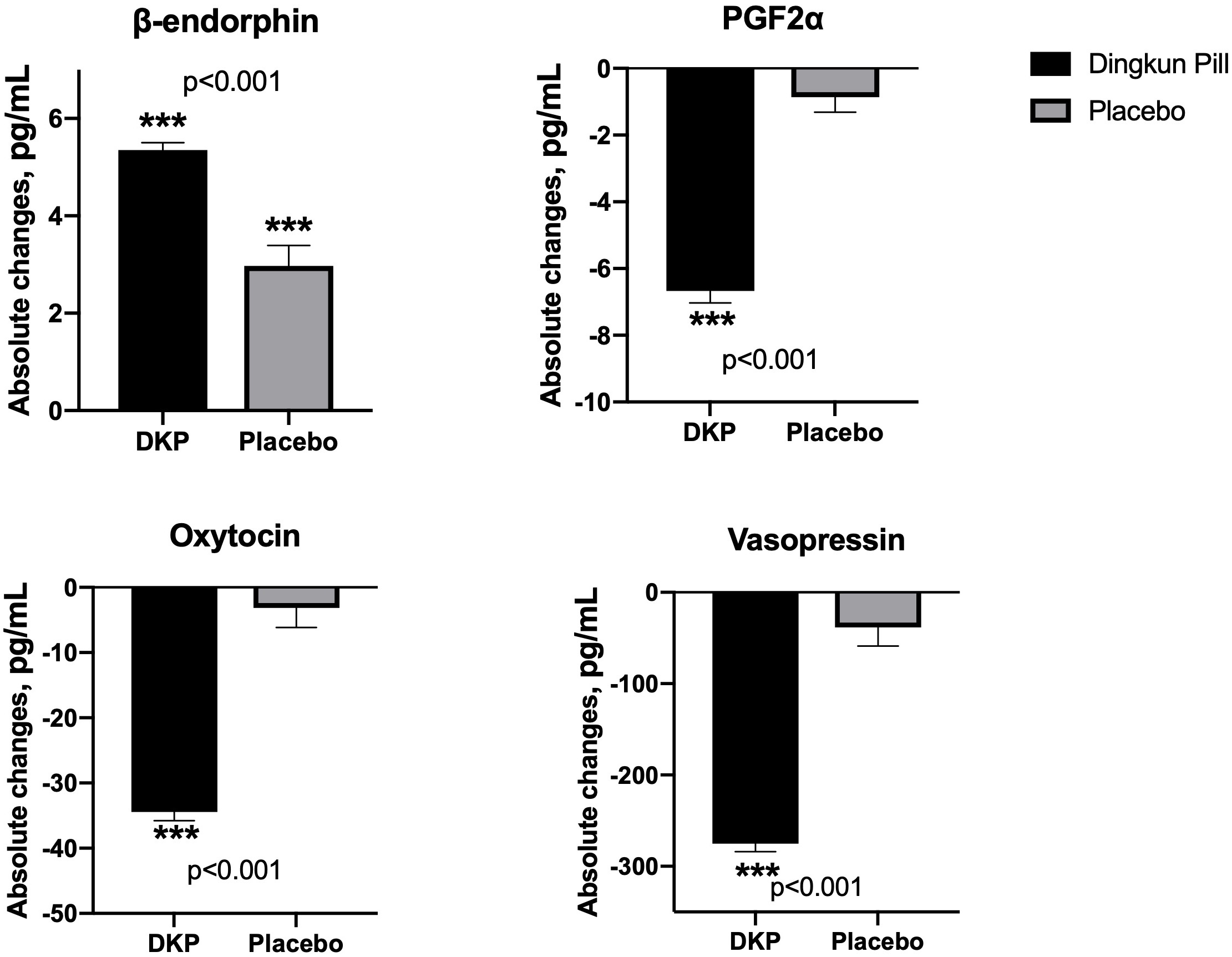
The absolute changes in the concentration of pain mediators are summarized in
Fig. 3. Compared with baseline values, both control and DKP groups demonstrated
significant modulation of beta-endorphin. The decline in concentrations of
PGF2
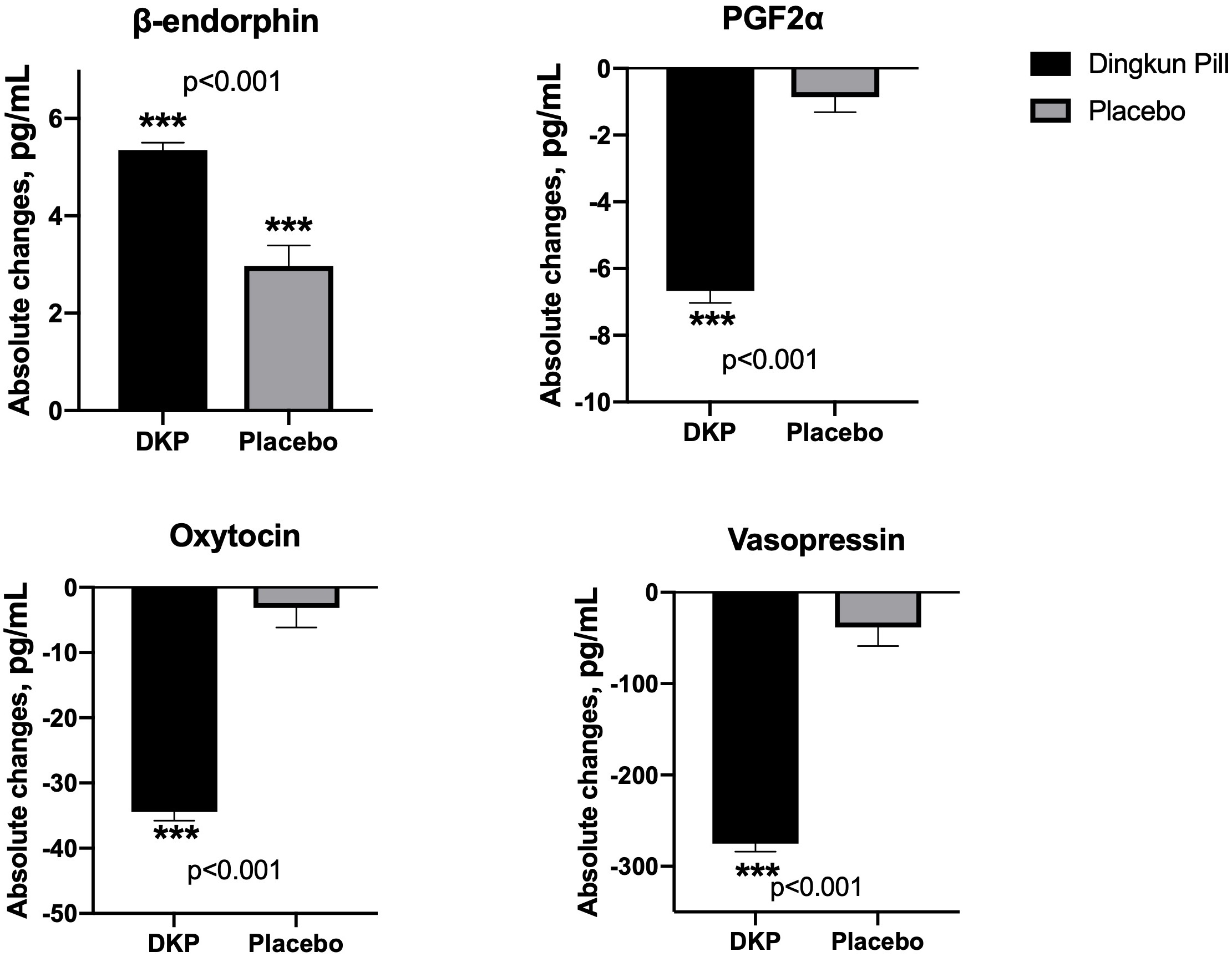 Fig. 3.
Fig. 3.Mean absolute changes in pain-associated biochemical factors in
Dingkun Pill group and placebo group. Data are presented as mean
On the other hand, the reproductive hormones in the early follicular phase
maintained a normal profile during the medical intervention (Table 2). Patients
treated with DKP showed a significantly lower level of FSH compared with patients
on placebo pills (6.33
| Hormone variables | DKP group | Placebo group | p value | |
|---|---|---|---|---|
| (n = 73) | (n = 69) | |||
| FSH, IU/L | ||||
| Baseline | 6.57 |
6.71 |
0.641 | |
| 12th week | 6.33 |
7.11 |
0.037 | |
| LH, IU/L | ||||
| Baseline | 4.50 |
4.59 |
0.813 | |
| 12th week | 4.53 |
5.05 |
0.228 | |
| Estradiol, pg/mL | ||||
| Baseline | 37.86 |
37.14 |
0.815 | |
| 12th week | 47.04 |
48.50 |
0.815 | |
| Testosterone, ng/mL | ||||
| Baseline | 0.40 |
0.41 |
0.765 | |
| 12th week | 0.41 |
0.40 |
0.905 | |
Data are expressed as mean
Abbreviations: DKP, Dingkun pill; FSH, follicular stimulation hormone; LH,
luteinizing hormone. *p
Bilateral uterine arteries were visualized in all participants. The mean values of PI, RI, and S/D of uterine ascending artery flow during pain-free follicular phrases are presented in Table 3. All indices responded to DKP with significantly reduced resistance of uterine blood flow, which indicated improved perfusion. No significant alteration was detected in the control group.
| Uterine blood flow indices | DKP group | Placebo group | p-value | |
|---|---|---|---|---|
| (n = 73) | (n = 69) | |||
| Left PI | Baseline | 2.57 |
2.54 |
0.860 |
| 12th week | 2.02 |
2.48 |
0.001 | |
| Right PI | Baseline | 2.61 |
2.67 |
0.635 |
| 12th week | 2.17 |
2.50 |
0.011 | |
| Left RI | Baseline | 0.85 |
0.85 |
0.758 |
| 12th week | 0.78 |
0.85 |
||
| Right RI | Baseline | 0.83 |
0.86 |
0.546 |
| 12th week | 0.80 |
0.81 |
0.784 | |
| Left S/D | Baseline | 7.00 |
6.96 |
0.930 |
| 12th week | 5.16 |
6.68 |
||
| Right S/D | Baseline | 7.00 |
6.87 |
0.882 |
| 12th week | 5.13 |
6.68 |
0.001 | |
Data are presented as mean
Abbreviations: DKP, Dingkun pill; PI, pulsatility index; RI, resistance index; S/D, the ratio between peak systolic and end-diastolic flow velocity.
Intragroup significance **p
No patient reported serious adverse effects during the study process. Changes in blood pressure, heart rate, blood cell count, and functional indicators of the liver and kidney are shown in Table 4. No meaningful abnormality was identified after the intervention.
| Parameters | DKP Group | Placebo Group | ||||
|---|---|---|---|---|---|---|
| (n = 73) | (n = 69) | |||||
| Baseline | Week 12 | p-value | Baseline | Week 12 | p-value | |
| SP, mmHg | 107.9 |
106.7 |
0.338 | 108.4 |
108.1 |
0.771 |
| DP, mmHg | 67.6 |
69.3 |
0.108 | 69.2 |
69.6 |
0.775 |
| HR, bpm | 77.2 |
76.8 |
0.369 | 76.3 |
76.8 |
0.346 |
| WBC, 10 |
5.51 |
5.57 |
0.741 | 5.48 |
5.46 |
0.884 |
| N, % | 58.84 |
60.21 |
0.162 | 57.84 |
58.66 |
0.243 |
| HGB, g/L | 128.91 |
130.79 |
0.062 | 129.75 |
128.70 |
0.160 |
| PLT, 10 |
242.18 |
243.32 |
0.780 | 245.45 |
257.56 |
0.015 |
| ALT, IU/L | 11.45 |
11.65 |
0.693 | 11.20 |
11.75 |
0.430 |
| AST, IU/L | 16.11 |
15.96 |
0.839 | 16.02 |
16.03 |
0.976 |
| BUN, mmol/L | 3.90 |
3.91 |
0.970 | 4.02 |
3.98 |
0.767 |
| Cr, umol/L | 59.10 |
59.90 |
0.320 | 59.59 |
57.42 |
0.012 |
Variables are described as mean
Abbreviations: DKP, Dingkun pill; SP, Systolic pressure; DP, Diastolic pressure; HR, heart rate; WBC, White blood cell; N, neutrophil; HGB, Hemoglobin; PLT, Platelet; ALT, Serum alanine aminotransferase; AST, Serum aspartate aminotransferase; BUN, Urea nitrogen; Cr, Creatinine.
It was the first randomized, double-blinded, placebo-controlled study that assessed the clinical effectiveness and safety of DKP in the treatment of PD. Comprehensive indicators including pain scores, pain biochemical mediators, ovarian hormonal profiles, and uterine artery flow indices were evaluated. We found that DKP produced significantly more relief of menstrual pain compared with the placebo. The levels of pain mediators in the DKP group were significantly improved in comparison with those in the placebo group, which further validated the subjective pain score with objective parameters. No abnormal results were found in safety indexes after the intervention. Therefore, our study suggests that DKP has an analgesic effect on dysmenorrheal pain with little systemic toxicity.
DKP has exhibited diverse pharmacological activities by targeting multiple
pathways through its multiple ingredients [21, 22]. Previously, 234 chemical
compounds were isolated and identified from the 30 crude ingredients of DKP; most
of the ingredients were triterpenoid saponins, flavonoids, and alkaloids [21].
Notably, in vitro experiments suggested the triterpenoid saponins
extracted from Ilex oubescens roots were able to inhibit nitric oxide (NO) and
prostaglandin E
Although DKP has a wide clinical application in polycystic ovarian syndrome,
female subfertility, and menstrual disorders, studies investigating the efficacy
of DKP in PD are extremely scarce [25, 26]. There is only one randomized
controlled study that investigated the role of DKP in dysmenorrhea [20]. Ma
et al. [20] found that DKP remarkably relieved the pain severity in
women experiencing dysmenorrhea; a significantly higher proportion of
participants in the DKP group achieved over 50% reduction in VAS scores compared
with the control group. In addition, the DKP group also showed a more significant
decrease in the serum levels of PGF2
Based on previous findings, many components in DKP have been found involved in the symptomatic regression for pain management. For example, the biologically active constituents such as baicalein, ethyl acetate fraction, and glabridin in Radix Scutellariae, Angelicae Sinensis, and Radix glycyrrhizae have been found to reduce the COX-2 mRNA or protein expression, and to inhibit the release of PG [28, 29, 30]. In vitro experiments indicated both Radix Scutellariae and Radix Glycyrrhizae had a spasmolytic effect on the smooth muscle of rat uterine tissue [31, 32]. Furthermore, as the primary extract of Rhizoma Corydalis, tetrahydropalmatine displays antinociceptive action on different types of pain syndromes by inhibiting the protein expression of inducible nitric oxide synthase (iNOS) which is responsible for the inflammatory cascade [33, 34]. Future research is needed to unravel the intricate molecular mechanism behind the antinociceptive effect of DKP in PD.
Though the pathogenesis of PD has not been fully elucidated to date, previous
studies have shown that this complicated process is modulated by many biological
factors [35]. In general, PGF2
Conversely, beta-endorphin, a neurotransmitter primarily produced in the
anterior lobe of the pituitary gland, has morphine-like effects on opioid
receptors throughout the body by inhibiting the irritation of peripheral
somatosensory fibers [38]. It’s been believed that anxiety disorders induced by
the painful event are related to decreased peripheral beta-endorphin [39]. Fedele
et al. [27] discovered women suffering from PD had positive feedback to
placebo and postulated that a surge in endogenous beta-endorphin might result in
short-term attenuation. Intriguingly, we indeed detected a significant elevation
of 3.05
Doppler sonographic studies suggest dysmenorrheal women have higher uterine impedance than eumenorrheic women throughout the whole menstrual cycle [40]. In the present study, patients in the DKP group showed favorable changes in hemodynamic parameters, inhibition of vasoconstrictors, and improvement in uterine blood flow (spasmolytic effect of DKP). Similar to our findings, a previous study observed that DKP treatment decreased PI and RI of the uterine artery [20, 41]. However, it remains unclear whether this vasodilative function is a direct effect of DKP or secondary sequalae indirectly mediated by the diminishing levels of vasoconstrictors.
Up to date, our study is the first and largest, randomized, double-blinded, placebo-controlled trial investigating the analgesic effect of DKP on PD based on a clinical setting. Notably, in this research, not only the subjective pain rating score but also the objective indicators, biochemical substances involved in pain pathogenesis and uterine artery flow indices reflecting vascular contractility, were evaluated thoroughly, which provided a more comprehensive understanding of the bioactive effect of DKP. Additionally, we included patients with different severity ranging from mild to severe pain and performed subgroup analysis. This approach ensured less selection bias and offered more pragmatic information of reference in daily practice.
Despite those advantages mentioned above, the results of our study should be interpreted with caution considering the following limitations. First, we took the blood sample for the measurement of biochemical parameters associated with pain perception, which might to some extent cause potential bias since the serum concentration of these biofactors could be regulated by systemic metabolic status. Further clinical trials investigating samples from menstruation blood or endometrial biopsy should be encouraged because they might provide more specific details on the local effect of DKP on the uterus. Second, the subjects who participated in our study are all Chinese Han women, which make it hard to extrapolate these therapeutic effects to all women of different ethnicity. Third, although the PD diagnosis in our study has been clinically verified via personal history, pelvic examination, and ultrasonographic screening, some pathological conditions that may cause secondary dysmenorrhea, like peritoneal endometriosis, can barely be identified at the very early stage. Therefore, the possibility of recruiting a dysmenorrheic woman with organic pathological conditions cannot be completely ruled out. Fourth, no pilot study has been done to conducted in this study. Last but not least, there are still major gaps in knowledge about the mechanism of the antinociceptive effect that DKP had on PD. In the current study, we did not explore the intervention of active components in DKP on the targets of different biological functions involved in this painful disorder. Additional bench research on the DKP targets of pain genesis is expected to elucidate its potential pain-killing mechanism. Hopefully, based on that, further trials might be proposed to explore whether PD patients who are refractory to first-line treatment could benefit from DKP treatment.
In conclusion, a pronounced reduction of pain intensity was observed in patients
with PD administrating DKP, compared with the placebo, especially for patients
with moderate to severe symptoms. With the elevation in beta-endorphin and
inhibition of PGF2
PD, Primary dysmenorrhea; DKP, Dingkun pill; VAS, visual analog scale; PG,
prostaglandins; PGF2
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
AS contributed to the conceptualization and design of the study, the data collection and interpretation. SZ and XD wrote and editing the manuscript. SZ and XM contributed to the protocol planning, data collection and analysis. SZ, XD, JG, YD and YW contributed to patient recruitment and data collection. All authors contributed to editorial changes in the manuscript. All authors read and approved the final version of the paper.
All patients recruited were fully informed, and the informed consent was signed by each participant. he protocol of this trial was evaluated and approved by the Ethics Committee of Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Science (No.ZS-1913).
We are grateful to all the women who participated in our study, and appreciate the arduous work performed by the clinicians and nurses in cooperating hospitals, namely Beijing Obstetrics and Gynecology Hospital, First People’s Hospital of Hangzhou affiliated to Zhejiang University, the Second Affiliated Hospital of Nanchang University, the Hubei Maternal and Child Health Hospital, the First Affiliated Hospital of Chongqing Medical University, Fujian Maternal and Child Health Hospital, and Liuzhou Maternal and Child Health Hospital.
This study received funding from the Capital’s Funds for Health Improvement and Research (CFH: 2020-2-40113), the Natural Science Foundation of China (No. 82074143), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (NO. 2020-PT320-003).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.