1 Department of Breast Surgery, Luoyang Central Hospital Affiliated to Zhengzhou University, 471000 Luoyang, Henan, China
2 Department of Stomatology, Luoyang Central Hospital Affiliated to Zhengzhou University, 471000 Luoyang, Henan, China
Abstract
Background: Neoadjuvant chemotherapy has become the standard treatment for patients with locally advanced breast cancer. However, patients with hormone receptor positive (especially human epidermal growth receptor 2 negative) breast cancer show low response rate to neoadjuvant chemotherapy. Whether neoadjuvant chemo-endocrine therapy (NCET) can improve the pathological complete response (pCR) rate of these patients remains controversial. Methods: A systematic literature search was conducted in the PubMed, Embase, and Cochrane databases. Pooled odds ratio (OR) with 95% confidence intervals (CI) was calculated. Results: Five randomized controlled trials were included (N = 566). NCET did not significantly improve pCR (OR 1.35, 95% CI 0.77–2.38, p = 0.30). Conclusions: NCET did not to improve the pCR rates in patients with hormone receptor positive breast cancer.
Keywords
- breast cancer
- neoadjuvant chemotherapy
- neoadjuvant chemo-endocrine therapy
- pathological complete response
Breast cancer is a common tumor among women worldwide. In addition to surgery, chemotherapy, anti-human epidermal growth receptor 2 (HER2) therapy and endocrine therapy are currently the main treatment methods for breast cancer and has been demonstrated to significantly improve the prognosis for breast cancer patients [1]. At present, anthracyclines and taxanes are the main drugs for chemotherapy of breast cancer, while endocrine therapy mainly includes tamoxifen and aromatase inhibitors. Neoadjuvant chemotherapy (NCT) has become the standard treatment for patients with locally advanced breast cancer [2]. It improves the breast conservation rate at surgery and significantly improves the number of patients who achieve pathological complete response (pCR). In the neoadjuvant randomized controlled trials (RCTs), a strong association at the patient level between pCR and the clinically relevant survival type end points, indicates that patients who achieve a pCR also have significantly better long-term survival compared with patients who do not have pCR. Patients with hormone receptor (HR) positive tumors, especially HER2 negative breast cancer, account for the largest proportion of breast cancer patients (about 70%) [3]. Their tumor sensitivity to NCT is low, with a lower pCR ranging from 5% to 10% [4, 5, 6]. Therefore, new adjuvant therapy strategies are urgently needed to improve the overall tumor response.
Hormone receptor positive breast cancer is more sensitive to endocrine therapy. It is possible that neoadjuvant chemo-endocrine therapy (NCET) may become a new treatment strategy to improve the pCR rate of these patients and further improve their prognosis. However, based on current clinical trials [7, 8, 9, 10, 11, 12, 13, 14, 15], the answer is unknown at this time.
We performed a systematic review and meta-analysis of RCTs to estimate the effects of NCET in women with estrogen receptor positive breast cancer.
An extensive literature search was performed in PubMed, Embase and Cochrane databases from through September 2022 without restriction in language. We used the following Medical Subject Heading terms and/or text words: ‘breast carcinoma’, ‘breast neoplasm’, ‘breast cancer’, ‘breast tumor’, ‘breast malignant tumor’, ‘mammary cancer’, ‘neoadjuvant systemic therapy’, ‘neoadjuvant treatment’, ‘neoadjuvant therapy’, ‘neoadjuvant chemotherapy’, and ‘pathologic complete response’.
We only included those RCTs that compared the effects of concurrent neoadjuvant chemo-endocrine therapy (experiment group) to the sole use of neoadjuvant chemotherapy (control group). The inclusion criteria were as follows: (1) divided into two intervention groups (concurrent neoadjuvant chemo-endocrine and neoadjuvant chemotherapy alone), (2) subjects were adults, and (3) patients with estrogen receptor positive breast cancer. The exclusion criteria were as follows: (1) unavailability of relevant data, (2) inclusion of patients with estrogen receptor negative tumors, and (3) metastatic or locally advanced breast cancer.
The screening of the databases was performed by two authors independently based on the above-mentioned criteria. Cases of disagreement were resolved through discussion and consensus without the use of a third investigator.
The following variables were extracted from each study: (1) baseline demographics, including the authors of the study, publication year and country; (2) characteristics of the study, including therapy regimens, HER2 status and sample size.
The risk of bias for the eligible studies was evaluated according to the guidelines in the Cochrane Reviewers’ Handbook. Six dimensions (selection bias, detection bias, performance bias, reporting bias, attrition bias and other bias) were appraised. The risk of bias was categorized into three levels: high, low, and uncertain.
Publication bias was evaluated by funnel plots, which were measured with Egger’s
test in Stata version 15.1 software (Stata, College Station, Austin, TX, USA). A
t-test was performed to determine the significance of the intercept, and
p
Our meta-analysis was carried out using Review Manager 5.3 (Cochrane Tech,
London, UK). In our study, the pooled odds ratio (OR) with 95% corresponding
confidence intervals (CI) was used to calculate for its effect on pCR. A Chi
squared-based Q statistic test was performed to describe the heterogeneity
qualitatively. When p
A total of 6410 records were identified for evaluation, of which 5 RCTs and 566 participants were eligible for meta-analysis. One of these 5 RCTs included more than 2 therapy regimen groups. Only data on eligible groups were extracted and considered for separate study. Fig. 1 depicts the process of identification and selection of eligible trials and Table 1 (Ref. [7, 8, 9, 10, 11]) summarizes the characteristics of the 5 eligible trials.
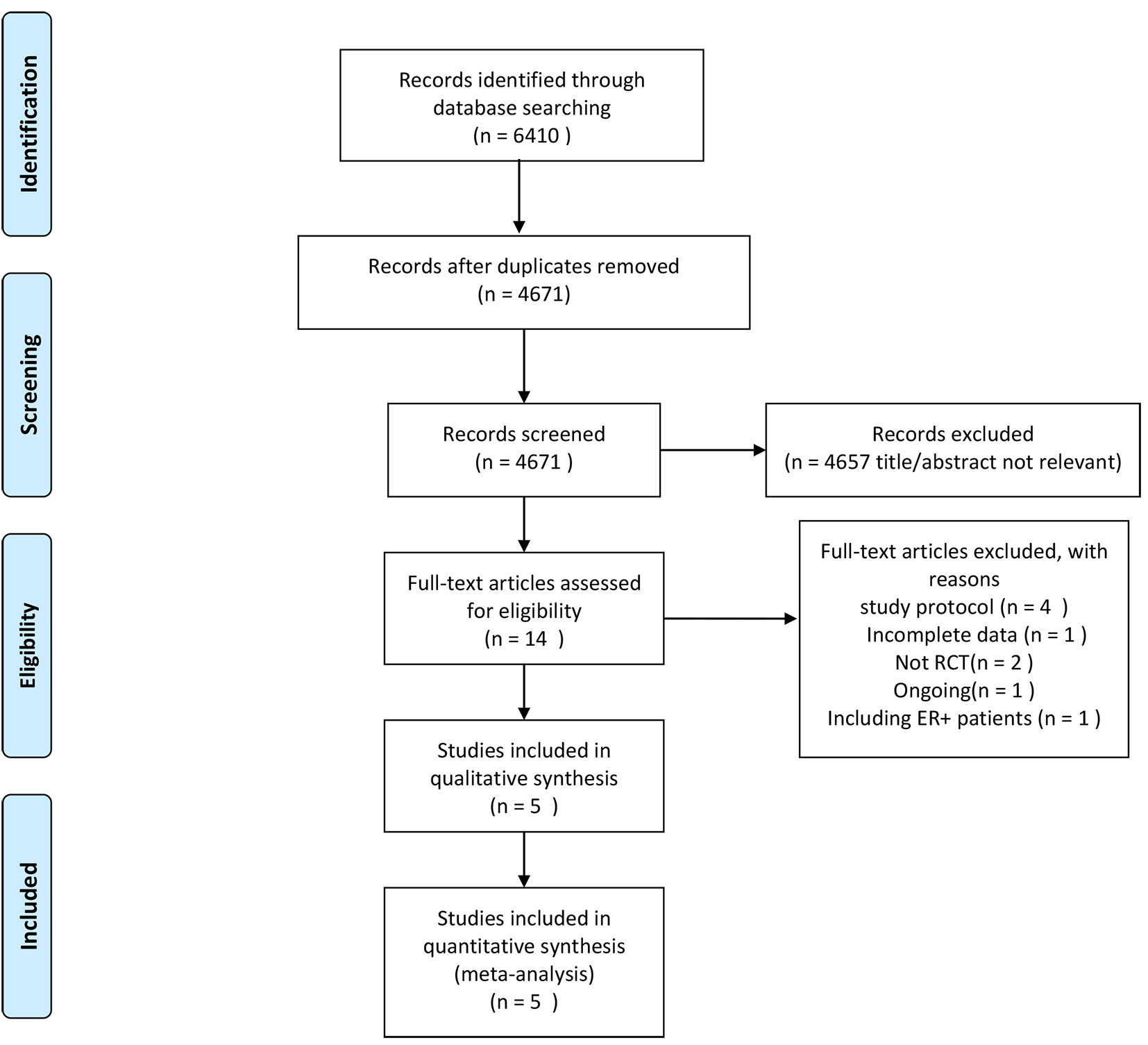 Fig. 1.
Fig. 1.Flow-chart of the literature search.
| Study | Year | Country | Regimen | HER2 | pCR | Clinical stage | Number of participants | ||
|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | Endocrine therapy | NCET | NCT | ||||||
| Ke-Da Yu, et al. [7] | 2019 | China | EC*4-T*4/FEC*3-T*3 | Leuprorelin + letrozole/letrozole | – | secondary endpoint | T1-4N0-3M0 | 115 | 116 |
| Sugiu K, et al. [8] | 2015 | Japan | P*12-FEC*4 | LEUPLIN/exemestane | –/+ | primary endpoint | T1-4N0-2M0 | 16 | 12 |
| Masuda N, et al. [11] | 2018 | Japan | P*12 | Leuprorelin + tamoxifen/letrozole | + | primary endpoint | T1-3N0-1M0 | 80 | 41 |
| Murray N, et al. [9] | 2022 | Australia | NA | NA | – | secondary endpoint | NA | 81 | 41 |
| Matsunuma R, et al. [10] | 2020 | Japan | P*12-EC*4 | Leuprorelin + anastrozole/anastrozole | – | primary endpoint | T1-4N0-2M0 | 33 | 31 |
Abbreviations: C, cyclophosphamide; E, epirubicin; F, 5-fluorouracil; HER2, human epidermal growth receptor 2; P, paclitaxel; pCR, pathological complete response; NCET, neoadjuvant chemo-endocrine therapy; NCT, neoadjuvant chemotherapy.
Overall, the present meta-analysis included a total of 566 participants, of whom 325 were in the neoadjuvant chemo-endocrine therapy (NCET) group and 241 were in the neoadjuvant chemotherapy (NCT) group.
The risk of bias for the 5 included studies was evaluated according to the Cochrane Risk of Bias Tool. Fig. 2 shows the details of the risk of bias of an eligible single trial. All 5 studies randomly allocated participants to the treatment groups, but 2 did not specify the exact randomization method utilized. Four studies provided registration information. In summary, the study design bias was regarded as moderate (Fig. 3).
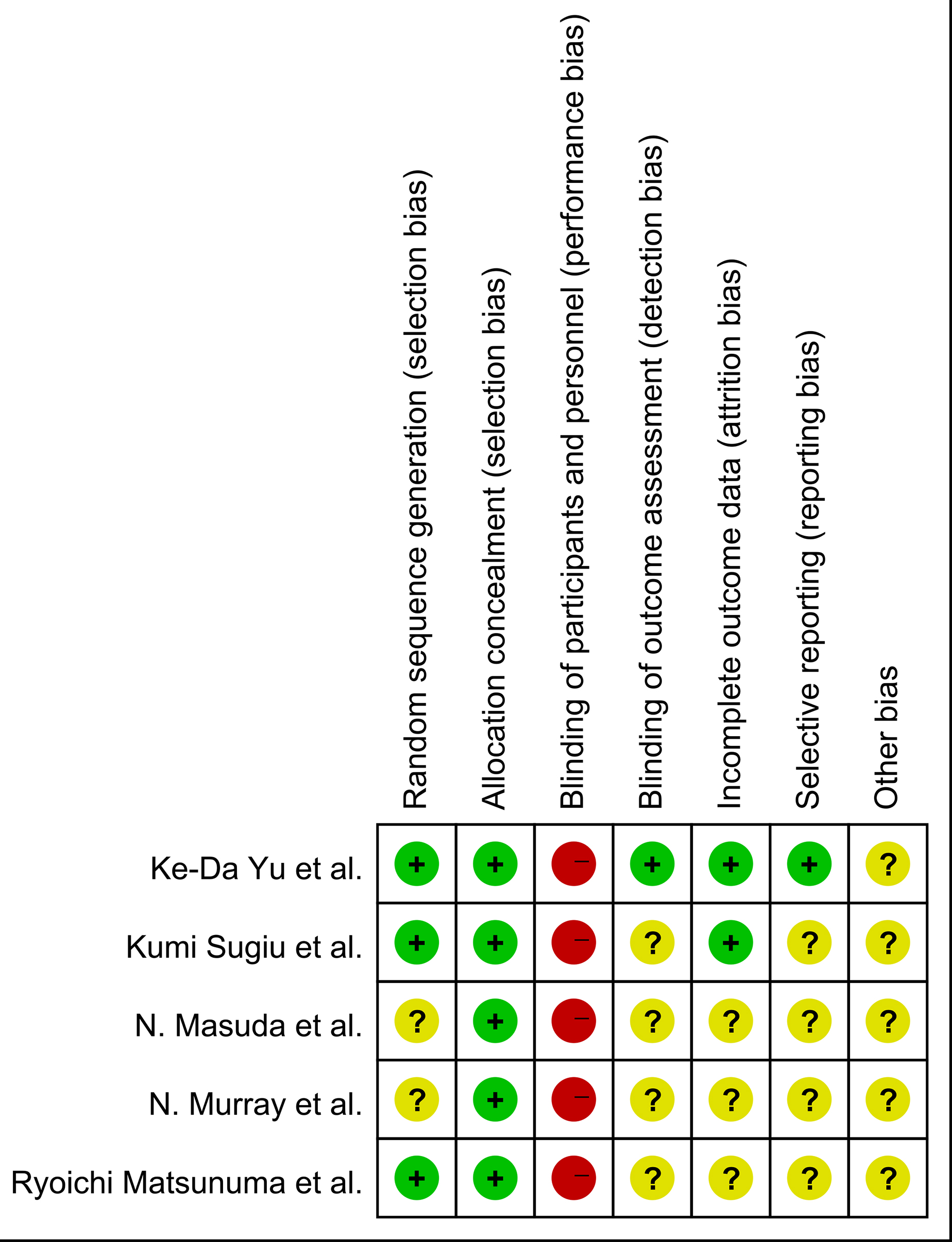 Fig. 2.
Fig. 2.Risk of bias summary.
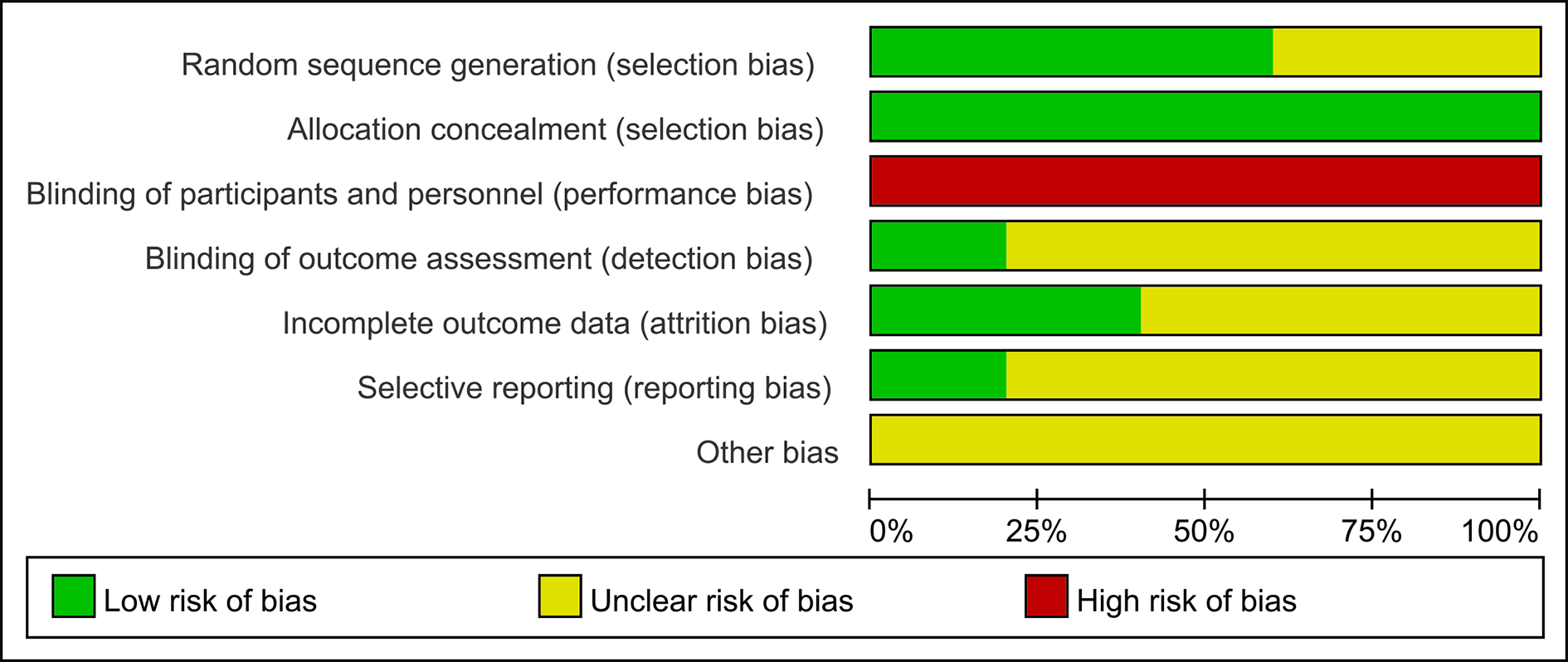 Fig. 3.
Fig. 3.Graphs of risk of bias.
Overall, including all the 5 studies, 70 of 566 (12.4%) patients achieved a pCR
after neoadjuvant treatment, while 47 of 325 (13.4%) patients in the neoadjuvant
chemo-endocrine therapy group and 23 of 241 (9.5%) patients in the neoadjuvant
chemotherapy group achieved a pCR. There was no significant statistical
differences (OR 1.35, 95% CI 0.77–2.38, p = 0.30) between the groups.
Studies had low heterogeneity (I
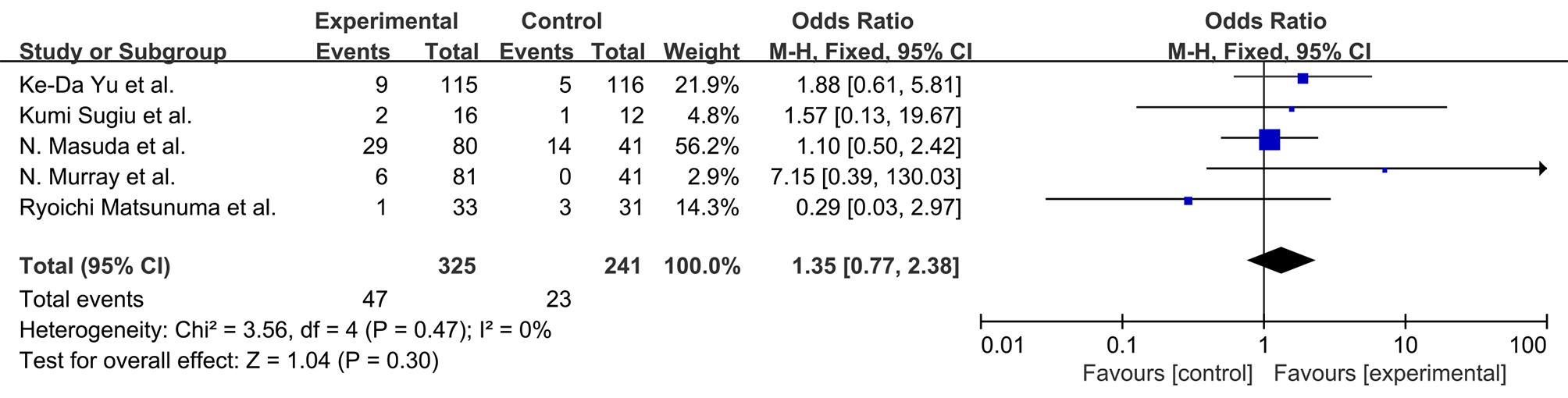 Fig. 4.
Fig. 4.Odds ratio for neoadjuvant chemo-endocrine therapy versus neoadjuvant chemotherapy.
The sensitivity analysis was conducted by excluding each study one at a time. As shown in Fig. 5, it demonstrated a stability of pooled OR estimates.
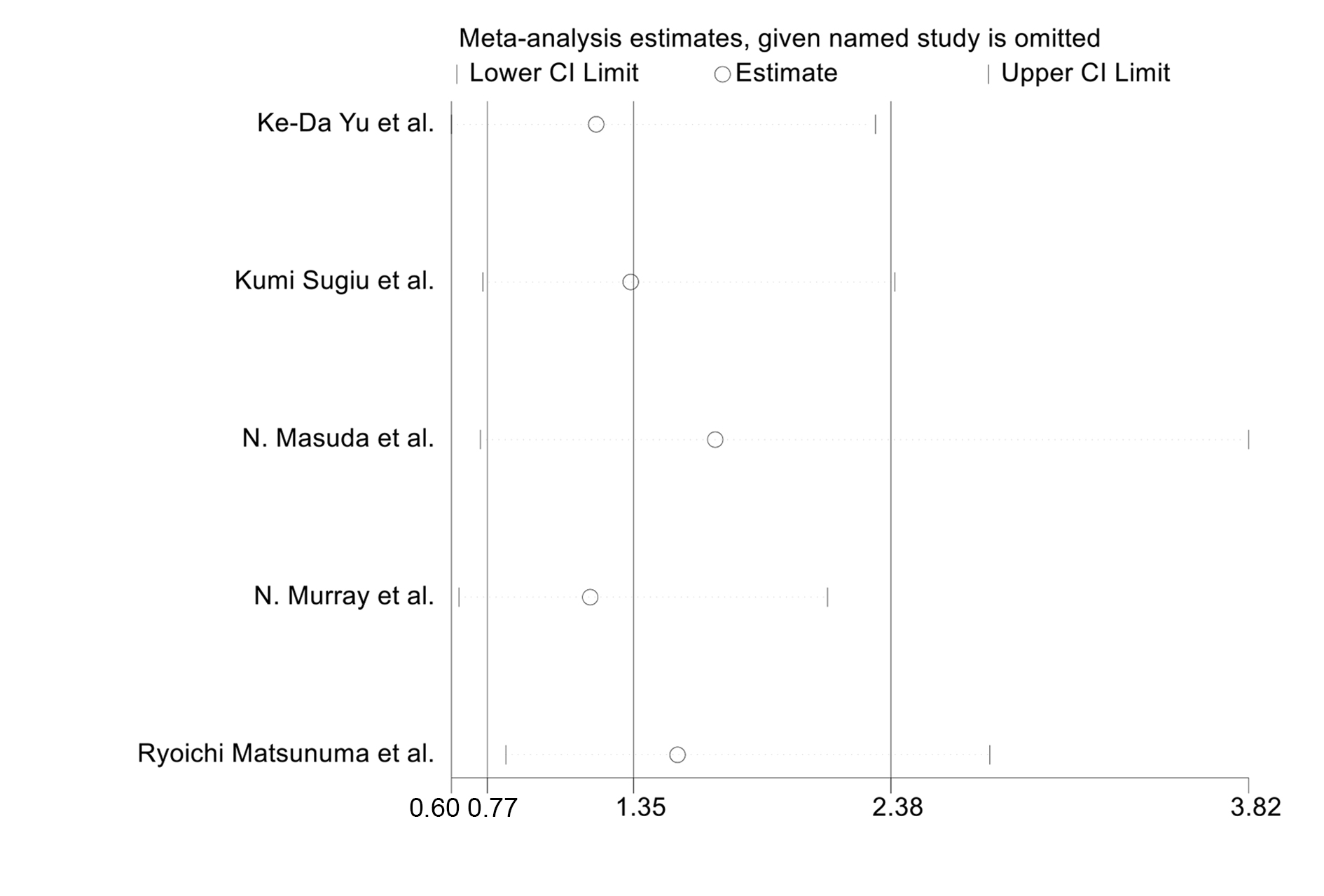 Fig. 5.
Fig. 5.Forest plot for sensitivity analysis.
As shown in Fig. 6, there was no significant publication bias (Egger’s test, p = 0.327).
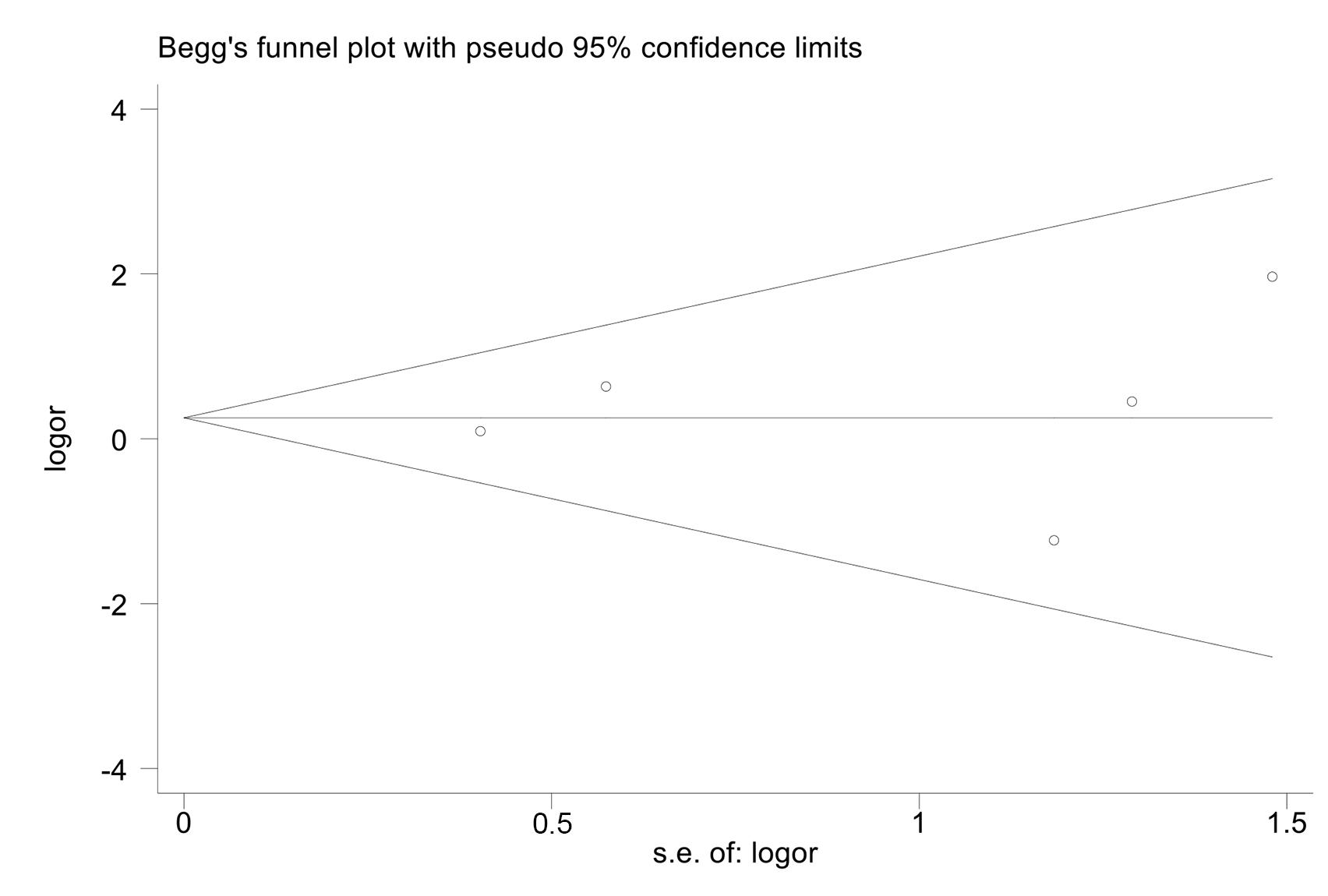 Fig. 6.
Fig. 6.Funnel plots for publication bias.
To assess the effects of concurrent neoadjuvant chemo-endocrine therapy for women with estrogen receptor positive breast cancer, we conducted a systematic review and meta-analysis. Our results revealed that concurrent neoadjuvant chemo-endocrine therapy did not significantly increase the pCR rate among these patients.
For breast cancer patients with hormone receptor positive tumors who need adjuvant chemotherapy, breast cancer guidelines recommend that the administration of adjuvant chemotherapy should not be concomitant with endocrine therapy. However, based on the current clinical data, the recommendation remains controversial [16, 17, 18]. In addition, tamoxifen was used as adjuvant endocrine therapy in the above studies, lacking data on aromatase inhibitors. This is the first meta-analysis assessing the efficacy of preoperative concurrent endocrine therapy with chemotherapy in estrogen receptor positive breast cancer. Endocrine therapy for breast cancer is mainly antiestrogen, which can slow down the transformation of the cell cycle by causing the delay of G1 phase transformation, thus leading to the accumulation of cells in G1 phase [19]. At the cost of S phase and G2M phase, it reduces the sensitivity of cells to S phase specific cytotoxic agents [20]. These considerations have led to the hypothesis that simultaneous endocrine therapy and chemotherapy may have an antagonistic drug interaction and therefore not further improve their efficacy. Several in-vitro and clinical studies have reported findings that support this opinion [8, 9, 12, 21]. Likewise, this meta-analysis suggests that patients with estrogen receptor positive breast cancer will not benefit from the addition of neoadjuvant endocrine to neoadjuvant chemotherapy. No significant increase in the pCR rate was observed (OR 1.35, 95% CI 0.77–2.38, p = 0.30) in this analysis.
There are important limitations to our meta-analysis. First, all of the 5 trials included in our meta-analysis were open-label. Second, the sample size is not large enough. Third, the use of pCR as a reliable surrogate for overall survival benefit remains controversial [22, 23]. Due to the lack of long-term follow up in these studies, it is not possible to determine whether concurrent neoadjuvant chemo-endocrine therapy will improve long-term outcomes, such as overall survival (OS) and disease-free survival (DFS). Fourth, this study included a small number of HER-2 positive patients, without consideration of its impact on targeted therapy. Fifth, we did not conduct an individual data meta-analysis. Sixth, the population included in this study was mainly from Asia. Therefore, future research should address the design of randomized controlled trials, such as strict blinding and concealment of allocation, have sufficient sample size and perform adequate long term follow-up.
From the data evaluated, although an improvement trend was noted, the administration of neoadjuvant chemotherapy concurrently with neoadjuvant endocrine did not improve the pCR rate in patients with estrogen receptor positive breast cancer. Nevertheless, further high-quality RCTs are necessary to support this conclusion.
ZYL designed the study, YLD and XZC conducted the selection of relevant studies and data extraction separately, ZZ and SSR evaluated the quality of each study independently, YLD and XZC performed the statistical analysis. ZZ and SSR drafted the manuscript. ZYL contributed to the interpretation of the results and critically reviewed the manuscript. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Not applicable.
We would like to express my gratitude to all those who helped me during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






