1 Gynecological Unit, Department of Surgical Sciences, San Marco Hospital, 95121 Catania, Italy
2 Gynecological Unit, Department of Surgical Sciences, ARNAS Garibaldi Hospital, 95123 Catania, Italy
3 Gynecological Unit, Department of Surgical Sciences, Valdarno Hospital, 52025 Montevarchi, Italy
4 Gynecological Unit, Department of Surgical Sciences, University of Rome “Tor Vergata'', 00133 Roma, Italy
Academic Editor: Valerio Gaetano Vellone
Abstract
Background: Bartholin’s gland cysts are one of the most frequent masses involving vulva. They may start as cysts without symptoms but if untreated, they may grow, leading to infection and consequent surgical treatment. Up to noe the giant and dumb-bell shaped presentation of Bartholin’s gland cyst is reported once in literature. Methods: We performed an online systematic literature review, using PubMed and Medline search to identify all listed publications around giant dumb-bell shaped Bartholin’s gland cyst larger than 10 cm. We identified a total of 3 cases. Of these 3 cases, 2 cases were giant Bartholin’s duct cyst and only 1 case a giant and dumb-bell shaped Bartholin cyst. We analyzed the diagnosis and the treatment of these 3 cases. Thus, we described our case of a giant and dumb-bell shaped Bartholin cyst and its treatment performed at the Department of Obstetrics and Gynecology of San Marco Hospital in Catania Sicily. After that, we first summarized the large Bartholin’s gland cyst hitherto reported and next we summarized the current knowledge of clinical handling of Bartholin’s gland cyst in general. Results: Apart from clinical examination, perineal ultrasound was very useful in diagnosis, because of providing more information concerning the dimensions, shape and extent of the cyst. The case presented with two large swellings with a main mass superiorly and a smaller one inferiorly and perineal ultrasound allowed us to realize that the two masses of the cyst were connected each other through a narrow neck. The ultrasound scan was useful also during the marsupialization of the cyst. Conclusions: In the current literature this type of giant and dumb-bell shaped presentation of Bartholin’s gland cyst is uncommon and has reported just one time. In addition we highlitght the utility of perineal ultrasound in the management of Bartholin’s gland cyst in order to define a right differential diagnosis between cystic and solid vulvar masses.
Keywords
- Bartholin's gland cyst
- dumb bell shaped
- giant
- ultrasound
The Bartholin’s glands are the greater vestibular glands. They may be single or bilateral and measure about 0.5 cm, located at the 5 o’ and 7 o’ clock positions at the base of the labia minora, with a 2 cm duct opening into the vestibule, slightly higher than the glands, between the hymen and labia minora at 4 o’ and 8 o’ clock position. The glands are located lateral to the bulbocavernosus muscle and receive blood supply from the external pudendal artery. They are innervated by the pudendal nerves and the lymphatic drainage includes superficial inguinal and pelvic nodes. In the absence of disease or infection the glands and ducts are typically impalpable [1, 2]. The epithelium of the gland and the duct is columnar and squamous respectively , allowing the possibility of squamous cell carcinoma or adenocarcinoma development [3].
The Bartholin gland is a mucus-secreting gland , important for vaginal lubrication [4]. Due to the presence of other glands, such as Skene glands, removal of a Bartholin gland does not impact on lubrication.
Two percent of women develop a Bartholin’s gland cyst or gland abscess in their lifetime, occurring more likely in sexually active women [3], and very rare in prepuberal age with only few reported cases in current literature [5]. Bartholin’s gland cysts measure typically 1 to 4 cm, but it can reach 8 to 10 cm of diameter.
Usually, Bartholin’ gland cyst is round or ovoid and irregular shape (such as, dumb-bell shape) is uncommon. This might lead to diagnostic dilemma when physicians encounter irregular (not round not ovoid) shape of cystic mass in the corresponding region. The present case is unique in that it was very large and it was dumb-bell shape.
A systematic review of the literature was performed to summarize the management of the large Bartholin’s gland cyst hitherto reported and next we summarize the current knowledge of clinical handling of Bartholin’s cyst in general (not confined to a gigantic or irregular shaped one). Furthermore, we highlight the utility of perineal ultrasound in the diagnosis and treatment of this condition.
In January 2022, we performed an online systematic literature review, using PubMed and Medline search to identify all listed publications using search terms established: (“giant Bartholin’s gland cyst” or “giant Bartholin cyst”) and (“dumb-bell shaped Bartholin cyst” or “dumb-bell shaped Bartholin gland cyst” or “atypical Bartholin cyst” or “large Bartholin cyst”). Using a combination of these proposed key words, we found a total of 48 articles.
The inclusion criteria were the presence of a giant Bartholin’s gland Cyst and the description of its treatment. The definition was a Bartholin’s gland cyst larger than 10 cm in the longitudinal diameter. The diagnosis was established based on the authors’ report. We selected the articles that included these factors. Two reviewers (F.G.M and S.C.) screened the titles and abstracts that pertained to our systematic review and selected 2 articles that included 3 cases. Of these 3 cases, 2 cases were giant Bartholin’s gland cyst and only 1 case was a giant and dumb-bell shaped Bartholin’s gland cyst. The data elements extracted from the articles included: (1) presentation of the case; (2) diagnostic management; (3) surgical treatment; (4) histopathological examination.
A 41-years-old, obese (Body Mass Index (BMI) 38; obesity class II) woman came to the Department of Obstetrics and Gynecology with chief complaint of a large swelling near the introitus in the left labial region, present and growing gradually in several years. She referred that initially it was small and painless and then gradually increased in size with pain and discomfort also during sitting or walking. Over the past years she underwent several incisions to drain the cyst.
The patient suffered from hypertension, depression and anxiety under therapy with vortioxetine, lorazepam, nebivolol and hydrochlorothiazide. Her obstetric history counts of three pregnancies, one miscarriage and two caesarean sections (the last one in 2011) and breastfeeding. She also underwent cholecystectomy in 2006.
On clinical examination, there were two masses located in the left labia majora extending from the level of pubic bones down to just above the vulvar fourchette inferiorly. The masses were tense with a smooth surface and no local warmth. It was subcutaneous, extending deep into tissue planes. Fluctuation was present. No enlargement of regional lymph nodes was noted.
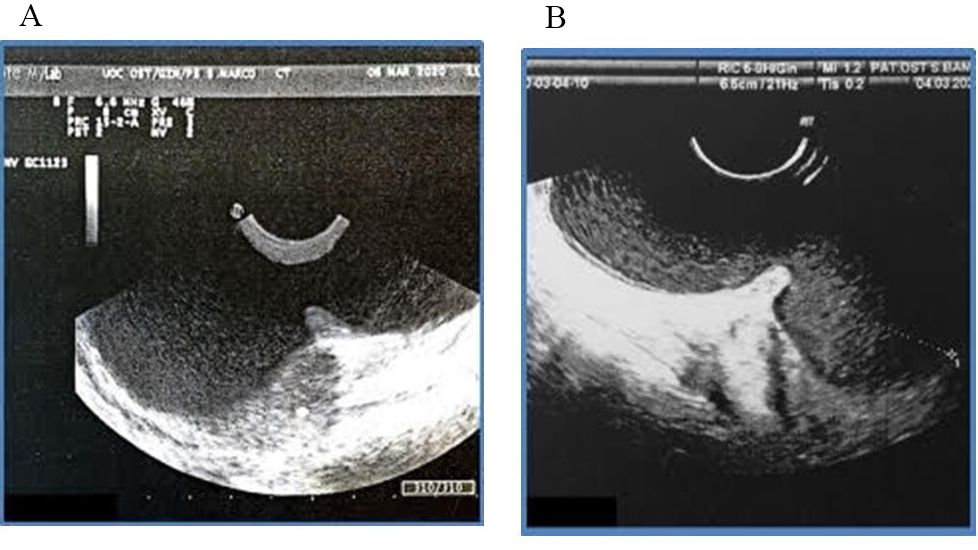
Perineal ultrasound (Fig. 1) was performed and showed evidence of two very large masses connected by a narrow neck of 10.6 cm in longitudinal diameter, configuring an unusual dumb-bell shaped presentation of Bartholin’s gland cyst. Cross fluctuation between them was noted.
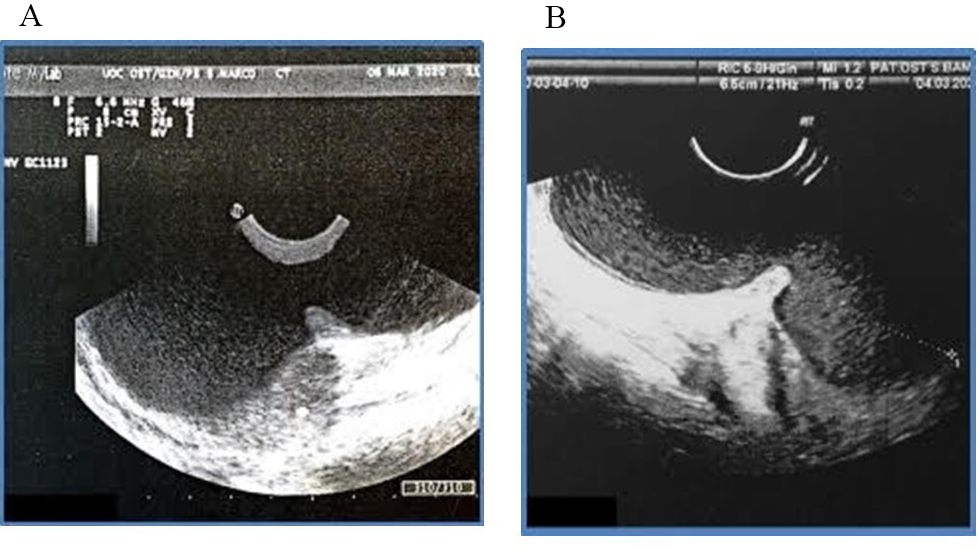 Fig. 1.
Fig. 1.Perineal Ultrasound scan with dumb-bell shape of the bilobar Bartholin’s cyst. (A) Perineal ultrasound of dumb-bell shape. (B) Longitudinal diameter of the bilobar Bartholin’s gland cyst.
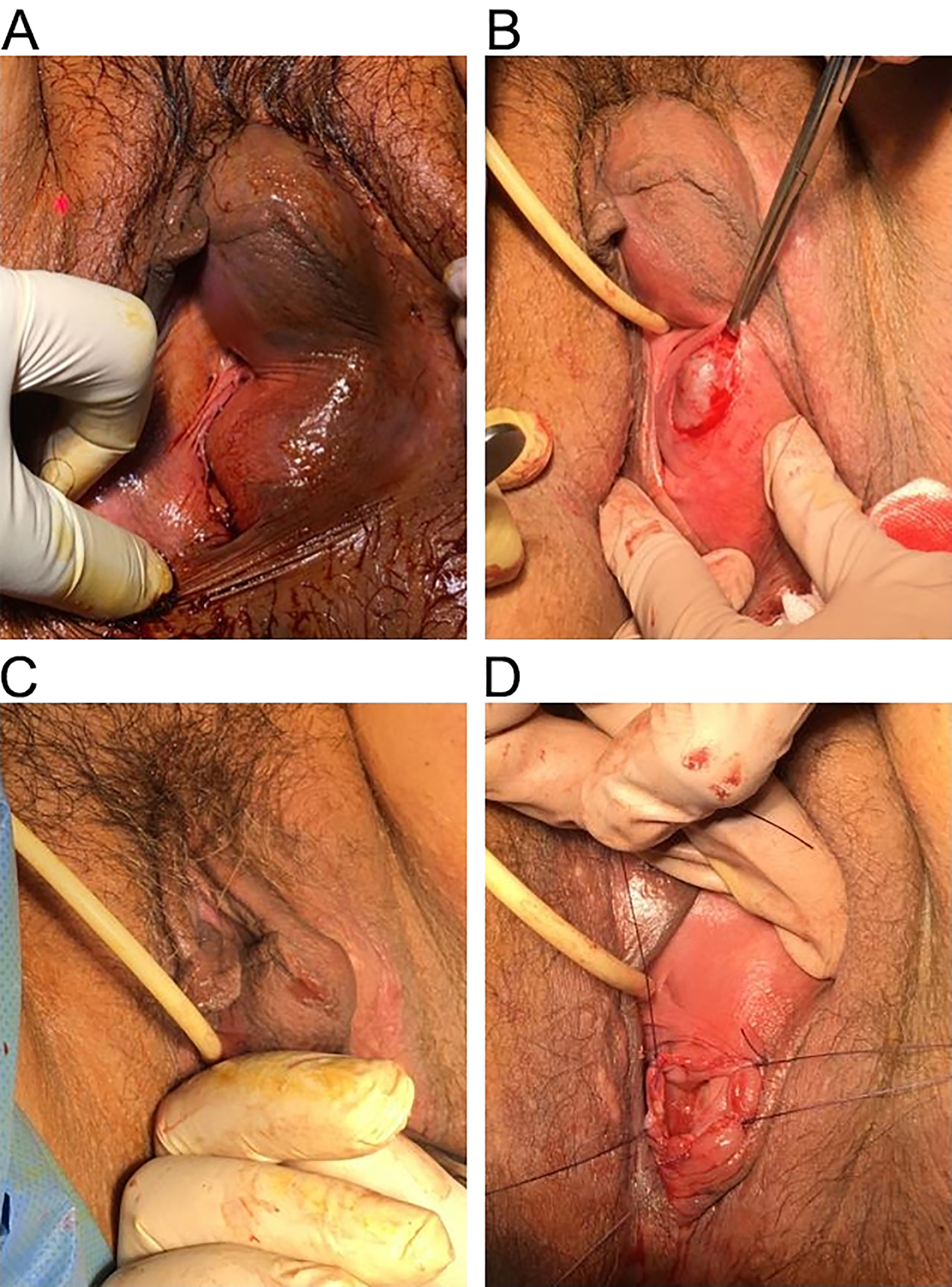
Diagnosis was made by clinical examination and the use of perineal ultrasound, which allowed us to confirm that the two masses were connected, figuring a dumb-bell shaped of the cyst. We took consent of the patient and planned her for surgery. Pre-operative antibiotic prophylaxis with cefazolin 2 gr was performed. Under spinal anesthesia, with patient in lithotomy position and a urethral Foley’s catheter in situ we everted the labia majora and performed a 3-cm vertical incision of the mucosa over the cyst near the hymenal ring. The cyst wall was grasped with small forceps to perform dissection on all sides of the cyst using scalpel and scissors in order to free the cyst of mucosa. So we completely opened the cyst and drained nearly 200 mL of thick brown colored fluid. Digital exploration of the bilobal cavity confirmed the connection between the two masses. We performed marsupialization of the cyst by everting the cyst wall and suturing its edges with the vestibular mucosa. and applied iodoformic gauze inside the empty cavity left by the cyst.
Steps of the marsupialization are illustrated in Fig. 2. Antibiotic coverage with amoxicillin-clavulanate 1 gr two times daily for 6 days was prescribed during the postoperative period . Postoperative period was uneventful and the patient recovered fast and was discharged on second postoperative day with satisfactory result. Removal of the iodoformic gauze, irrigation with saline solution and disinfection with hydrogen peroxide and replacement of a new iodoformic gauze was performed every day also after discharge for 6 days. The wound healed well and she was comfortable when she came for checkup. Up to date there was no report of recurrence in 1 year of follow up. We diagnosed this condition as benign Bartholin’s gland cyst (we denied the possibility of malignancy) and thus we did not perform specimen taking for pathological diagnosis.
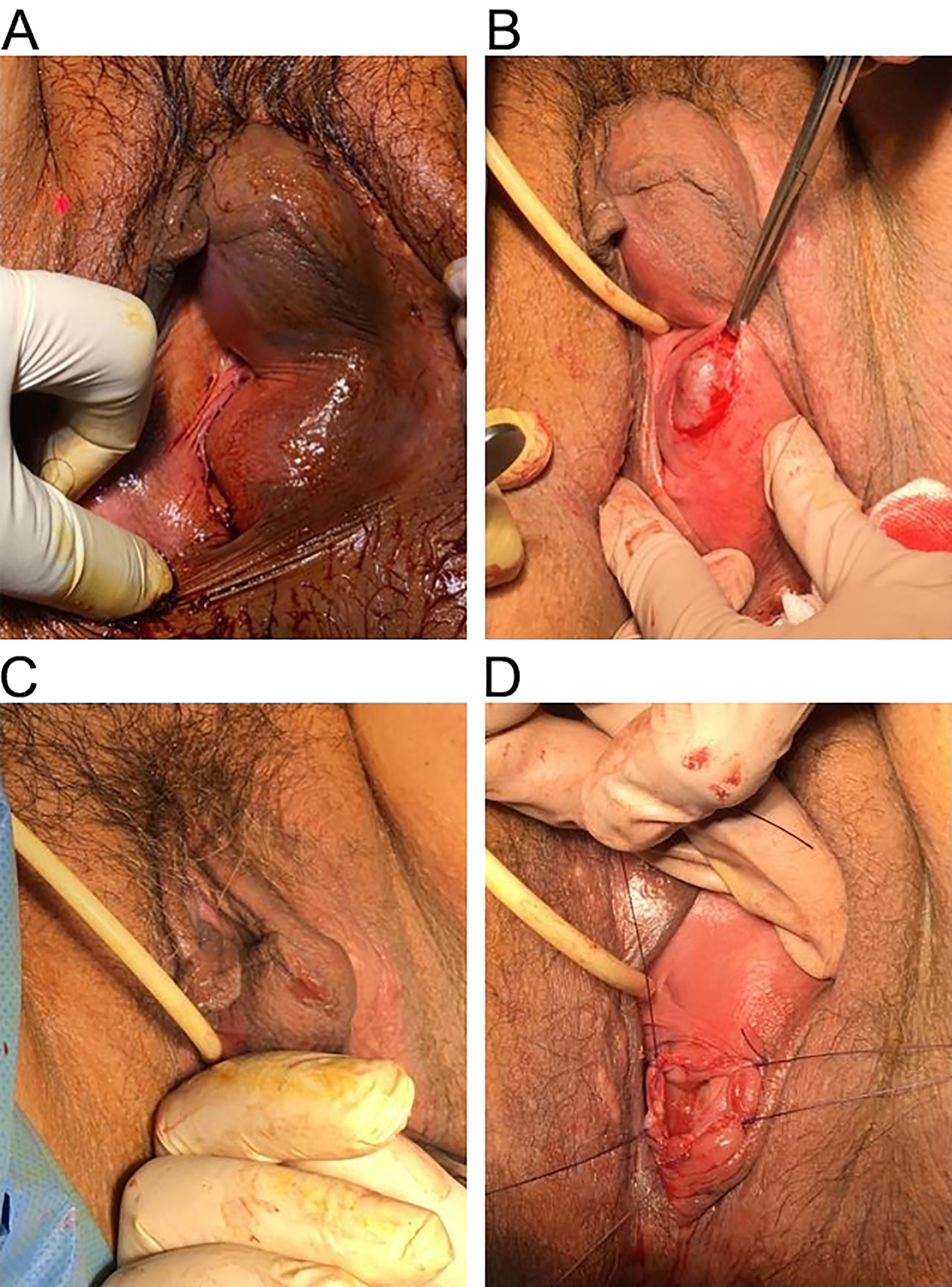 Fig. 2.
Fig. 2.Phases of marsupialization. (A) Pre-operative. (B) Vertical incision. (C) Digital exploration of the empty cavity. (D) Marsupialization.
In the current literature, only three cases of giant Bartholin’s gland cyst
larger than 10 cm in the longitudinal diameter were reported. In 2008 Kozawa
et al. [6] reported the first one case in a 63 old woman with a vulvar
mass on the left vulvar wall of 10.8
Bartholin’s gland cysts origins from dilatation of the duct, as consequence of blockage of the duct opening. This situation may lead to infection and abscess. Abscesses are three times more common than cysts [7]. A Bartholin’s duct cyst is not always an antecedent for a Bartholin’s duct abscess.
In this kind of abscess the following pathogens may be involved: S. faecalis, E. coli or S. aureus. Sexually transmitted infections like N. gonorrhea or C. trachomatis are rare. Instead, respiratory organism such as S. pneumoniae and H. influenzae are becoming more common, possibly related to the practice of oral sex. However, the majority of Bartholin’s gland abscesses contain polymicrobial vaginal flora, including anaerobic microbes [1, 3].
The involution of the gland begins by 30 years of age. For this reason, any increase in women older than 40 should pay attention in a suspicion of malignancy and biopsy is mandatoy in these cases, even if Bartholin’s gland cancers represent only 5% of vulvar carcinomas [3]. The highest incidence is among women of 60 years, usually adenocarcinoma and squamous cell carcinoma histotypes [2]. Probably due to rarity of Bartholin’s gland carcinoms, there is not unanimous consensus on treatment and guidelines are still on the way. In a review published in 2017 reporting a total of 275 cases the histological type of cancer were squamous cell carcinoma (30.7%), adenoid cystic carcinoma (29.6%) and adenocarcinoma (25.0%). At a multivariate analysis adenocarcinoma histotype and positive lymph node were associated with worse prognosis [8].
The diagnosis of a Bartholin’s gland cyst or gland abscesses origins from history and physical examination [1]. Cysts are usually asymptomatic, small and unilateral, without tenderness or signs of inflammation. They grow slowly, drain spontaneously and the fluid inside is free of infection. Sometimes women can present dyspareunia and discomfort or pain during walking or sitting [1]. Abscesses grow rapidly, are larger, more painful and present signs of inflammation, such as warmth, tenderness, edema, erythema and sometimes fever. When it grows to the upper labia it may drain spontaneously with sudden relief of pain [2]. A painless and fix mass in the vulvar region should raise suspicion of malignancy. In addition to physical examination, ultrasound and MRI may be performed [7].
There are many conditions that mimic Bartholin gland’s diseases, such as inclusion cysts, folliculitis, Gartner duct cysts, lipoma and carcinoma [9].
Management is guided by the cyst dimension, symptoms, patient’s age and history of recurrence [2]. Asymptomatic cysts in woman younger than 40 years can be left untreated, recommending only comfort measures like sitz baths, particularly for abscesses that tend to spontaneous rupture, good hygiene and analgesics and offering follow up in 2 to 4 days to determine if the condition is worsening. In the absent of sexually transmitted infections, urinary tract infection or cellulitis antibiotics are not necessary [1, 3]. Simple and quick methods of providing relief for the patient are needle aspiration and incision and drainage of the infected area followed by a suture closure, but these methods have a high recurrence rate. Antibiotic coverage is indicated in case of moderate to severe conditions, in adjunction to surgery, systemic infection and fever, cellulitis and in pregnant woman [2]. Antibiotics should cover staphylococcal, particularly methicillin-resistant Staphylococcus aureus, and Streptococcal species, as well as enteric Gram-negative aerobics, like Escherichia coli [4]. An example of antibiotic use in absent of a culture could be metronidazole 500 mg every 8 hours plus levofloxacin 500 mg every 24 hours for 7 to 10 days.
A conservative surgical method for treating Bartholin’s gland cysts and abscesses that can be performed in the office with a local lidocaine anesthesia is given by fistulization, introducing a foreign body which prevents wound closure and so creates a new outflow tract given by an epithelialized fistula that allows the lesion to continuously drain and heal from the inside. Fistulization is achieved by small and linear incision and drainage, with placement of a Word catheter or Jacobi ring. Purulent discharge evacuated may be sent to the laboratory for cultures and at this time also a biopsy may be performed. The balloon tip of the Word catheter is located in the abscess cavity and inflated with 3 to 5 mL of saline water and the end of the catheter is in vagina, remaining in place for 4 to 6 weeks to permit reepithelization [3, 4]. In a randomized clinical trial, the outcomes using Jacobi ring or Word catheter were comparable, but patient’s satisfactory were higher with the Jacobi ring [10].
Fistulization is not appropriate for deep cysts and is contraindicated in case of latex allergy, since the Word catheter and Jacobi ring are composed of latex [3, 4].
Marsupialization is the preferred treatment for recurrent lesions, but it is also appropriate for larger primary lesions. This procedure requires spinal, epidural or general anesthesia and is therefore done by a gynecologist or a surgeon in the operating room. An incision (from 1.5 to 3 cm) above the cyst wall is performed and its edges are sutured open with interrupted absorbable sutures to evert the cyst wall and promote continuous drainage and epithelization. Main complications of this procedure are hematoma, dyspareunia and infections, but the recurrence rate is low [1, 2, 3].
Inthe WoMan-trial, published in September 2016, 161 women were randomized to treatment with Word catheter or marsupialization. Primary outcome was recurrence rate of the cyst or abscess within 1 year of treatment. The secondary outcomes were pain during and after treatment and use of analgesics. Recurrence rate was 12% in Word catheter arm versus 10% in marsupialization arm. 24 hours after treatment, 33% used analgesics in the Word catheter group versus 74% in the marsupialization. The trial reported that treatment with Word catheter and marsupialization were comparable in recurrence rates [11]. Another study comparing Word catheter fistulization with marsupialization concluded that fistulization has a higher recurrence rate than marsupialization, but it is easier and more quickly to perform and also less expensive [12].
Sclerotherapy is another treatment possibility. It consist in chemical destruction of the epithelial lining of the lesion. Alcohol sclerotherapy had a healing time of 5 days vs. 10 days using silver nitrate [3]. In a randomized prospective study comparing marsupialization and silver nitrate sclerotherapy, both seem equally effective, but using silver nitrate less scar formation was reported [7].
Excision of the whole gland is recommended after recurrence and should be taken into account in patients older than 40 years [2, 3], even if this treatment may be complicated by hematoma, hemorrhage, damage to surrounding structures and a compromised moisture to the distal region of the vulva [11].
Hemorrhage is the primary complication of this method, so the Atlas of Pelvic Surgery call this tecnique the bloodiest little operation in gynecology. In a case report of 2017, preoperative transarterial embolization was performed before the excision of the gland with good results and a low estimated blood loss [13].
Carbon dioxide laser might be a less invasive method avoiding complications of
traditional surgery [2, 4]. In fact, this procedure may be done in the office,
consisting in an incision on the cyst and the vaporization of the cyst wall from
inside [1]. Cases of literature, report no scar formation and low recurrence
incidence [14, 15]. In a study in 2012 among 127 patients 86.6% needed a single
laser treatment [16]. Management with CO
Pregnant women should be treated the same way as non-pregnant woman with Bartholin abscess, with the exception of gland excision due to the increased risk of bleeding [4].
Immunocompromised women, such those with diabetes mellitus or HIV, pregnant women or those taking high dose steroid, are at greater risk of cellulitis and necrotizing fasciitis. Therefore, they should be admitted in an inpatient setting for management and close observation [1].
In presence of unusual case of Giant Bartholin’s gland it’s important to exclude malignancies and to perform the right management considering the conservative surgical approach as the marsupialization, especially in young women. Ultrasound evaluation is very useful to evaluate the presence of solid lesions, the vascularization and the consequently risk of malignancy. Therefore, is useful to evaluate the real size and the deep of the lesion and planning the appropriate surgical treatment. Marsupialization can be the preferred treatment for larger primary lesions because preserves the function and reduces the damage of the surrounding structures.
Although giant Bartholin gland cysts are reported in some case reports, they remain rather rare conditions and dumb-bell shaped presentation has only been reported once in the whole literature. In our case we performed diagnosis through physical examination and perineal ultrasound, which was fundamental to discover the connection between the two masses. In addition perineal ultrasound evaluation is very useful to perform a right differential diagnosis between cystic and solid vulvar lesions. In case of suspicion of malignancy, surgical excision is recommended to obtain histopathological examination. In our case we decided to perform marsupialization on basis of prior ultrasound evaluation that excluded malignancy risk factors. We performed the surgical treatment under antibiotic coverage to reduce the recurrence of the cyst with good outcomes.
FG, SS and FGM designed the research study. EL and AT performed the research. FAG, FCan and FCat analyzed the data. MAP and SC wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors contributed equally. All authors read and approved the final manuscript.
The enrolled patient gave her informed consent before the clinical treatment to allow the use of her data. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of San Marco Hospital under number of register 00128/2020).
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.