1 Department of Ultrosound, West China Second University Hospital, Sichuan University, 610000 Chengdu, Sichuan, China
2 Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, 610000 Chengdu, Sichuan, China
3 Department of Gynaecology and Obstetrics, West China Second University Hospital, Sichuan University, 610000 Chengdu, Sichuan, China
Academic Editor: Paolo Ivo Cavoretto
Abstract
Background: Intrahepatic cholestasis of pregnancy (ICP) is a disorder
specifically associated with pregnancy. Recent evidence suggests that the T
helper 17 (Th17) cell population is related to a maternal and foetal immune
imbalance associated with ICP. However, there has been insufficient attention
paid to the potential roles of signal transducer and activator of transcription 3
(STAT3) and RAR-related orphan receptor gamma (ROR
Keywords
- correlation
- intrahepatic cholestasis of pregnancy
- signal transducer and activator of transcription 3
- T helper cell-17
During pregnancy, intrahepatic cholestasis (ICP) causes excessive pruritus without a rash and elevations of total bile acids (TBAs) [1]. Incidences of ICP vary by geographic location and ethnicity, ranging from 0.2% to 2% [2]. After delivery, the mother’s symptoms usually disappear, whereas foetal exposure to ICP results in perinatal and long-term complications, including premature delivery, neonatal respiratory distress syndrome, sudden intrauterine foetal demise, stillbirth, and metabolic syndrome later in life [3, 4]. However, the aetiology of ICP remains unclear. Researchers recently found that the placenta’s core genes are largely responsible for regulating immune function; expression of these genes is upregulated in mild ICP, and in severe cases the expression is further increased, which indicates immune system might play a major role in ICP [5]. Recently, the T helper 17 (Th17) cell in pregnant women has received attention. ICP patients’ peripheral blood and placentas were reported to be significantly elevated in interleukin (IL)-17, a cytokine of Th17 cell [6, 7].
There are both positive and negative impacts on the immune system caused by Th17
cell, a relatively new subset of CD4
Women were recruited from August 2020 to October 2020 at West China Second Hospital, affiliated with Sichuan University. Our study was approved by the hospital’s ethics committee. Clinical information and samples were anonymized for statistical workup. Pregnancy healthcare profiles were created for all patients and they underwent routine pregnancy checks at our hospital. In the experimental and control groups were nine patients with singleton ICP and nine patients with normal-term singleton pregnancy,respectively. Delivery of all patients in both groups was by caesarean section. Based on Chinese Medical Association’s “Guidelines for diagnosis and treatment of intrahepatic cholestasis of pregnancy (2015)”, ICP was diagnosed.
Inclusion criteria for ICP group included the following: elevated TBA or aminotransferase; signs of prutitus or jaundice; no history of skin disease, gallstones, cholecystitis, liver cirrhosis, autoimmune diseases, acute and chronic infectious diseases, no history of infection with hepatitis virus, Epstein Barr virus, and cytomegalovirus; without any other obstetric complication. For the control group, the following criteria were used: normal liver or renal function; no history of gallstones, cholecystitis, liver cirrhosis, autoimmune diseases, acute and chronic infectious diseases, without history of infection with hepatitis virus, Epstein Barr virus, cytomegalovirus are not permitted; without any obstetrics complication.
Before the operation, 2 mL elbow venous blood was collected.Placental tissue was
collected within 5 min of delivery. We incubated the paraffin-embedded placental
sections with primary antibodies: anti-STAT3 (1:150), anti-IL-17A (1:200)
(Proteintech Group, Chicago, IL, USA), and anti-ROR
We homogenized placental tissues in ice-cold lysis buffer. Protein were
subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membranes (STAT3: 100 V, 90 min; ROR
The whole blood was diluted using RPMI 1640 medium and incubated with stimulant
and blocker in turn. The blood was then incubated for 5 h at 37 °C with
5% CO
Analysis of the data were conducted using IBM SPSS 23 software and R version
4.0.4 (IBM corp., Armonk, NY, USA). For the quantitative variables, the results
were presented as the mean value
Comparison of clinical and obstetric characteristics between groups is provided
in Table 1 and the comparison of biochemical indicators is provided in Table 2.
Regarding clinical and obstetric data, both groups showed some similar
characteristics. However, significant differences in gestational days, foetal
weights and ratio of placental weight and neonatal weight were noted in. ICP was
associated with earlier gestational days, lower neonatal weights and higher ratio
of placental weight and neonatal weight, which may reflect impaied placenta
function. A few maternal serum biochemical parameters did not differ
significantly between the two groups. However, there were much higher levels of
maternal serum alanine aminotransferase (ALT), aspartate aminotransferase (AST),
TBA, direct bilirubin (DBIL), and alkaline phosphatase in the ICP group than in
the control group (all p
| Characteristic | Control (n = 9) | ICP (n = 9) | t | p |
| Age (years) | 32.33 |
31.00 |
–0.81 | 0.43 |
| Weight gain (kg) | 12.78 |
12.89 |
0.06 | 0.95 |
| Body mass index (cm/kg |
26.30 |
25.86 |
–0.36 | 0.72 |
| Gravidity | 2.22 |
2.11 |
–0.27 | 0.79 |
| Parity | 1.67 |
1.44 |
0.70 | 0.37 |
| Gestational day (day) | 274.89 |
262.22 |
–4.3 | 0.02* |
| Neonatal weight (g) | 3216.67 |
2922.67 |
–1.71 | 0.03* |
| Umbilical artery S/D ratio | 2.20 |
2.45 |
1.39 | 0.13 |
| Placental weight (g) | 560.78 |
563.56 |
0.11 | 0.92 |
| Placental weight/neonatal weight | 0.19 |
0.17 |
2.88 | 0.02* |
| 10 min Apgar score | 10 |
10 |
1.00 | |
| History of ICP | 0% (0/9) | 33.3% (3/9) | 0.21 | |
| Preterm birth | 0% (0/9) | 33.3% (3/9) | 0.21 | |
| Intrauterine foetal stress | 0% (0/9) | 1.1% (1/9) | 1.00 | |
| Meconium-stained amniotic fluid | 0% (0/9) | 11.1% (1/9) | 1.00 | |
| Neonatal intensive care unit | 0% (0/9) | 0% (0/9) | 1.00 | |
| Perinatal death | 0% (0/9) | 0% (0/9) | 1.00 | |
| Note: * Compared with those in the control group,
gestational age, foetal weight and placental weight/neonatal weight were
significantly decreased (p Abbreviations: S/D, ratio of maximum systolic flow velocity (S) to end diastolic flow velocity (D) of umbilical artery. | ||||
| Indicator | Control (n = 9) | ICP (n = 9) | t | p |
| Alanine aminotransferase (U/L) | 14.67 |
196.33 |
3.03 | 0.01* |
| Aspartate aminotransferase (U/L) | 17.89 |
127.44 |
2.86 | 0.02* |
| Total bile acid ( |
1.98 |
43.32 |
4.76 | |
| Total bilirubin ( |
9.84 |
12.94 |
1.54 | 0.14 |
| Indirect bilirubin ( |
7.98 |
7.89 |
–0.09 | 0.93 |
| Direct bilirubin ( |
1.86 |
5.05 |
2.53 | 0.03* |
| 14.67 |
42.22 |
2.13 | 0.06 | |
| Albumin (g/L) | 38.27 |
38.14 |
–0.13 | 0.90 |
| Globulin (g/L) | 28.49 |
28.60 |
0.08 | 0.94 |
| Alkaline phosphatase (U/L) | 107.62 |
198.00 |
2.89 | 0.02* |
| Lactate dehydrogenase (U/L) | 178.33 |
200.44 |
1.15 | 0.27 |
| Fasting plasma glucose (mmol/L) | 4.30 |
4.42 |
0.54 | 0.62 |
| Prealbumin (mg/L) | 226.78 |
207.11 |
–1.12 | 0.26 |
| Note: * Compared with those in the control group, TBA,
ALT, AST, and DBIL levels in the ICP group were significantly higher (p | ||||
In the placenta, cytotrophoblasts and syncytiotrophoblasts were the main sites
of STAT3, ROR
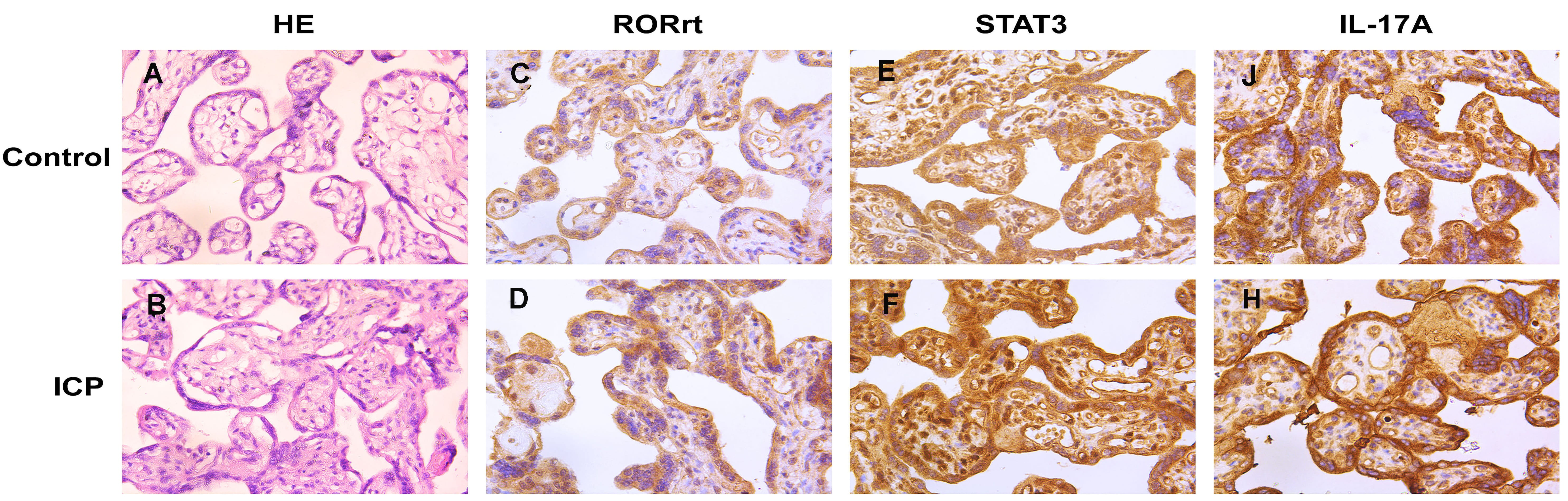 Fig. 1.
Fig. 1.STAT3, ROR
Comparatively to the control group, placental levels of STAT3, ROR
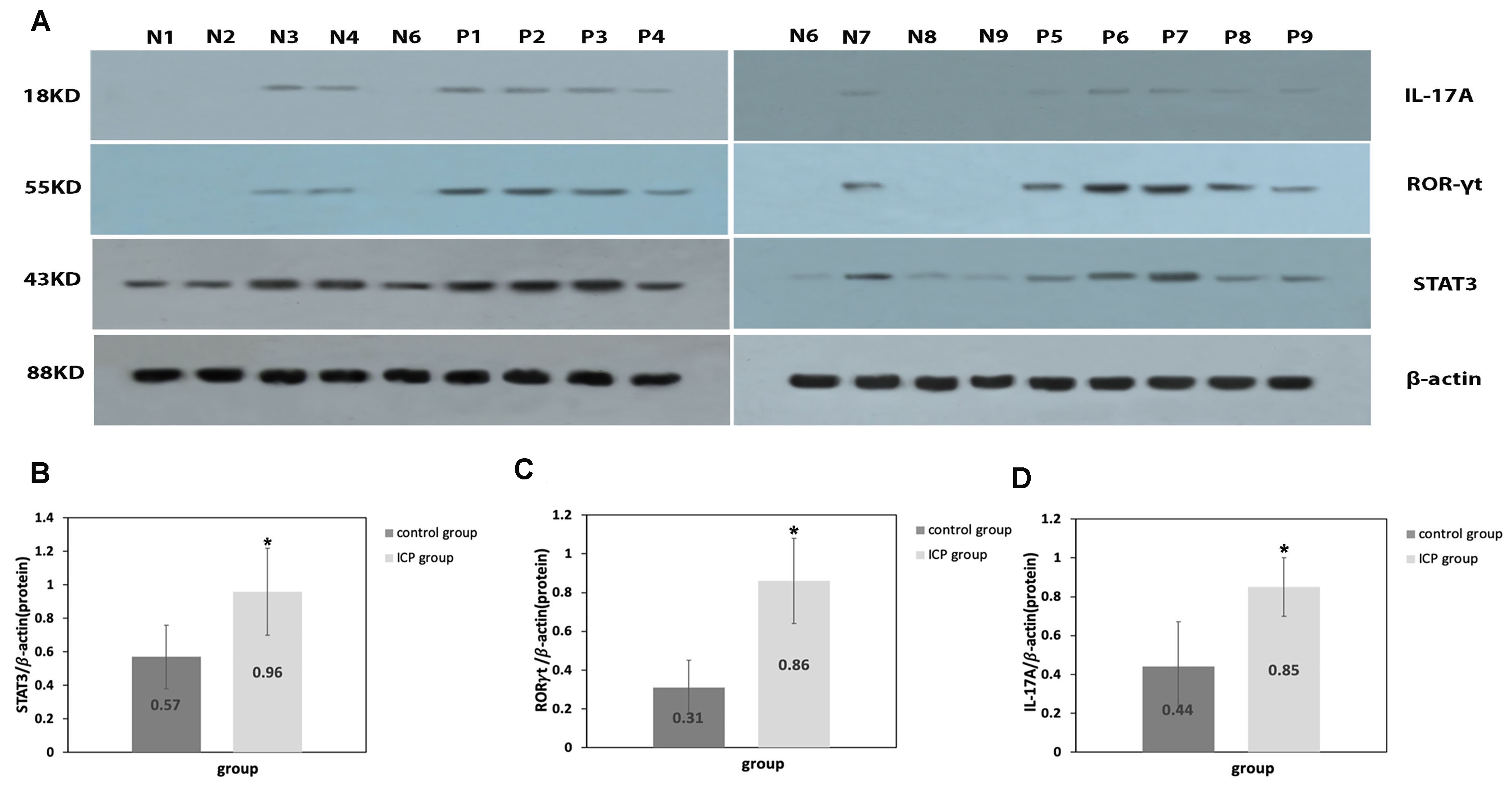 Fig. 2.
Fig. 2.Protein expression levels of IL-17A, ROR
Flow cytometry showed that comparatively to the control group, the proportion of Th17 cell in the peripheral blood was significantly higher in ICP group (p = 0.01; Fig. 3).
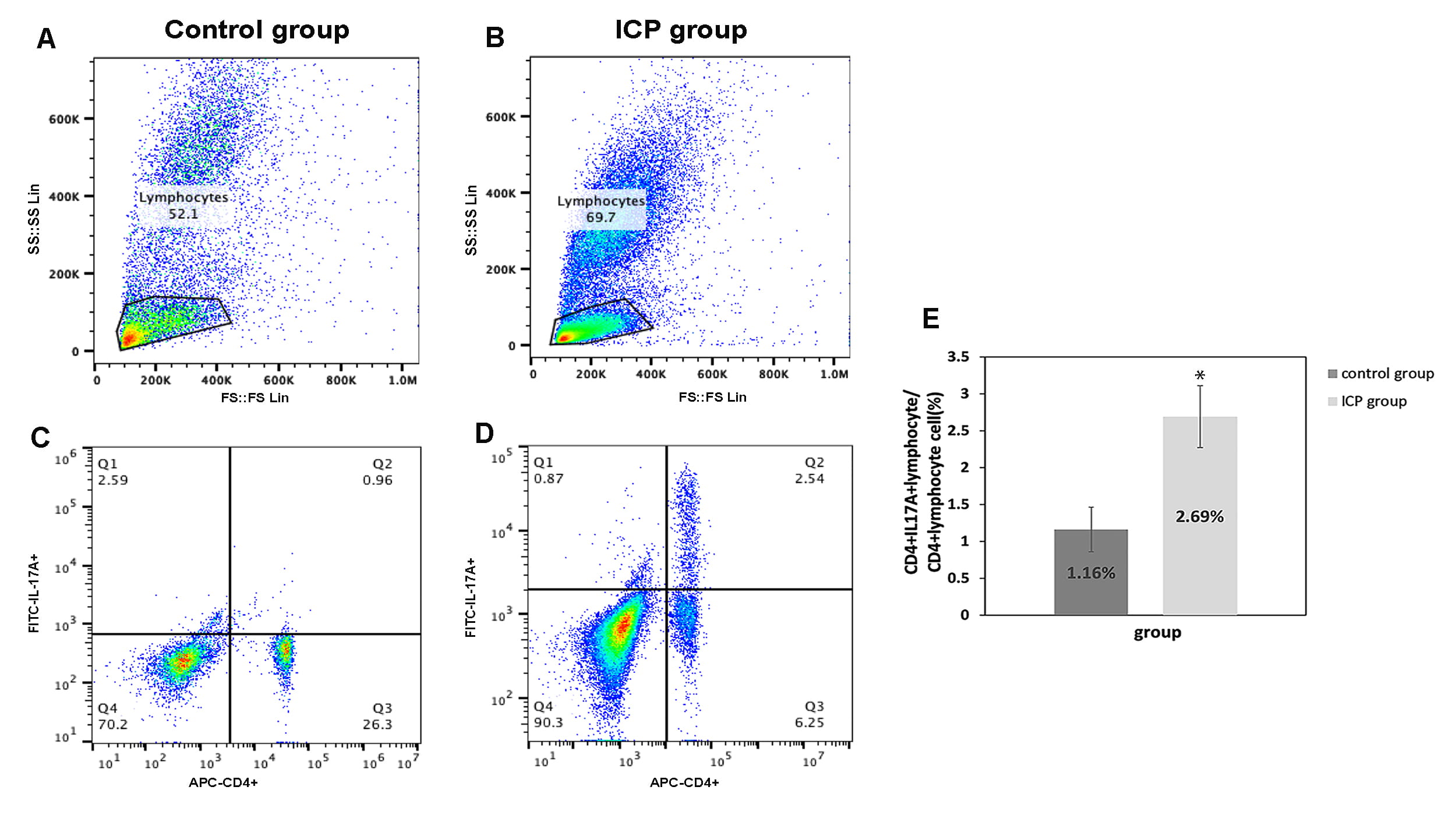 Fig. 3.
Fig. 3.The proportion of CD4
Data of maternal peripheral blood Th17 cell and placental STAT3,
ROR
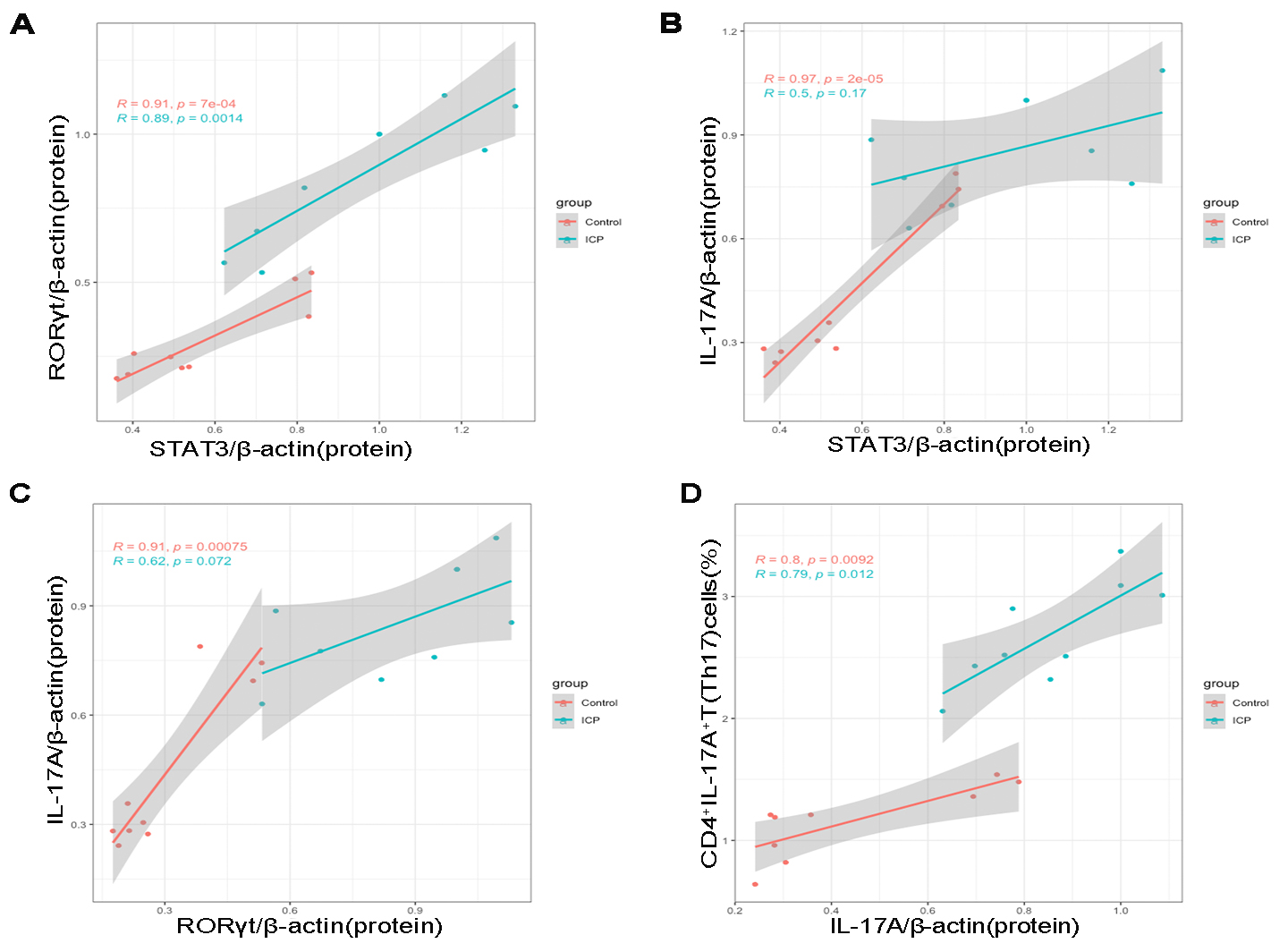 Fig. 4.
Fig. 4.Pearson correlation scatterplots of placental STAT3, ROR
The correlation matrix was constructed by testing Pearson parametric
correlations among maternal serum biochemical indices related to hepatic and
biliary parameters, matrnal peripheral blood Th17 cell; and levels of placental
STAT3, ROR
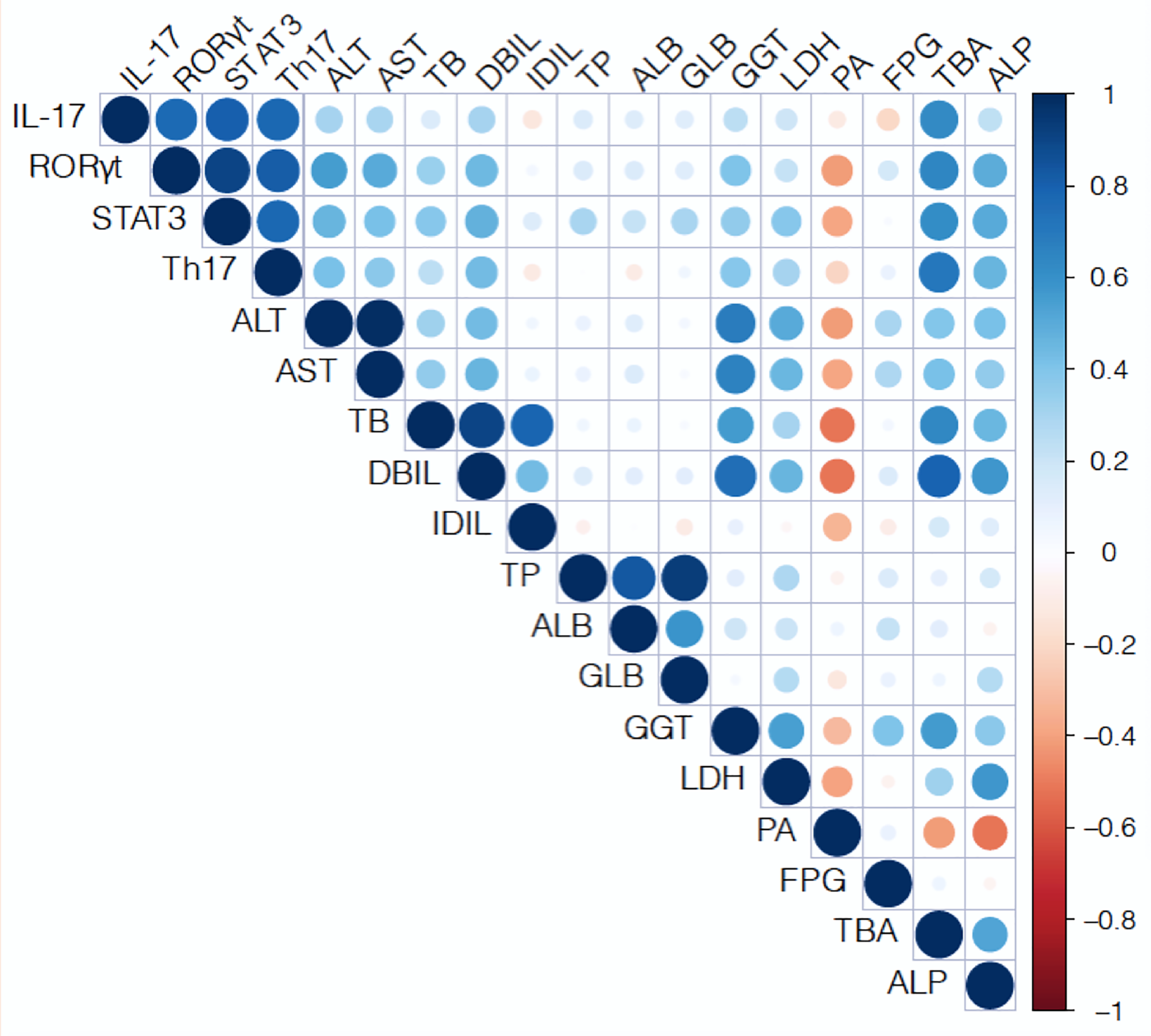 Fig. 5.
Fig. 5.Pearson correlation matrix of peripheral blood Th17 cell, and
levels of placental STAT3, ROR
Our study highlights the alters of STAT3, ROR
As a key player that defends against external pathogens and mediates immune
rejection in the host, Th17 cell are vital in mediating maternal-foetal immunity.
Recent studies have shown that an increased Th17 cell count is associated with
ICP as it disrupts the delicate immunity and tolerance balance at the
maternal-foetal interface [6, 7]; however, the mechanism underlying
differentiation and functional regulation of Th17 cell in ICP remains unclear. We
found that STAT3/ROR
STAT3 is indispensable for the differentiation of Th17 cell. Our study revealed
that STAT3 protein was distributed in the nucleus and cytoplasm of trophoblasts.
Shochet et al. [18] found that STAT3 promoted the invasion of
trophoblast cell and facilitated embryo implantation in primary pregnancy.
Additionally, Ye et al. [19] demonstrated that STAT3 is mostly located
in trophoblasts and ICP participants have higher STAT3 levels compared to
controls.Our study demonstrated increased expression levels of STAT3 protein in
the placenta of the ICP group, which is consistent with earlier studies. STAT3
can regulate Th17’s differentiation via directly binding to the ROR
ROR
IL-17 is an important inflammatory mediator of immunity and several studies have
focused on its role in ICP. Ren et al. [6, 25] found
that IL-17 levels in the decidual tissue and
peripheral blood of ICP patients were significantly elevated compared with
healthy pregnancy; they positively correlated with serum TBA. However, Kirbas
et al. [17] found serum IL-17 level was significantly increased in ICP
patients than normal pregnant patients, but no significant relationship between
IL-17 and TBA was abserved. Based on these results, no definite conclusion could
be reached on the correlation between IL-17 and TBA ICP patients. We found that
placental IL-17A protein levels and peripheral blood Th17 cell levels were
significantly higher in ICP group than control group. Our results are similar to
previous studies [6, 25]. Additionally, our study also revealed positive
correlations among maternal serum TBA, DBIL, AST, and ALT and placental STAT3 and
ROR
Bile acid can induce the production of inflammatory mediators like IL-23 [30], and IL-17 can promote the these inflammatory mediators production, which in turn induces Th17 cell expansion through a positive feedback loop [31]. IL-17 could act on bile duct epithelial cells and promot e the Th17 cell differentiation and aggregation. Th17 cells around the bile duct, which may interfere with bile acid metabolism and induce ICP [30]. Moreover, the expansion in Th17 cell might further aggravate the inflammatory response at the maternal-foetal interface [32]. We found significant positive correlations between placental IL-17A protein and the proportion of Th17 in both the ICP and control groups. These results indicated the proportion of peripheral blood Th17 could reflect immune activity at the maternal-foetal interface.
Our study revealed ICP patients were associated with earlier gestational days, lower neonatal weights and higher ratio of placental weight and neonatal weight comparing with normal pregnancy. The difference in gestational days may affected the comparisons’ accuracy of Th17 cell between normal and ICP groups. However, previous and recent studies both found Th17 cell count keep stable during all pregnancy [33, 34]. Additionlly, the mean gestational days in both groups was over 37 weeks. So the gestational days difference may has little effect on Th17 cells in our study. The difference of ratio of placental weight and neonatal weight in two group may reflect impaied placenta function,which need further study.
Our study presents some limitations. We studied the expression levels of
STAT3/ROR
In summary, our data indicated that with an increase of Th17 cells, the roles of
STAT3 and ROR
YXW is responsible for research performing and manuscript writing; YYH is responsible for the design of the research study; QC is responsible for the data analysis; QHC and DS is responsible for the help and advice. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was approved by the Ethics Committee of West China Second Hospital, Sichuan University, Chengdu, China and the Ethics board number is 2020-67. All patients signed an informed consent form.
We are very grateful to the patients and staff participated in this study.
This work was supported by Applied Research in China Sichuan Province [grant number 2022YFS0043]; the Applied Research in China Sichuan Province [grant number 2020CDZG-23]; Clinical research fund of West China Second University Hospital [grant number KL107].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





