1 Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, Seoul Medical Center, 02053 Seoul, Republic of Korea
2 Department of Obstetrics and Gynecology, Ilsan Paik Hospital, InJe University College of Medicine, 10380 Goyang, Republic of Korea
3 Department of Pediatrics, Ilsan Paik Hospital, InJe University College of Medicine, 10380 Goyang, Republic of Korea
4 Division of Biology and Public Health, Mokwon University, 35349 Daejeon, Republic of Korea
5 Department of Emergency Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University College of Medicine, 05278 Seoul, Republic of Korea
Academic Editor: Michael H. Dahan
Abstract
Background: Preterm premature rupture of membranes (PPROM) is a major
cause of preterm birth. There are few reports on vitamin D deficiency associated
with PPROM. We aimed to investigate the association between PPROM and vitamin D
levels in maternal plasma and the umbilical cord blood of newborns.
Methods: This prospective study included 355 pregnant women who
delivered live infants between March 2017 and December 2018 at a medical center.
Vitamin D levels were measured in the maternal plasma at the first, second, third
trimesters of pregnancy and just before delivery, and in the umbilical cord blood
of newborns at birth. In addition, we evaluated the pregnancy and neonatal
outcomes according to vitamin D status. Results: The rate of PPROM in
the vitamin D deficiency group (25(OH)D
Keywords
- preterm premature rupture of membranes
- vitamin D deficiency
- cord blood
- inflammation
- oxidative stress
Preterm premature rupture of membranes (PPROM) is defined as a spontaneous rupture of the membranes during pregnancy before 37 weeks’ gestation and at least 1 h before the onset of contractions [1]. The incidence of PPROM ranges from 3.0% to 10.0% of all deliveries. Approximately 40% of PPROM cases result in preterm delivery, contributing significantly to increased neonatal morbidity and mortality [2, 3, 4]. The main causes of PPROM are infection/inflammation, decidual bleeding, uterine overdistention (e.g., polyhydramnios, twins), genetic predispositions, and smoking [5]. The risk factors for PPROM are generally similar to those for preterm birth (PTB), including preterm spontaneous labor with intact membranes [6]. The known risk factors for preterm birth are short cervical length, polyhydramnios, PPROM, assisted reproductive technologies (ART) procedure, prior PTB, pregnancy induced hypertension, placenta previa, placental abruption, fetal growth restriction, urinary tract infections, complex autoimmune diseases with polytherapy, maternal anxiety, obesity, and malnutrition [7, 8, 9, 10]. There are a few reports on maternal vitamin D insufficiency or deficiency associated with PTB [11, 12, 13]. Woo et al. [14] reported a positive association between vitamin D deficiency and PTB. In contrast, a prospective cohort study reported no significant difference in vitamin D levels between the PTB group and full-term delivery group [15], this association remains conflicting. However, there are few reports that have studied the relationship between PPROM and vitamin D deficiency so far. Therefore, we investigated the association between PPROM and vitamin D levels in maternal plasma and umbilical cord blood of newborns with possible circumstances excluding factors affecting PTB.
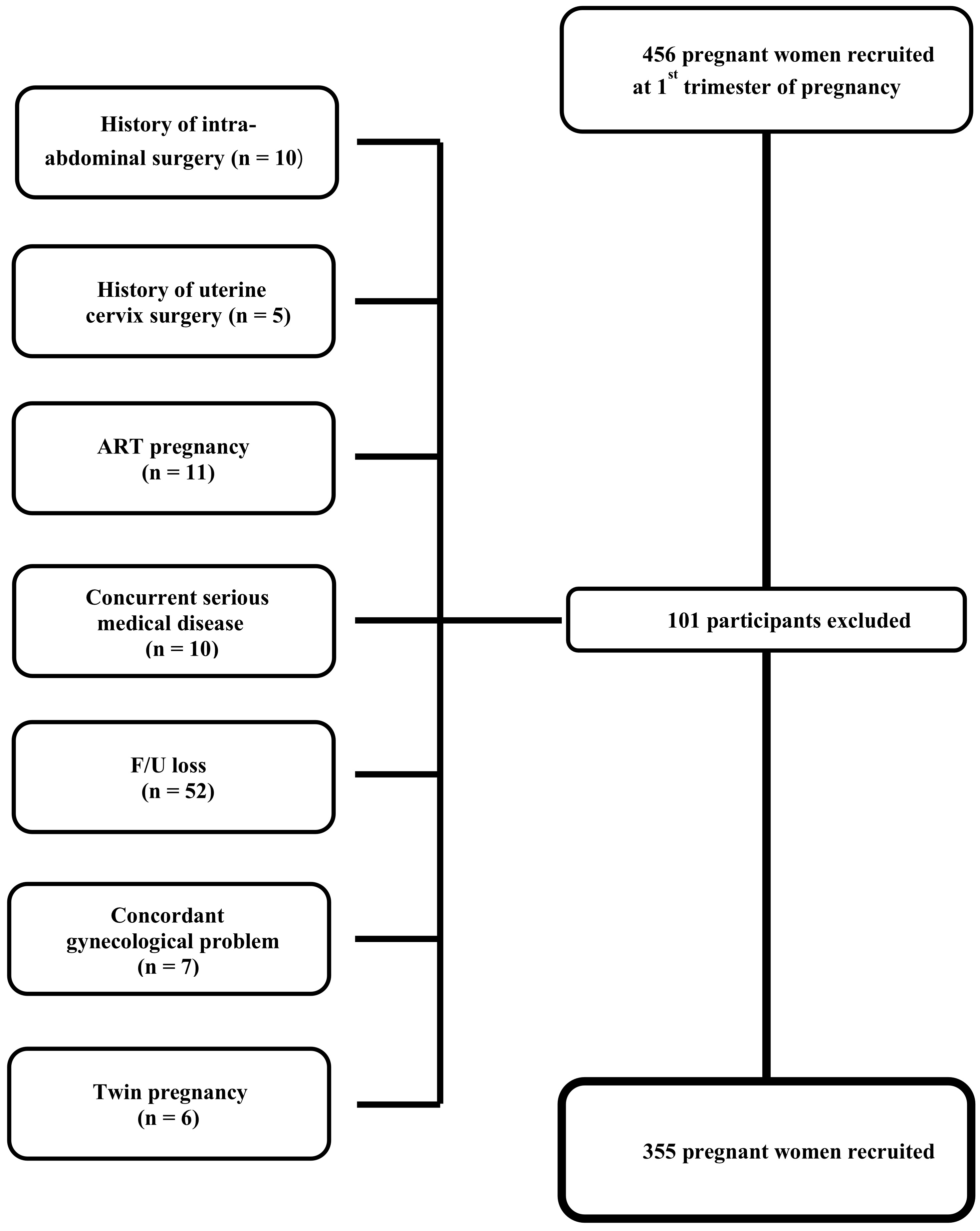
During the period of March 2017–December 2018, 456 pregnant women were
prospectively recruited and followed in the first trimester of pregnancy. Among
these women, 101 were excluded for one of the following reasons which could have
affected pregnancy outcomes: ART pregnancy (n = 11), twin pregnancy (n = 6),
history of intra-abdominal surgery (n = 10), history of uterine cervix surgery (n
= 5), concurrent serious medical disease that could affect pregnancy outcomes
(for example, type 1 diabetes mellitus, uncontrolled thyroid disease, rheumatoid
arthritis, renal disease, uncontrolled hypertension and systemic lupus
erythematosus) (n = 10), concordant gynecological problem that could affect
pregnancy outcome (leiomyoma
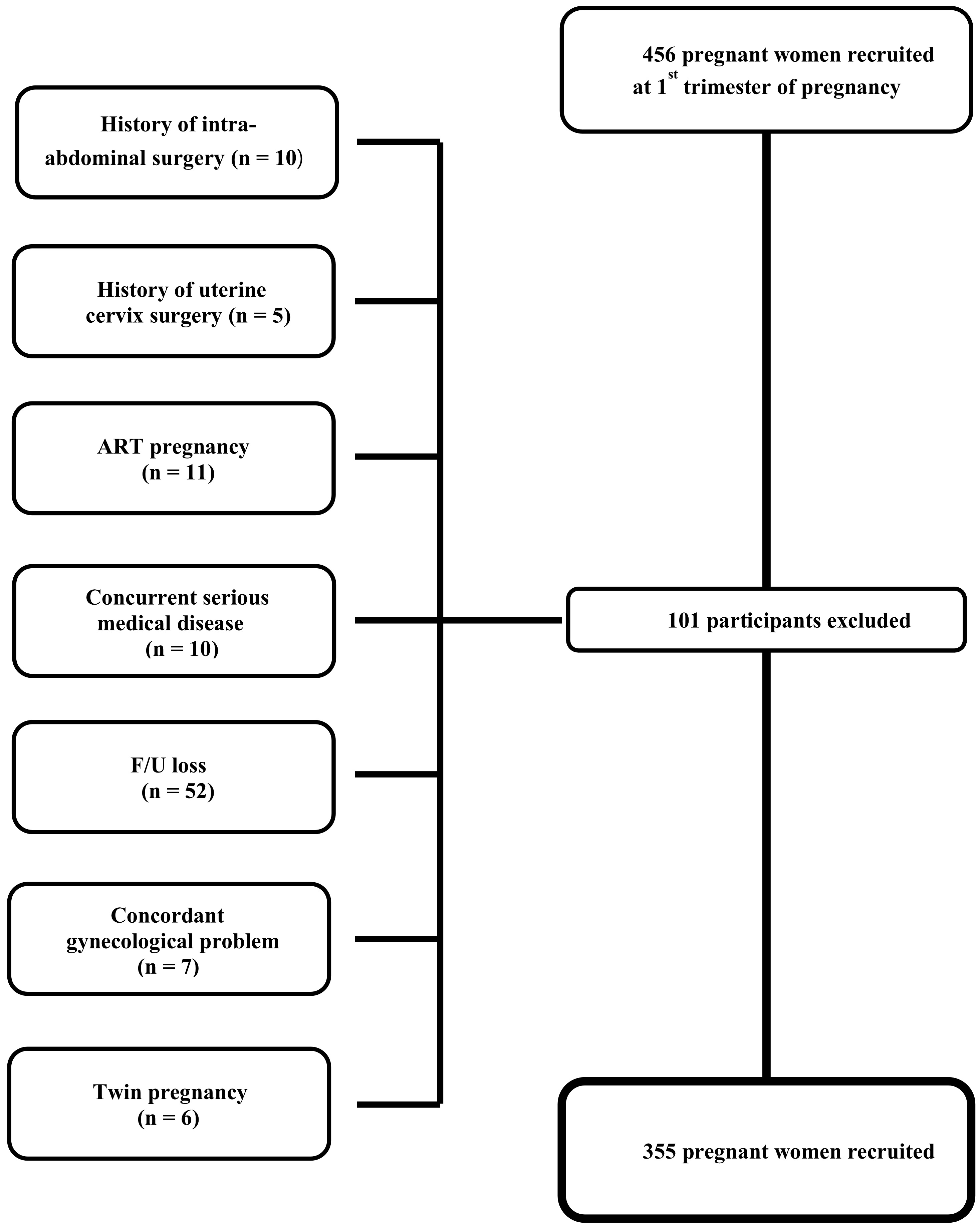 Fig. 1.
Fig. 1.Flowchart of study participants.
Participant’s demographic characteristics such as age, medical history, operation history, medication, and obstetrical and gynecological history including gravidity and parity were gathered by obstetrical doctors and nurses at the first prenatal consultation. The body mass index (BMI) before pregnancy was collected from the participants’ self-reported records and the BMI just before delivery was measured at their admission for delivery. Gestational age was calculated from the last menstrual period and ultrasound measurements. The information regarding the dosage and duration of vitamin D supplementation was collected from the participants’ self-reports. Additionally, the birth season was categorized into winter (October to March) and summer (April to September).
We followed the criterion of PPROM that was defined by a suggestive anamnestic
history of vaginal fluid leakage or sonographic evidence of oligohydramnios,
followed by documentation of fluid passing through the cervix on sterile speculum
examination and a positive nitrazine test (Bristol-Myers Squibb, Princeton, NJ,
USA), AmniSure rupture of fetal membrane test (placental alpha microglobulin – 1
or Ferning test) [16]. Gestational diabetes mellitus (GDM) was defined as
diabetes diagnosed by glucose tolerance test between 24 and 28 weeks of
gestation, excluding overt diabetes mellitus. Pregnancy associated hypertension
(HTN) included all hypertensive disorders and their complications during
pregnancy. Thyroid disorder included all thyroid diseases diagnosed during
pregnancy which were treated with medication. A preterm birth (PTB) was defined
as a birth at
Maternal venous blood (5 mL) was collected in the first trimester (5~9 weeks of gestation), second trimester (24~28 weeks of gestation), third trimester (35~37 weeks of gestation), and just before birth (at their admission for delivery) to measure the levels of vitamin D. Additionally, 5 mL of umbilical venous blood was collected during birth for measuring the levels of vitamin D of newborn baby. Total 25-hydroxyvitamin D (25(OH)D) levels were measured using an electrochemiluminescence binding assay with an automated analyzer (Cobas C 602; Roche Diagnostics, Seoul, Republic of Korea).
Vitamin D deficiency is usually defined as a 25(OH)D level of less than 20 ng/mL
(50 nmol/L) [17, 18]. The definition of vitamin D deficiency has been a point of
debate [19]. The United States Institute of Medicine (USIM) has recently defined
levels of serum 25(OH)D above 50 nmol/L (or 20 ng/mL) as adequate in pregnant
women [20]. We employed the USIM cutoff for vitamin D deficiency (25(OH)D
Continuous variables are presented as medians and interquartile ranges.
Categorical variables are presented as numbers and percentages. The patients were
divided into two groups (Non-PPROM vs. PPROM and vitamin D
This prospective study was conducted after obtaining ethical approval from the Institutional Review Board (IRB) of Seoul Medical Center (2017-03-015). All participants provided written informed consent after receiving a complete explanation of this study.
A total of 355 pregnant women participated in this study from the 1st trimester of pregnancy to delivery, and 12 (3.38%) of them suffered PPROM. Table 1 shows the difference in general characteristics between the PPROM and non-PPROM groups. There was no difference in age, prepregnant BMI, and gravidity between the two groups, however, the incidence of PPROM was significantly higher in the nulliparous group. There was no difference between the two groups in adverse pregnancy outcomes such as GDM, HTN, and thyroid disorders, in birth season and in mode of delivery (Table 1).
| Variables | PPROM | Non-PPROM | p | |
| (N = 12) | (N = 343) | |||
| Age, years, median (IQR) | 32.5 (27.5–35.5) | 33 (30–36) | 0.332 | |
| BMI, kg/m |
21.3 (19.8–28.6) | 21.6 (19.7–24.2) | 0.533 | |
| Gravidity, N (%) | 0.134 | |||
| 0 | 9 (75.0) | 182 (53.1) | ||
| 3 (25.0) | 161 (46.9) | |||
| Parity, N (%) | 0.014 | |||
| 0 | 11 (91.7) | 175 (56.1) | ||
| 1 (8.3) | 137 (43.9) | |||
| GDM, N (%) | 0 (0) | 18 (5.2) | 0.999 | |
| HTN, N (%) | 0 (0) | 10 (2.9) | 0.999 | |
| Thyroid disorder, N (%) | 0 (0) | 21 (6.1) | 0.999 | |
| Vitamin D intake, N (%) | 5 (41.7) | 189 (56.6) | 0.306 | |
| Birth season, N (%) | 0.558 | |||
| Winter | 5 (41.7) | 115 (33.5) | ||
| Summer | 7 (58.3) | 228 (66.5) | ||
| Mode of delivery, N (%) | 0.068 | |||
| Vaginal delivery | 2 (16.7) | 148 (43.1) | ||
| Cesarean section | 10 (6.9) | 198 (56.9) | ||
| 25(OH)D levels in maternal plasma, ng/mL, median (IQR) | 0.034 | |||
| 0.042 | ||||
| 1st trimester | 8.8 (7.3–12.2) | 14.1 (10.3–19.0) | 0.02 | |
| 2nd trimester | 12.6 (9.9–24.7) | 23.1 (15.9–31.2) | 0.029 | |
| 3rd trimester, | 17.8 (13.5–26.9) | 24.1 (15.6–33.1) | 0.192 | |
| Just before birth | 14.6 (11.8–20.3) | 22.4 (14.1–31.0) | 0.015 | |
| Cord blood of newborn, ng/mL, median (IQR) | 13.4 (9.0–18.1) | 20.5 (13.2–28.5) | 0.006 | |
| PPROM, preterm premature rupture of membranes; BMI, body mass index;
GDM, gestational diabetes mellitus; HTN, pregnancy associated
hypertension; 25(OH)D: 25-hydroxyvitamin D. IQR, interquartile range; | ||||
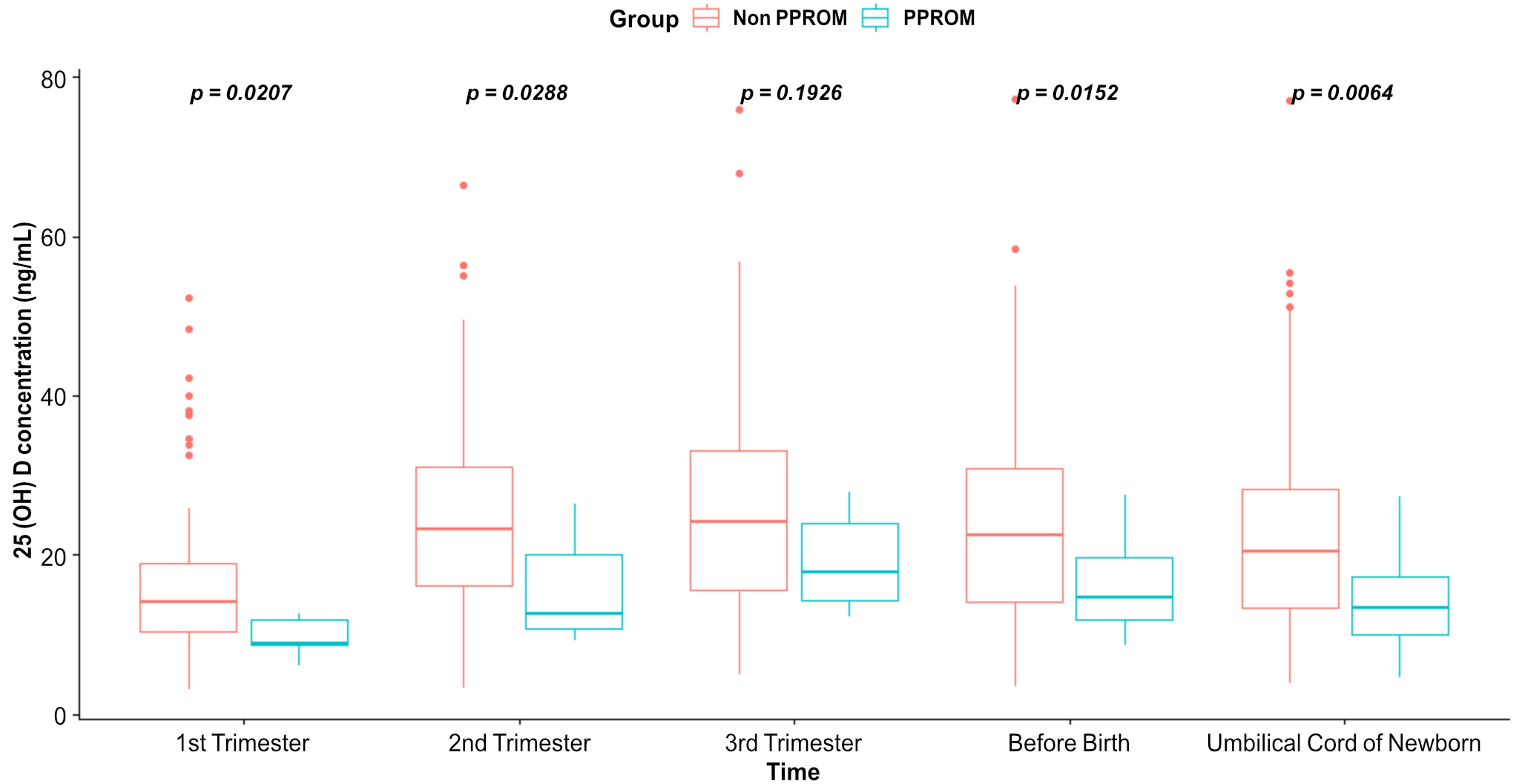
Vitamin D levels in the maternal plasma and umbilical cord blood of newborns were significantly different between the PPROM and non-PPROM groups in the first trimester (p = 0.02), second trimester (p = 0.029), before birth (p = 0.015), and in the umbilical cord blood at birth (p = 0.006) (Fig. 2).
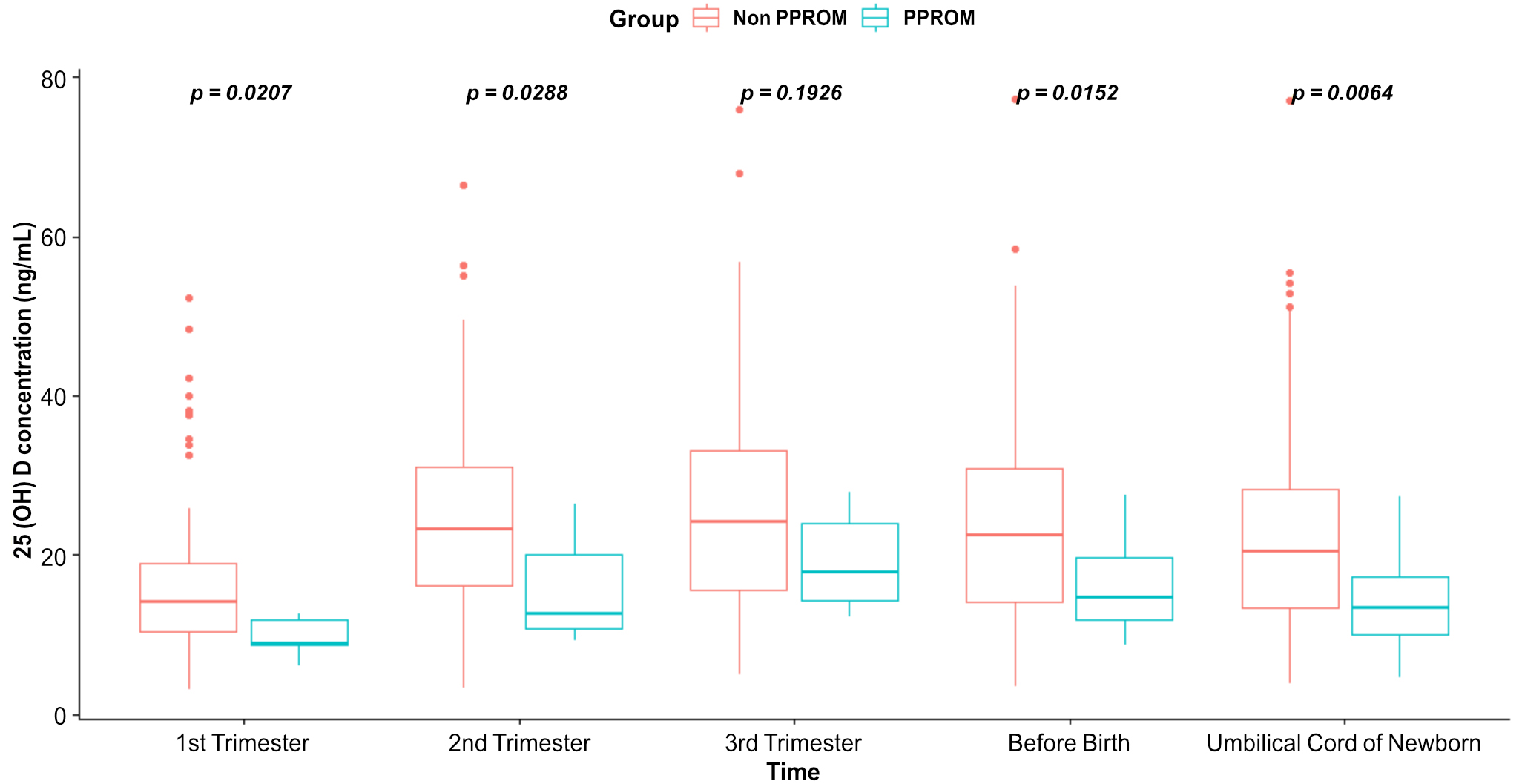 Fig. 2.
Fig. 2.Comparison and boxplot of vitamin D levels in the maternal plasma and in the umbilical cord blood of newborns.
The weight at birth (p
| Variables | PPROM | Non-PPROM | p | |
| (N = 12) | (N = 343) | |||
| Sex, N (%) | 0.134 | |||
| Male | 9 (75.0) | 182 (53.1) | ||
| Female | 3 (25.0) | 161 (46.9) | ||
| Birth weight, g, median (IQR) | 2925 (2550–3315) | 3190 (2920–3500) | 0.036 | |
| Preterm birth | ||||
| No | 4 (33.3) | 302 (88.0) | ||
| Yes | 8 (66.7) | 41 (12.0) | ||
| Apgar score at 1 minute | 8 (8–8.75) | 8 (8–8) | 0.661 | |
| Apgar score at 5 minutes | 9 (9–9.75) | 9 (9–9) | 0.962 | |
| Neonatal jaundice | 0.140 | |||
| No | 7 (58.3) | 273 (79.6) | ||
| Yes | 5 (41.7) | 70 (20.4) | ||
| PPROM, preterm premature rupture of membranes; IQR, interquartile range. | ||||
Outcomes of pregnancies and newborns were compared by creating two groups based
on the vitamin D status of cord blood; the vitamin D deficiency group (
| Variables | 25(OH)D |
25(OH)D |
p | |
| (N = 176) | (N = 179) | |||
| Age, years, median (IQR) | 33 (30–36) | 32 (30–36) | 0.509 | |
| BMI, kg/m |
||||
| Prepregnant | 22.5 (20.3–25) | 20.9 (19.4–23.4) | ||
| Just before birth | 27.1 (24.7–29.7) | 25.4 (23.8–28.2) | ||
| Gravidity, N (%) | 0.002 | |||
| 0 | 80 (45.5) | 111 (62.0) | ||
| 96 (54.5) | 68 (38.0) | |||
| Parity, N (%) | 0.014 | |||
| 0 | 60 (34.1) | 84 (64.9) | ||
| 116 (65.9) | 95 (53.1) | |||
| PPROM, N (%) | 11 (6.3) | 1 (0.6) | 0.003 | |
| GDM, N (%) | 9 (5.1) | 9 (5.0) | 0.971 | |
| HTN, N (%) | 4 (2.3) | 6 (3.4) | 0.539 | |
| Thyroid disorder, N (%) | 8 (4.5) | 13 (7.3) | 0.278 | |
| Vitamin D intake, N (%) | 68 (40.0) | 126 (71.6) | ||
| Birth season, N (%) | 0.433 | |||
| Winter | 56 (31.8) | 64 (35.8) | ||
| Summer | 120 (68.2) | 115 (64.2) | ||
| Mode of delivery, N (%) | 0.113 | |||
| Vaginal delivery | 67 (38.1) | 83 (46.4) | ||
| Cesarean section | 109 (61.9) | 96 (53.6) | ||
| PPROM, preterm premature rupture of membranes; GDM, gestational diabetes mellitus; HTN, pregnancy associated hypertension. | ||||
Multiple logistic regression analysis was performed to eliminate the confounding
effects. After adjusting for other confounders (age, gravidity, parity, and BMI),
a higher vitamin D level showed a lower rate of PPROM (p
| Variables | Odds ratio | Confidence interval | p |
| Age (1 year increase) | 0.961 | 0.855–1.081 | 0.508 |
| Gravidity ( |
0.305 | 0.074–1.258 | 0.999 |
| Parity ( |
1.783 | 0.453–7.020 | 0.408 |
| BMI (1 kg/m |
1.040 | 0.910–1.189 | 0.563 |
| 25(OH)D levels in the cord blood of the newborn (1 ng/mL increase) | 0.907 | 0.836–0.983 | 0.018 |
| PPROM, preterm premature rupture of membranes; BMI, body mass index. | |||
The results were analyzed only for primigravidas to prevent the previous histories of preterm labor or PTB from affecting the PPROM rate. Among 191 primigravidas, the rate of vitamin D deficiency was 70%. There was no difference in age, BMI, rate of GDM, HTN or thyroid disorders between the vitamin D deficiency and non-deficiency groups, however PPROM occurred only in the vitamin D deficiency group (Table 5).
| Variables | 25(OH)D |
25(OH)D |
p | |
| (N = 80) | (N = 111) | |||
| Age, years, median (IQR) | 31.5 (28–35.7) | 31 (30–35) | 0.225 | |
| BMI, kg/m |
||||
| Prepregnant | 22.4 (19.8–24.9) | 20.8 (19.6–23.5) | 0.029 | |
| Just before delivery | 26.9 (24.4–29.6) | 25.5 (24.1–28.6) | 0.097 | |
| PPROM, N (%) | 9 (11.3) | 0 (0) | ||
| GDM, N (%) | 4 (5.0) | 6 (5.4) | 0.999 | |
| HTN, N (%) | 2 (2.5) | 5 (4.5) | 0.701 | |
| Thyroid disorder, N (%) | 5 (6.3) | 8 (7.2) | 0.796 | |
| Vitamin D intake, N (%) | 36 (45.6) | 88 (80.0) | ||
| Birth season, N (%) | 0.609 | |||
| Winter | 31 (38.8) | 39 (35.1) | ||
| Summer | 49 (61.3) | 72 (64.9) | ||
| Mode of delivery, N (%) | 0.415 | |||
| Vaginal delivery | 25 (31.3) | 41 (36.9) | ||
| Cesarean section | 55 (68.8) | 70 (63.1) | ||
| PPROM, preterm premature rupture of membranes; BMI, body mass index; IQR, interquartile range; GDM, gestational diabetes mellitus; HTN, pregnancy associated hypertension. | ||||
Our study was conducted prospectively, and all participants with risk factors
that could affect PPROM were excluded. All participants were recruited in the
first trimester of pregnancy, and blood was collected for vitamin D level
analysis at predetermined periods (the first, second, third trimester of
pregnancy and just before delivery). Additionally, vitamin D levels were measured
from the blood taken from the umbilical cord of the newborn. Our study showed
that the occurrence of PPROM in cases with vitamin D deficiency (
The rate of PPROM in this study was 3.38% (12/355), with the rate in the vitamin D deficiency group being 6.3% (11/176) and 0.6% (1/179) in the non-deficiency group. Of these, 66.7% (8/12) led to PTB. In the United States, the PTB rate is approximately 10% [21]. PPROM is responsible for 30%–40% of PTBs [22]. Similar to in the United States, PTB rate is approximately 7% in South Korea [23]. Therefore, reducing vitamin D deficiency during pregnancy might lead to a reduction of PTB due to PPROM.
There are several hypotheses for how vitamin D deficiency can affect PPROM. First, vitamin D is involved in several processes leading to an inflammatory response. The fetal membrane not only acts as a barrier to the invasion of microorganisms [24, 25, 26], but also provides immune protection [27, 28, 29]. Impairment of these functions of the fetal membrane can lead to microbial invasion in the genital tract as well as triggering of inflammatory processes, resulting in PPROM through collagenolysis [24, 30, 31, 32]. Some studies have reported that inflammation with bacterial infection is the main cause of PPROM [33], and recent studies have reported that sterile inflammation of the fetal membrane without microbial infection is the predisposing cause of PPROM [34]. Liu et al. [35] reported that vitamin D regulates both acquired and innate immune responses at the fetal-maternal interface. Vitamin D could act as an intracrine regulator of cyclic adenosine monophosphate in trophoblasts and regulate innate immune response in the placenta [36, 37]. In the placenta, 1,25-dihydroxyvitamin D induces the production of cathelicidin, an antimicrobial peptide, and consequently is associated with a reduction of the risk of bacterial vaginosis. Dunlop et al. [38] reported that vitamin D deficiency during pregnancy is more likely to cause bacterial vaginosis. Moreover, sufficient vitamin D levels during pregnancy could reduce the risk of bacterial vaginosis, thereby reducing the incidence of PTB [39, 40]. Therefore, maternal vitamin D could function as an immune regulator in pregnancy and have anti-inflammatory effects which reduce the risk of PPROM by decreasing bacterial colonization of the genital tract.
Second, vitamin D is a potent antioxidant. One of the pathophysiologies of PPROM
is oxidative stress (OS) mediated by reactive oxygen species (ROS). Collagen is
the primary target of ROS. In healthy pregnancies, the ROS and antioxidants are
balanced [41, 42, 43, 44, 45]. However, this balance is disturbed in case of increase in ROS
(high O
Overall, PPROM is associated with an inflammatory response by infection and directly with OS-inducing chorioamniotic collagenolysis. Vitamin D can regulate the immune response in the placenta and decrease bacterial colonies in the genital tract. Furthermore, vitamin D functions as an antioxidant that upregulates antioxidative enzymes, triggering the expression of Nrf2 that suppresses ROS via genetic mechanism.
We collected pre-pregnancy BMI and measured BMI just before delivery, and Table 3 showed that prepregnant BMI and just before birth BMI were statistically significantly higher in the vitamin D deficiency group than in the normal group. As mentioned in several studies, it was reported that the higher the BMI, the lower the vitamin D blood concentration [50], and there is also a research result that the bioavailability of vitamin D is lowered in the obesity group [51].
Our study has several advantages. This study was prospectively conducted. Therefore, it was possible to exclude factors that could affect PPROM. Additionally, to exclude previous PTB and preterm labor history, a second sub-analysis was performed with only primigravidas. Our sample size of 355 pregnant women was relatively larger than that of previous studies. Moreover, this study investigated the association between PPROM and vitamin D deficiency in the umbilical venous cord blood of newborns. To the best of our knowledge, previous studies on this association are scarce.
However, our study has some limitations. The sample size was too small to allow the generalizability of our research outcomes. Additionally, we could not accurately analyze the correlations between the vitamin D level in the maternal plasma, umbilical cord blood, and vitamin D intake (through vitamin D therapy or diet during pregnancy). In the future, further study on PPROM and vitamin D levels, considering fetal infection and vitamin D intake, is required to study the detailed mechanism of PPROM associated with vitamin D deficiency.
PPROM accounts for a considerable portion of PTB, which increases fetal morbidity and mortality. In this study, we found that vitamin D deficiency had a significant association with PPROM. We expect that the incidence of PPROM could be considerably reduced if vitamin D intake is recommended before and during pregnancy. For this to be achieved, further large-scale research needs to be conducted.
HJL—Designed the research study, acquired patient data, and drafted the manuscript; JYH—Conception, design, statistical analysis, created figures and tables, provided guidance, responsible for the accuracy and integrity of the work presented here; JHH—Statistical analysis and data extraction; HYK—Interpretation of data and statistical analysis; HZC—Statistical analysis and data extraction, created figures and tables. All authors contributed to editorial changes in the manuscript. All authors read and approved the final version of the manuscript.
This prospective study was conducted after obtaining ethics approval from the institutional review board of Seoul Medical Center (2017-03-015). All participants provided written informed consent after receiving a complete explanation of this study.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This study was supported by the Seoul Medical Center, Korea (17-C09, 18-C04). The sponsor played no role in the design, collection, analysis and interpretation of data, writing the manuscript, or deciding to submit the manuscript for publication. The article contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsor.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.