1 Department of Clinical Experimental and Biomedical Sciences “Mario Serio”, University of Florence, 50134 Florence, Italy
2 Obstetrics and Gynecology, Department of Maternity and Infancy, AOU Careggi, 50134 Florence, Italy
Academic Editors: Simone Garzon and Andrea Tinelli
Abstract
Objectives: Endometriosis is an inflammatory disease characterized by a frequent association with gynecologic and systemic comorbidities. Our aim was to evaluate which gynecologic and systemic comorbidities occur in women affected by endometriosis and their impact on quality of life and global health. Mechanism: A literature search of the PubMed, Cochrane Library, Scopus and Web of Science databases was performed to identify the relevant studies published before December 31, 2021. We selected clinical studies, systematic reviews, and meta-analyses in English. Findings in Brief: Endometriosis is strongly associated with gynecologic (adenomyosis, uterine fibroids, polycystic ovarian syndrome-PCOS) and systemic (autoimmune, inflammatory, psychiatric and neurological disorders) comorbidities that impair women quality of life and global health through multiple mechanisms, influencing everyday life and work activities. Conclusions: Endometriosis is a chronic disease, impairing multiple functioning areas and affecting women’s health and everyday life. Considering the co-existence of multiple both gynecological and non-gynecological disorders, endometrisois needs a multidisciplinary approach. Thus, specialized referral centres are warranted for a personalized management, focused on patient symptoms and comorbidities.
Keywords
- endometriosis
- pain
- infertility
- uterine fibroids
- adenomyosis
- polycystic ovary syndrome
- autoimmune diseases
- bowel diseases
- mental health disorders
- migraine
- quality of life
- integrated management
Endometriosis is a gynecologic endocrine and inflammatory disease affecting about 10% of fertile age women. Multiple factors are involved in the pathogenesis, with a major role of sex steroid hormones, i.e., progesterone resistance and increased estrogen receptor sensitivity. Other mechanisms are involved in endometriosis development, in particular tissue invasion, cellular proliferation, adhesion formation, neoangiogenesis and apoptosis [1, 2].
In a group of patients other gynecologic disorders coexist with endometriosis contributing to impair fertility, other than causing additional symptoms, such as pain and abnormal uterine bleeding [3, 4, 5]. Moreover, the concomitance of systemic comorbidities may further affect quality of life (QoL) and global health of women with endometriosis [6].
The present narrative review will report and discuss which gynecologic (adenomyosis, uterine fibroids, polycystic ovarian syndrome-PCOS) and systemic (autoimmune, inflammatory, psychiatric and neurological disorders) comorbidities are commonly described in patients with endometriosis and their effect on QoL and global health.
The PubMed, Cochrane Library, Scopus and Web of Science databases were used to perform a literature search on relevant studies (clinical studies, systematic reviews, and meta-analyses) in English published before December 31, 2021 on the field. The research strategy included the combinations of the following Medical Subject Heading (MeSH) and non-MeSH terms: adenomyosis, uterine fibroids, polycystic ovary syndrome, autoimmune diseases, inflammatory diseases, mental health disorders, migraine in combination with endometriosis, quality of life, health, pelvic pain, abnormal uterine bleeding, infertility. No additional inclusion or exclusion criteria were used. The reference list of all identified studies was systematically revised to identify other eligible publications. Table 1 (Ref. [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31]) shows the characteristics of the analysed studies.
| Author(s) (y) | Patients (n) | Sub types of endometriosis and patients affected (n) (%) | Mean age(y) (mean SD) | Associated disorders | Endometriosis diagnosis | Study design | |
| Uimari O (2011) [10] | 558 | NR | NR | Uterine fibroid | Ultrasound | Retrospective | |
| Laparoscopy and histology | |||||||
| NaphatthalungW (2012) [11] | 220 | NA | 63 (28.6%) | 40–50 | Adenomyosis and/or | Laparoscopy and histology | Retrospective |
| Uterine fibroid | |||||||
| Di Donato N (2014) [7] | 586 | OMA | 280 | 35.2 |
Adenomyosis | Ultrasound | Retrospective |
| DIE | 652 | ||||||
| SUP | 686 | ||||||
| Maclaran K (2014) [13] | 212 | NA | 45 (21.2%) | 38.1 |
Uterine fibroid | Laparoscopy and histology | Prospective |
| Tanmahasamut P (2014) [12] | 331 | NA | 101 (30.5%) | NR | Uterine fibroids | Laparoscopy | Retrospective |
| Adenomyosis | |||||||
| Benign ovarian cyst | |||||||
| Capezzuoli T (2020) [8] | 586 | OMA | 244 | 35.6 |
Uterine fibroid | Ultrasound | Retrospective |
| DIE | 89 | Adenomyosis | |||||
| SUP | 86 | ||||||
| Alcalde AM (2022) [9] | 112 | DIE | 42 | NR | Adenomyosis | Questionnaire | Prospective |
| DIE+AD | 31 | ||||||
| Pasoto SG (2005) [16] | 45 | 33 |
Systemic Lupus Erythematosus | Laparoscopy and histology | Retrospective | ||
| Harris HR (2016) [15] | 6434 | NA | 37.8 |
Lupus erythematosus and Rheumatoid arthritis | Laparoscopy and histology | Retrospective | |
| Peyneau M (2019) [17] | 32 | SUP | 9 (21%) | 38.6 |
Thyroid dysimmunity | Laparoscopy and histology | Retrospective |
| OMA | 4 (12%) | ||||||
| DIE | 20 (60%) | ||||||
| Porpora MG (2020) [14] | 148 | ASRM Stage I | 6.1% | 31.6 | Autoimmune diseases: | Laparoscopy and histology | Retrospective |
| ASRM Stage II | 13.6% | Autoimmune thyroiditis | Diagnosis | ||||
| ASRM Stage III | 31.3% | IBD, SLE, Celiac Disorder | |||||
| ASRM Stage IV | 49% | ||||||
| Jess T (2012) [18] | 37,661 | NA | 38.6 |
Inflammatory bowel disease | Laparoscopy and histology | Retrospective | |
| Clinical | |||||||
| Chiaffarino F (2020) [19] | 15 (studies) | NA | NR | Inflammatory bowel disease | Laparoscopy and histology | Review | |
| Chiaffarino F (2021) [20] | 83,330 | NA | NR | Inflammatory bowel disease | Ultrasound | Review | |
| Mu F (2017) [22] | 116,430 | NA | 34.5 |
Cardiovascular risk | Laparoscopy and histology | Prospective | |
| Cirillo M (2021) [21] | 643 | ASRM Stage III/IV | 92 (14.3%) | 40.5 |
Cardiovascular risk | Ultrasound | Retrospective |
| Coratti F (2020) [23] | 149 | SUP | 34 | 25.6 | Appendectomy | Ultrasound | Retrospective |
| Clarizia R (2021) [24] | 196 | ASRM Stage 1 | 3% | 33 | Pelvic inflammatory disease | Laparoscopy and histology | Retrospective |
| ASRM Stage 2 | 3% | ||||||
| ASRM Stage 3 | 7% | ||||||
| ASRM Stage 4 | 88% | ||||||
| Wu C-C (2018) [25] | 9191 | NA | NR | Bladder pain syndrome | Clinical | Retrospective | |
| Vannuccini S (2018) [26] | 150 | OMA | 37% | 34.8 |
Mental health disorders | Ultrasound | Retrospective |
| DIE | 24% | ||||||
| OMA+DIE | 3.6% | ||||||
| OMA+SUP | 3% | ||||||
| Missmer SA (2021) [27] | 51 (studies) | NA | NR | Mental health disorders | Ultrasound | Review | |
| Laparoscopy and histology | |||||||
| Tietjen GE (2007) [31] | 171 | NA | 37.6 |
Migraine | Clinical | Cross-sectional study | |
| Miller JA (2018) [28] | 296 | NA | 17 | Migraine | Clinical | Prospective | |
| Laparoscopy and histology | |||||||
| Maitrot-Mantelet L (2020) [29] | 314 | NA | 31.04 |
Migraine | Laparoscopy and histology | Prospective | |
| Jenabi E (2020) [30] | 9 (studies) | NA | NR | Migraine | Laparoscopy and histology | Meta analysis | |
| Notes: DIE, deep infiltrating endometriosis; IBD, inflammatory bowel diseases; OMA, ovarian endometriosis; SUP, superficial endometriosis; SLE, systemic lupus erythematous. | |||||||
Adenomyosis is histologically defined by the ectopic localization of endometrium (glands and stroma) into the myometrium and dysmenorrhea and heavy menstrual bleeding (HMB) are the main symptoms [4]. Both infertility and recurrent pregnancy loss are also observed in patients with adenomyosis [6, 32].
The coexistence of endometriosis and adenomyosis has been evaluated by trans-vaginal ultrasound (TVUS) and magnetic resonance imaging and ranged between 21.2% and 89.4% [7, 33, 34, 35, 36]. The concomitance of the two diseases is described especially in women with severe dysmenorrhea and deep infiltrating endometriosis (DIE) is the most common phenotype [33, 34, 35, 36, 8]. Patients with concomitant adenomyosis and DIE present worse QoL compared to healthy group, showing a poorer sexual life and a high rate of severe pelvic pain in comparison to only DIE patients [9].
The concomitance of uterine fibroids and endometriosis is less studied and controversial. Considering patients operated for endometriosis, uterine fibroids were described in 25.8% of women [9, 10, 11]. In case of hysterectomy for benign uterine disorders or myomectomy in premenopausal women, the prevalence of endometriosis ranged between 21.2% and 28% of cases [12].
When evaluated by TVUS in infertile young endometriotic patients [8], the coexistence of uterine fibroids and endometriosis was found only in 3.1% of cases. The concomitance of endometriosis and uterine fibroids can determine a synergic negative effect on abnormal uterine bleeding (AUB), HMB, dysmenorrhea, chronic pelvic pain, or infertility, affecting global QoL [13, 37, 38, 39].
The association between endometriosis and PCOS has been poorly evaluated. In a recent study [40], a low incidence (7.7%) of asymptomatic endometriosis in women undergoing laparoscopic ovarian drilling for PCOS resulted to be associated with lower endometriosis stage (I and II) at American Society for Reproductive Medicine (ASRM) classification [41].
In patients with endometriosis, an increased incidence of some autoimmune diseases has been shown. In fact, endometriosis is characterized by altered immune surveillance with reduced cell-mediated immunity and higher humoral immune response. Moreover, a genetic predisposition HLA DQ7 related is hypothesized for endometriosis, similarly to several autoimmune diseases [14].
The presence of concomitant endometriosis and autoimmune diseases impair QoL because of the synergistic effect of severe pelvic pain and systemic inflammation.
The pathogenesis of celiac diseases includes the presence of an altered cell-mediated immunity with a critical role of interleukin-18 (IL-18) and interferon-c (IFN-c) in persisting Th1 activation after gluten exposure. In case of endometriosis, IL-18 and FN-c are involved in a Th1 imbalance, similarly to celiac disease. Particularly IL-18 is a key factor in endometriosis pathogenesis and related inflammation [14].
The strong relationship between systemic lupus erythematous (SLE) and endometriosis is supported by ANA autoantibodies production in some endometriosis patients [3]. Moreover, the association between SLE and endometriosis may be in part explained by the common humoral dysfunction and the common hormonal risk factors [15]. SLE is a heterogeneous disease with a significant impact on many physical, social and psychological aspects of patient QoL. Even when not diagnosticated with SLE, women with endometriosis have high frequency of generalized musculoskeletal manifestation, including the complete fibromyalgia syndrome [16, 42].
The dysregulation of humoral immunity seems to be a shared mechanism between endometriosis and autoimmune thyroid diseases. High levels of some autoantibodies (e.g., anti-thyroid peroxidase antibodies) in patients with endometriosis have been found. Another possible link between endometriosis and autoimmune thyroiditis may be due to an alteration of the expression of the estrogen receptor beta gene (ESR2), which is an important modulators of immune system [17, 43].
Inflammatory bowel diseases (IBD) are frequently associated with endometriosis, even after 20 years from diagnosis. As detected in a large Danish cohort study, the risk of Chron’s disease (CD) and ulcerative colitis is increased in women with endometriosis, with a standardized incidence ratio of 1.5 (95% CI 1.3–1.7) and 1.6 (95% CI 1.3–2.0) respectively. Considering epidemiological studies, the prevalence of IBD in patients with endometriosis ranged between 2 to 3.4%, whereas in women without endometriosis the risk of IBD was reduced (0–1%). Endometriosis and inflammatory bowel diseases are characterized by similar features and symptoms. In case of concomitancy, this results in an increased risk of delayed or indeterminate diagnosis. Dysregulation of the immune system and inflammatory cytokines are involved in the pathogenesis of both endometriosis and IBD. Moreover, endometriosis and IBD may overlap also in symptoms. Patients with bowel endometriosis may present functional symptoms (diarrhea, and cramping) originating from the inflammatory microenvironment, and mechanical obstructive symptoms (bloating, constipation, bowel occlusion) originating from occlusive nodules and scar tissue retractions [18, 19]. In case of endometriosis and coexistent IBD, bowel symptoms may be atypical and cyclic and the endometriosis-related fibrosis may worsen obstruction [18, 19].
Also irritable bowel syndrome (IBS) seems to be frequently associated with endometriosis and some studies reported an elevated IBS severity score in case of I–II endometriosis stages and a lower score in III–IV endometriosis stages [20].
Surprisingly, the risk of ischemic heart diseases is increased in women with endometriosis and probably this may be related to the inflammatory background and the increase of oxidative stress. Furthermore, the surgical treatment of endometriosis at young age, especially in case of oophorectomy or hysterectomy, may contribute to the increased rate of myocardial infarction and coronary disease (1.4–1.6) [3, 44, 21]. Moreover, women with endometriosis present a higher risk of hypercholesterolemia with RR 1.25 (95% CI, 1.21–1.30) or hypertension with RR 1.14 (95% CI, 1.09–1.18) [21, 22]. Also in this case, the altered hormonal and the chronic systemic inflammatory milieus are involved. On the other hand, the chronic inflammation related to hypertension and the elevated low-density lipoprotein in hypercholesterolemia may augment the risk of endometriosis [22].
Considering intra-pelvic inflammation conditions, superficial endometriosis shows an increased prevalence in women undergoing emergency surgery for appendectomy [23].
Moreover, endometriosis patients present more frequently with a history of pelvic inflammatory disease (PID), especially in case of severe disease. In those cases, the co-existence of PID and endometriosis may potentiate painful symptoms and lead to a more difficult surgery [24].
Also bladder pain syndrome and recurrent interstitial cystitis (BPS/IC) seems to be associated with endometriosis by common pathogenetic mechanisms involving the same chemokines or cytokines [25].
An association between psychiatric disorders and endometriosis has been described. In particular, depression, anxiety disorders, substance abuse, panic and somatoform disorders are more common. The association between chronic pelvic pain, infertility and psychiatric disorders may lead to disability and lower QoL, with a severe impairment of global functioning and significant disruption of daily life [26, 45, 27]. Education too may be interested because pain appears to be a negative driver of the educational impacts of endometriosis because of the frequent school absences due to dysmenorrhea. Moreover, women with endometriosis experienced altered career trajectories, because of education impairment, and a decrease of social interaction.
Finally, endometriosis seems to be associated with a higher risk of migraine, especially in adolescents (69.3% in young girls with endometriosis versus 30.7% in young girls without endometriosis) [28].
The systemic spreading of prostaglandins produced by endometriosis lesions may contribute to increase the prevalence of migraine in this population.
Another hypothesis postulates that the up-regulation or dysregulation of nitric oxide synthesis contribute to the pathogenesis of both migraine and endometriosis [29, 30].
Endometriosis is characterized by a significant psychological and social impact with a high annual economic burden, similar to those of other chronic diseases [46]. This is due to the significant social and psychological impact, impairing QoL.
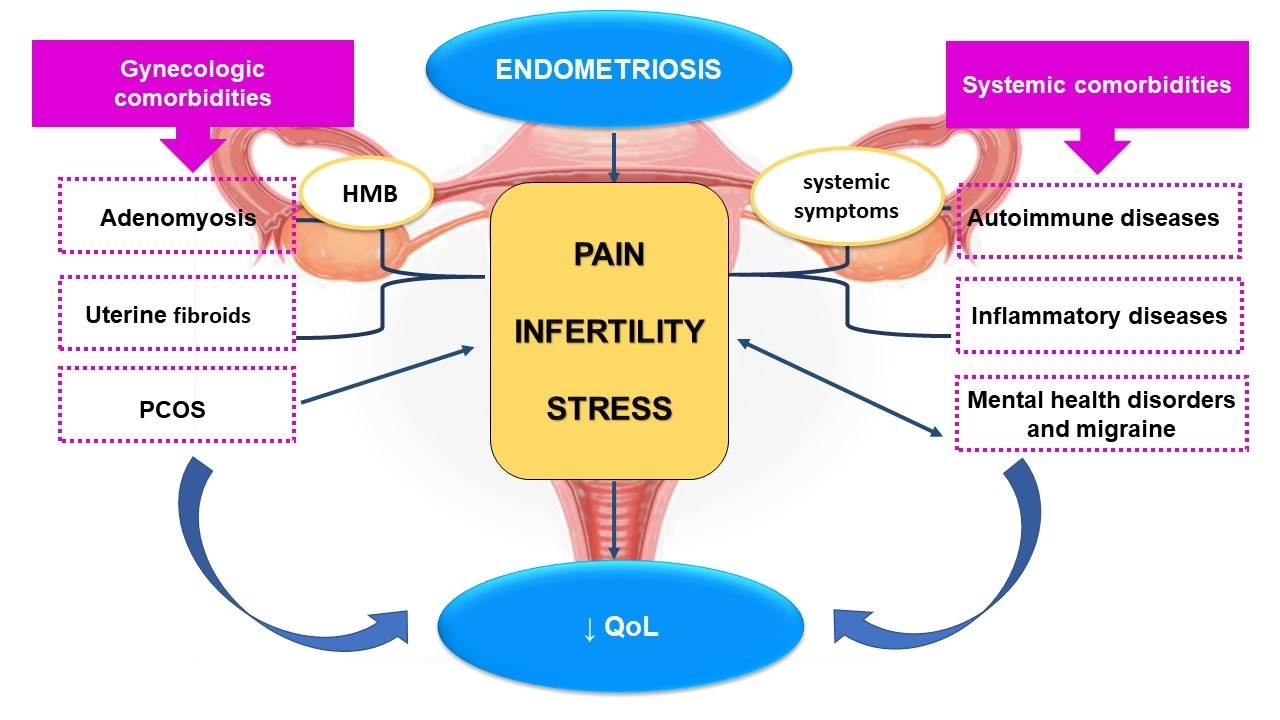
The concomitant presence of endometriosis and gynaecologic or systemic comorbidities have a synergistic effect in determining a worst QoL in affected women, interfering with everyday life and work activities (Fig. 1) [29, 31].
 Fig. 1.
Fig. 1.Gynecologic and systemic comorbidities in patients with endometriosis and their effect on quality of life. The main symptoms of endometriosis are pain and infertility, which contribute to stress and to a reduction in the QoL. Comorbidities associated with endometriosis may further reduce QoL by several mechanisms: adenomyosis and uterine fibroids causing pain, infertility and HMB; PCOS contributing to infertility; autoimmune and inflammatory diseases through a synergistic effect of severe pelvic pain, infertility and systemic symptoms. Finally, endometriosis is frequently associated with mental disorders which may worsen endometriosis related symptoms by creating a vicious circle. HMB, heavy menstrual bleeding; PCOS, polycistic ovary syndrome; QoL, quality of life.
This is particularly important in presence of DIE phenotype because it is more frequently linked to a more severe and aggressive clinical presentation, with an increased rate of intractable chronic pelvic pain, severe dyspareunia, bowel occlusion and hydroureteronephrosis [47].
A recent study has demonstrated that QoL of women with endometriosis may be related also to the ethnicity, the different health system organization and the social and cultural background. Italian women seemed to be more commonly affected by systemic comorbidities, especially inflammatory diseases and mental health disorders (such as depression and anxiety) compared to Chinese population [47].
For all these reasons it is very important to identify the co-existence of endometriosis and gynaecologic and systemic diseases in order to provide a global approach to the patient, considering QoL and global health.
Endometriosis treatment require an individual management centred on patient symptoms and priorities. A multidisciplinary approach considering associated disorders should be provided in specialized referral. Finally, the improvement of health system organization and social and cultural background seems to be crucial in the management of these complex and multifactorial conditions.
All the authors contributed to the intellectual content of the study and approved the final version of the article. TC and GO made substantial contributions to conception and design and acquisition, analysis and interpretation of data. SC, IP, FS and SV were involved in drafting the manuscript and revising it critically. FP gave final approval of the version to be published. FP agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. TC is serving as one of the Guest editors of this journal. SV is serving as one of the Editorial Board members and Guest editors of this journal. We declare that TC and SV had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to AT and SG.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.