1. Introduction
TNF- is a cytokine with multiple functions, such as participating in
proinflammatory responses, regulating cell differentiation, controlling tissue
renewal and restructuring, and so on, which depends on the cell type,
concentration, and receptor type present [1, 2, 3, 4]. It’s well known that
TNF- and its two types of receptors are expressed in the ovaries of
most species [3, 4, 5, 6]. TNF- is mainly expressed in human oocytes and
cumulus granulosa cells from aspirated follicles, and TNF-
immunoreactivity even can be observed in oocytes of human primordial follicles
[1, 3, 4]. In rodents, TNF- is observed not only in neonatal oocytes
but also in oocytes of all follicle stages in adults [1, 7]. Our previous studies
have demonstrated that granulosa cells in the ovary generally express Toll-like
receptor 3 (TLR3), retinoic acid-inducible gene I (RIG-I) and melanoma
differentiation-associated gene 5 (MDA5), which mediate innate activation, the
main source of TNF- in the ovaries [2, 3]. Therefore, TNF-
produced locally in the ovary rather than that from peripheral blood plays an
important regulatory role in ovarian function [1, 8].
Many studies have revealed that TNF- can regulate granulosa-luteal
cell growth and steroidogenesis in human and murine ovary [9, 10, 11, 12] and the
granulosa cells from small and large follicles exhibit differential response [13, 14]. TNF- consistently inhibits estrogen secretion from granulosa cells
of small follicles but stimulates progesterone secretion in granulosa cells of
large follicles, which may determine whether the follicle remains healthy or
becomes atretic during its course of development [14]. It is further verified
that TNF- represses P450 aromatase and inhibin -subunit
expression by activating the inducible repressor isoform of cAMP-responsive
element binding modulator in a MAPK dependent mechanism [15]. Further, high
levels of TNF- in follicular fluid (FF) and granulosa cells induce
oocyte, granulosa, and luteal cell death, which contribute to some extent to
polycystic ovary syndrome (PCOS) and premature ovarian failure (POF) [4, 16]. It
has been demonstrated that the effects of TNF- vary dramatically
depending on the dose [17, 18]. It has been reported that treatment of rats with
relatively low (0.1 ng/mL) and high (10 ng/mL, 50 ng/mL) doses of TNF-
has no effects on either oocyte/follicle numbers or apoptosis, while an
intermediate dose (1 ng/mL) significantly reduces oocyte/follicle numbers and
stimulates apoptosis [17]. However, no promoting effect on granulosa cell
proliferation has been observed within this concentration range.
The aim of this study was to evaluate the effect of TNF- on human
granulosa cell proliferation, P450 aromatase and inhibin A/B expression, and
estradiol and inhibin synthesis. Furthermore, the expression of two distinct
receptors, TNFR1 and TNFR2, was detected after TNF- treatment.
2. Materials and Methods
2.1 Ethics Statement
Our study group consisted of 20 infertile patients with PCOS without metabolic
syndrome and 19 normally ovulating women diagnosed with male factor or tubal
infertility who were undergoing their first cycle of in vitro fertilization (IVF)
or intracytoplasmic sperm injection (ICSI) from September 2019 to August 2020 in
the Reproductive Medical Center of Anhui Provincial Hospital. All patients
provided written informed consent before participation, and all procedures of the
study were approved by the human ethics committee of Anhui Provincial Hospital
(approve ID: 20170108). The ages and body mass index (BMI) values of the two
groups were comparable.
For each patient, FF samples were aspirated from follicles with a diameter
18 mm under the guidance of transvaginal ultrasound, and were all free of
blood contamination. After oocytes were isolated, the FF samples were transported
on ice to the laboratory immediately, and then centrifuged at 240 g at
4 C for 10 min to isolate supernatant for TNF- detection and
granulosa cells for culture.
Twelve histological specimens of normal ovaries were retrieved from storage at
the Department of Pathology of China-Japan Friendship Hospital. These ovarian
tissues were obtained from patients of childbearing age who underwent
hysterectomy and double appendectomy for invasive cervical cancer. The project
was approved by the Ethics Committee of China-Japan Friendship Hospital (approval
number: 2020-28-k20).
2.2 Cell Culture and Treatment
Granulosa cells from FF samples were washed twice with 3 mL culture medium
(serum-free DMEM/F12). After centrifuged at 1000 g at 4 C
for 10 min, the supernatant fluid was discarded and the cells were resuspended.
Cell viability and cell count were checked by 2% Trypan Blue staining. Then, the
granulosa cells were diluted and cultured in DMEM/F12 (11320-033, Gibco, USA)
containing 25 mM HEPES, 2 mM L-glutamine, and 10% fetal bovine serum in 6-well
plates (Nunc, Thermo Fisher, USA) at 37 C with 5% CO. When the
cells adhered to the bottom of the plates, the medium was refreshed with
serum-free DMEM/F12. As for E2 detection, 10 M testosterone was added as
the substrate. Then, the cells were treated with varying concentrations (0, 0.2,
0.4, 0.8, 2.0, 4.0 ng/mL) of recombinant human TNF- (Cat. 300-01A,
PeproTech, Rocky Hill, NJ, USA). After 48 h, the medium was collected as
conditioned medium, which was frozen at –70 C until E2 and inhibin
assays were performed.
2.3 Cell Viability
Granulosa cells with a concentration of 5 10 cells/well were
seeded in 96-well plates and treated with different concentration of
TNF- as described above. Cell viability was evaluated using a WST-8
Cell Counting Kit (CCK-8, Dojindo, Kyushu, Japan) per the manufacturer’s
instructions at 24, 48, 72, and 96 h.
2.4 Immunohistochemistry and Immunocytochemistry
Four-micrometer-thick tissue sections were cut from paraffin-embedded tissue
blocks, mounted onto glass slide, deparaffinized in xylene and rehydrated in
graded ethanol series sequentially. Then the slides were immersed in 0.01 M
citrate buffer (pH 6.0) buffer and placed in microwave oven for 10 min for
antigen retrieval. After cooling to 20–28 C, endogenous peroxidase was
blocked with 3% HO for 10 min. For immunocytochemistry, granulosa
cells were grown on coverslips to semiconfluency, fixed with cold acetone for 10
min and rinsed with PBS. After blocking non-specific sites with 5% normal goat
serum for 1 h at 20–28 C, the sections were incubated overnight at 4
C in a humidified chamber with primary antibodies as follows:
anti-TNF- at a 1:200 dilution (Rabbit monoclonal [TNF/1500R],
ab270264), anti-TNF receptor 1 at a 1:500 dilution (Rabbit polyclonal, ab19139),
anti-TNF receptor 2 at a 1:200 dilution (Rabbit monoclonal [EPR1653], ab109322),
and Ki-67 at a 1:200 dilution (Rabbit monoclonal [SP6], ab16667). All of the
primary antibodies were purchased from Abcam (USA). For the negative controls,
preimmune rabbit serum were used in place of the primary antibodies. After
rinsing with PBS, the sections were sequentially treated with biotinylated
secondary antibodies at 20–28 C for 1 h, and diaminobenzidine
substrate chromogen system to visualize specific staining. For counterstaining,
the sections were dipped in Mayer’s hematoxylin for 30 s.
2.5 Enzyme-Linked Immunosorbent Assay (ELISA)
The concentrations of TNF- in the supernatants of FF and the
concentrations of estradiol and inhibin in the conditioned medium were measured
with ELISA kits according to the manufacturers’ instructions. The kit for
TNF- (Cat. #BMS223HS, Thermo Fisher Scientific, Waltham, MA, USA) and
the kit for inhibin- (Cat. SEA395Hu, Cloud-Clone Corporation, Wuhan,
China) were sandwich enzyme immunoassay for quantitative determination while the
kits for estradiol (Cat. #KAQ0621, Thermo Fisher Scientific) was a competitive
binding immunoassay. Bring the strips and reagents of the kit and samples to
20–28 C before use. The serum-free medium and sample diluent were used
as sample control and blank, respectively. Each sample, standard, sample control
and blank were assayed in triplicate. At the end of reaction, all the wells were
analyzed by spectrophotometry and read the absorbance of each well at 450 nm. The
kit for TNF- had a sensitivity of 0.13 pg/mL and precision (CV%) of
8.5% (intra-assay) and 9.8% (inter-assay). The sensitivity, intra-assay and
inter-assay CV for the estradiol kit and inhibin- kit were 5 pg/mL,
4.3%, 6.1% and 5.5 pg/mL, 10%, 12%, respectively. Standard curves and
sample values were plotted using GraphPad Prism Software (Version 6.0, San Diego,
CA, USA).
2.6 RNA Isolation and Real-Time PCR Quantification
Total RNA was isolated with Trizol reagent (15596018, Life Technologies,
Carlsbad, CA, USA) and reverse-transcribed into the first strand of cDNA by using
SuperScript III reverse transcriptase (2680, TaKaRa, Kyoto, Japan). Then the cDNA
were used for real-time PCR with specific primers (Table 1). Real-time PCR was
performed on a CFX96 Touch instrument (Bio-Rad) using the iQ SYBR Green
Supermix kit (170-8880, Bio-Rad, USA). The cycling conditions of PCR included 95
C for 15 s, 60 C for 30 s, repeating 40 cycles. Data were
normalized to GAPDH levels [19].
Table 1.Primers used for real-time quantitative RT-PCR.
| Gene name |
Primer pairs (5′-3′) |
| Forward |
Reverse |
| TNFR1 |
GCTGCCACTGGAACCTACTT |
GGTTTTCTGAAGCGGTGAAGG |
| TNFR2 |
CGGGAGCTCAGATTCTTCCC |
CACTGTGAGCTGTGGTCAGA |
| P450arom |
TGCATGGGAATTGGACCCC |
GGTTGTAGTAGTTGCAGGCAC |
| Inhibin- |
GATGTCTCCCAAGCCATCCTTT |
CTGGCAGCTGACTTGTCCTCAC |
| Inhibin-A |
AGTGCCAATACCATGAAGAGG |
AATTCTCTTTCTGGTCCCCACTC |
| Inhibin-B |
GCGAGAACCCTCAACTGACA |
ACCGCATCCATTTGCTGGTA |
| GAPDH |
GGACCTGACCTGCCGTCTAG |
TAGCCCAGGATGCCCTTGAG |
2.7 Immunoblotting
Antibodies specific to TNF receptor 1 (Rabbit polyclonal, ab19139, 1:1000), TNF
receptor 2 (Rabbit monoclonal [EPR1653], ab109322, 1:1000), Caspase 3 (Rabbit
monoclonal [EPR18297], ab184787, 1:1000), and aromatase (Rabbit polyclonal,
ab18995, 1:500) were obtained from Abcam. Anti-GAPDH antibody (Rabbit monoclonal
[14C10], #2118, 1:2000) was purchased from Cell Signaling Technology.
The expression of these proteins was detected by immunoblotting as previously
described [20]. The granulosa cells were lysed using RIPA lysis buffer (Cat.
P0013C, Beyotime Biotechnology, Beijing, China). The protein concentration of the
whole cell lysates was measured with a bicinchoninic acid protein assay kit
(Pierce Biotechnology). Proteins (30 g) were mixed with 5
SDS-PAGE loading buffer, then loaded and separated on 10% SDS-PAGE gels and
subsequently electrotransferred to polyvinylidene fluoride (PVDF) membranes
(IPFL00010, Merck Millipore, Kenilworth, NJ, USA). After blocking with 5% nonfat
milk for 1 h at 20–28 C, the PVDF membranes were incubated with
primary antibodies on a rocker platform at 4 C overnight. Then, the
PVDF membranes were washed with 0.1% Tween 20-containing Tris-buffered saline
(TBS-T) three times, and incubated with HRP-linked Goat anti-Rabbit IgG antibody
(#7074, Cell Signaling) at 20–28 C for 1 hour, and developed with
chemiluminescent substrate (34080, Thermo) after washing with TBS-T four times.
Autoradiography was performed with chemiluminescence image analysis system (Tanon
5200, Beijing, China).
2.8 Apoptosis Assay
Cell apoptosis were detected with an Annexin V-FITC/PI Apoptosis Detection Kit
(Cat. BMS500FI, eBioscience). After treatment with varying concentrations of
recombinant human TNF- for 96 h, 2 10 granulosa cells
were harvested and stained with 5 L of Annexin V-FITC and 5 L of PI
for 15 min in the dark at 20–28 C. The cells were then washed,
resuspended with 1 PBS containing 2% FBS, and analyzed by flow
cytometry (FACS Calibur, BD).
2.9 Statistical Analyses
All the experiments were repeated three times. SPSS 19.0 software (IBM Corp.,
Chicago, IL, USA) was used for data analysis. The data are presented as the mean
standard deviation. As the concentration of TNF- in the
follicular fluid from PCOS patients was quite different, the data were checked
the normal distribution and variance homogeneity before comparisons. The
statistical significance of differences between groups was performed by Student’s
unpaired t test. One-way ANOVA was applied for multiple comparisons,
further LSD test was used to inter-group comparisons. A value of p
0.05 was considered as statistical significance.
3. Results
3.1 Expression of TNF- in Human Follicles
TNF- detection with immunohistochemistry staining in specimens of
normal ovaries showed that the level of TNF- increased gradually in
human follicles, in accordance with the development of follicles (Fig. 1A).
However, a significantly higher TNF- level in the FF was observed for
the PCOS group than for the normal group (Fig. 1B), which to some extent
contributed to granulosa cell apoptosis and follicular atresia.
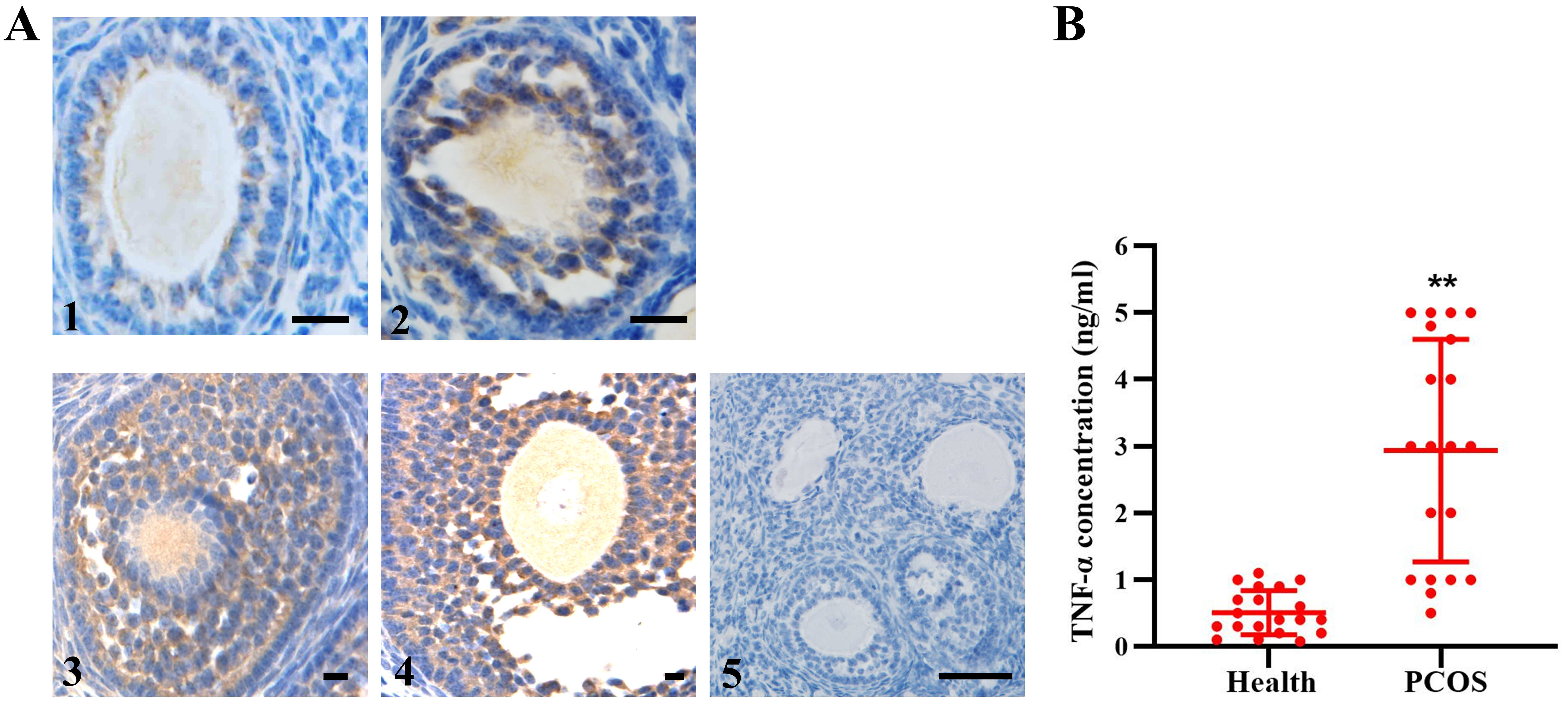 Fig. 1.
Fig. 1.
TNF- expression in human follicles. (A)
Immunohistochemistry showed TNF- expression in all stages of
developmental follicles (1–4). 5, negative control. n = 12. (B) Quantitative
detection of TNF- in FF from healthy people (n = 19) and PCOS patients
(n = 20). The data expressed as the mean SD. Bar = 20 m.
**, p 0.01, vs. health group.
3.2 TNF- does not Affect TNFR Expression
TNF- exerts its functions in the ovary through two types of receptors,
TNFR1 and TNFR2. As shown in Fig. 2A,B, the expression of TNFR1 and TNFR2 showed
no significant differences in human granulosa cells. Treatment with varying
concentrations of TNF- also caused no evident effect on the expression
of TNFR1 and TNFR2. However, the expression of TNFR1 appeared to increase in
accordance with follicular development, while the expression of TNFR2 remained
constant at all stages in follicles (Fig. 2C).
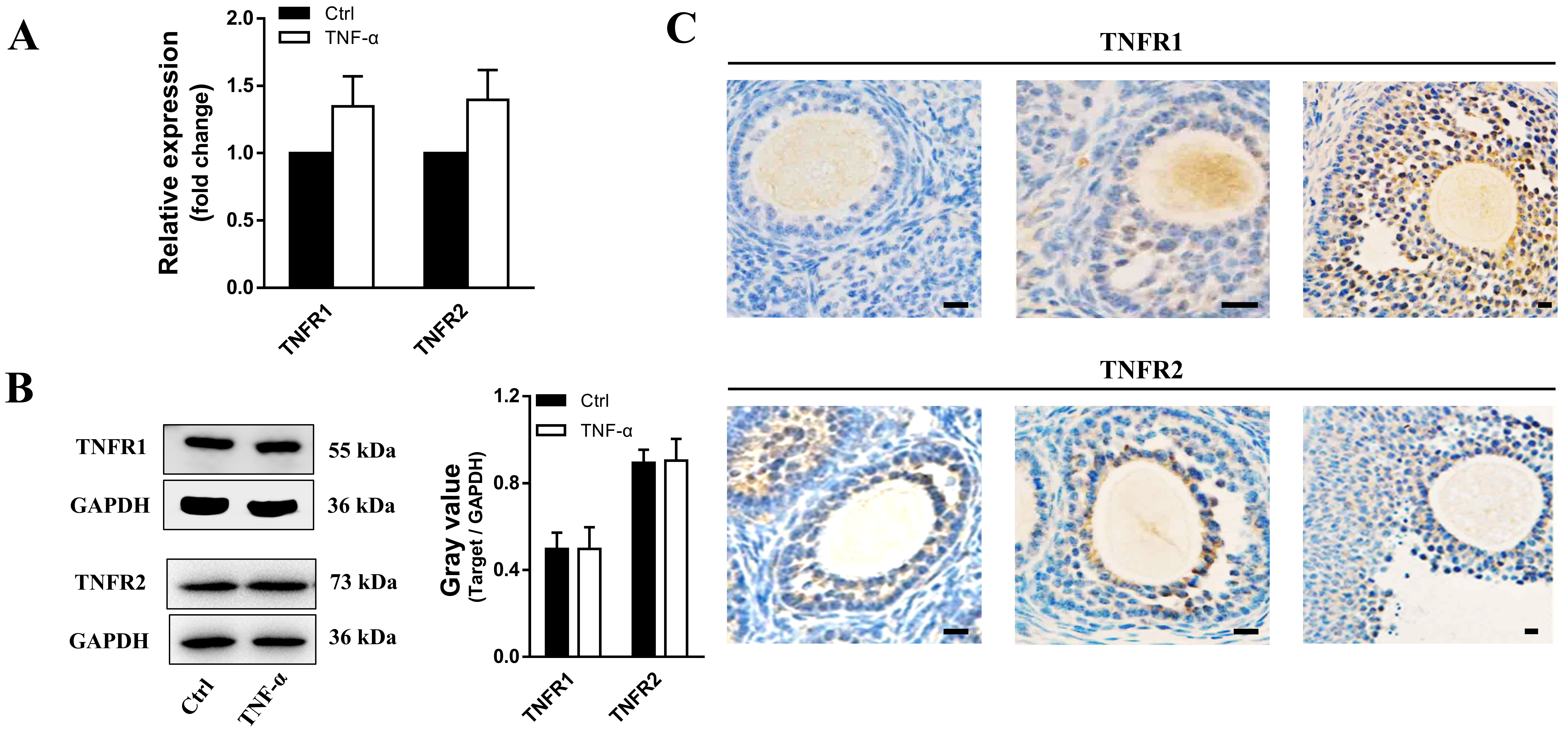 Fig. 2.
Fig. 2.
TNFR expression in cultured granulosa cells and
developmental follicles in ovarian tissues. (A) Transcript levels and (B)
protein expression of TNFR1 and TNFR2 in granulosa cells after TNF-
treatment. n = 19. (C) Immunohistochemistry showing TNFR1 and TNFR2 expression in
ovarian tissues. The data expressed as the mean SD. Bar = 20
m. n = 12.
3.3 TNF- Regulates Estradiol Synthesis in Human Ovarian
Granulosa Cells
As shown in Fig. 3A,B, both mRNA and protein expression of P450 aromatase
(P450arom), which catalyzes the formation of aromatic C18 estrogen from C19
androgens, was gradually inhibited by TNF- in a dose-dependent manner
(from 0.4 to 4 ng/mL) after 24 h of treatment. In addition, the concentration of
E2 was decreased in the same way in the conditioned medium 48 h after
TNF- treatment (Fig. 3C). However, surprisingly, P450arom expression
was significantly upregulated meanwhile E2 concentration was also increased by
treatment with 0.2 ng/mL TNF- for 48 h (Fig. 3A–C).
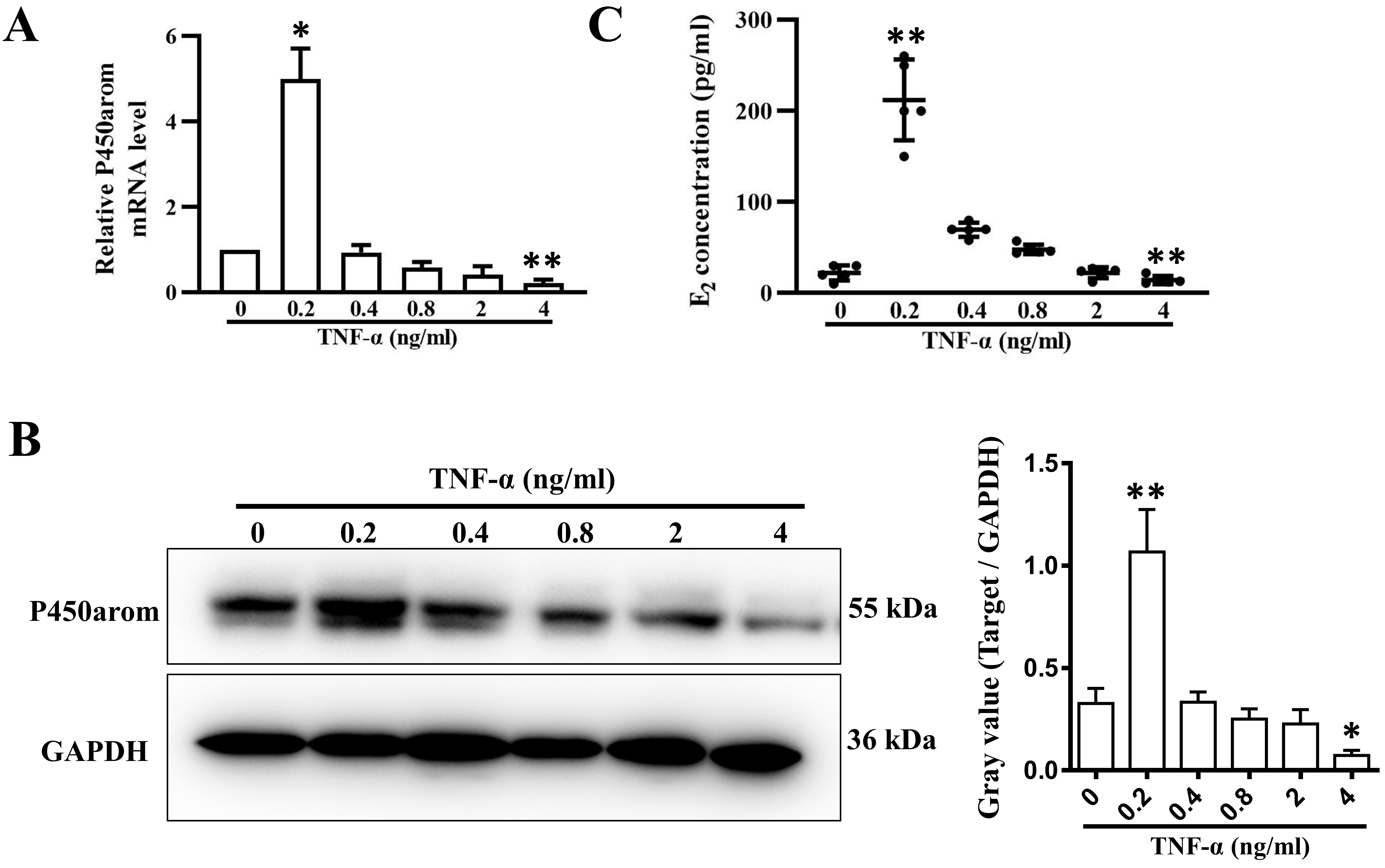 Fig. 3.
Fig. 3.
P450arom expression and E2 secretion in granulosa cells
treated with varying concentrations of TNF-. (A) Relative mRNA levels
and (B) relative protein levels of P450arom in granulosa cells treated with 0.2,
0.4, 0.8, 2, and 4 ng/mL TNF- for 24 h. (C) E2 concentrations in the
supernatants of cultured granulosa cells treated with 0.2, 0.4, 0.8, 2, and 4
ng/mL TNF- for 48 h. The data expressed as the mean SD. n = 5.
*, p 0.05; **, p 0.01, vs. untreated group.
3.4 Inhibin Secretion is Regulated by TNF- in a
Dose-Dependent Manner
In this study, our data showed that TNF- can regulate inhibin A
expression and secretion in granulosa cells in a dose-dependent manner, similar
to the regulation of P450arom expression (Fig. 4A,D). The transcription of
inhibin - and A-subunit were elevated significantly in
granulosa cells treated with 0.2 ng/mL TNF- for 24 h and decreased
dramatically as the dose of TNF- increased (Fig. 4B,C). At a
concentration of 4 ng/mL, inhibin A was significantly inhibited by
TNF-. The expression of B subunit mRNA, to some extent, was
also altered by TNF-, although no significant difference was observed.
Accordingly, the secretion of inhibin A was also markedly increased by 0.2 ng/mL
TNF- treatment for 48 h, but it decreased with increasing
TNF- concentrations (Fig. 4D).
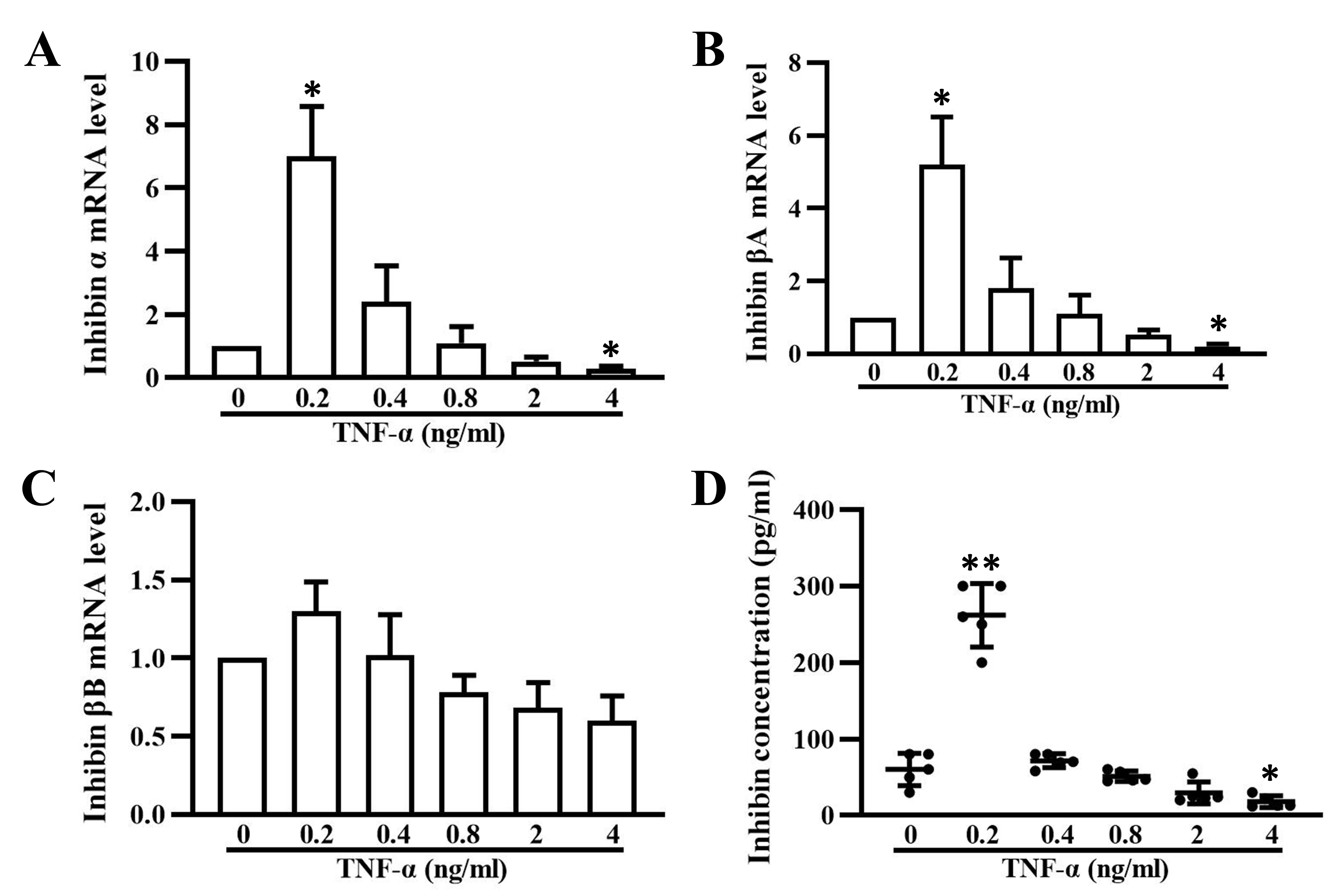 Fig. 4.
Fig. 4.
Inhibin A expression and secretion in granulosa cells treated
with varying concentrations of TNF-. Relative mRNA levels of subunits
(A), A (B), and B (C) of inhibin in granulosa cells
treated with 0.2, 0.4, 0.8, 2, and 4 ng/mL TNF- for 24 h. (D) Inhibin A
concentrations in the supernatants of cultured granulosa cells treated with 0.2,
0.4, 0.8, 2, and 4 ng/mL TNF- for 48 h. The data expressed as the mean
SD. n = 5. *, p 0.05; **, p 0.01, vs.
untreated group.
3.5 Different Concentrations of TNF- Induce Different
Effects on Cell Proliferation and Apoptosis
To assess the effect of TNF- on granulosa cell growth, a CCK-8 assay
was used to evaluate cell viability at different time points. The data showed
that 0.2 ng/mL TNF- significantly promoted cell proliferation in a
time-dependent manner, while a high dose of 4 ng/mL led to evident cell
inhibition. Compared with the untreated group, the group treated with the medium
dose (0.8 ng/mL) showed no apparent difference in cell growth (Fig. 5A). Ki-67 in
granulosa cells was expressed in a similar way after exposure to varying
concentrations of TNF- for 96 h (Fig. 5B). Furthermore, the apoptosis
assay results showed that 4 ng/mL TNF- induced significant apoptosis
and upregulation of cleaved-caspase3 (Fig. 5C,D,E), which may have contributed to
some extent to the notable decreases in E2 and inhibin A secretion.
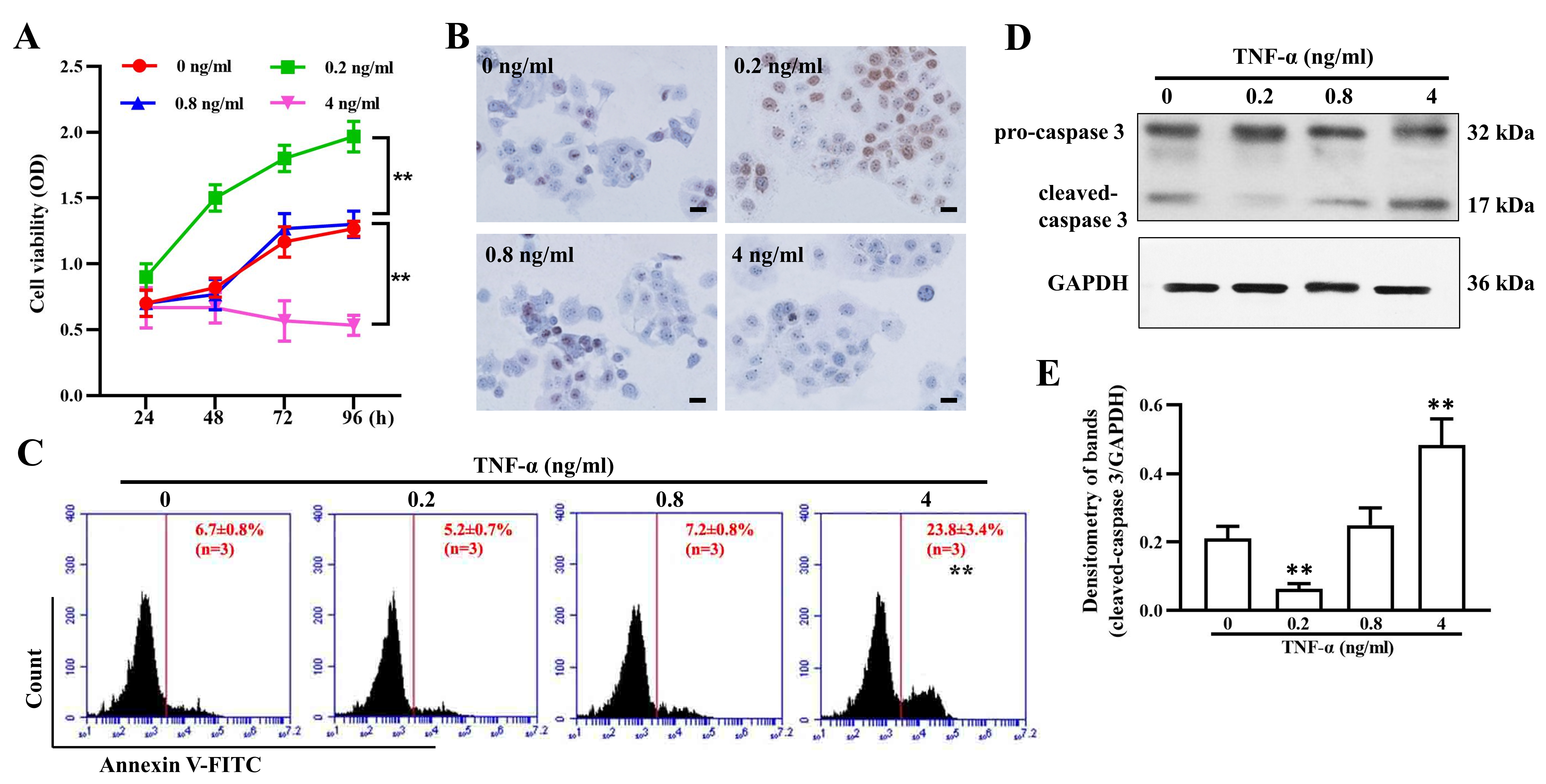 Fig. 5.
Fig. 5.
TNF- regulates granulosa cell proliferation and
apoptosis. (A) Cell viability of granulosa cells treated with 0, 0.2, 0.8, and 4
ng/mL TNF- for 24, 48,72, and 96 h. Ki67 expression (B), cell apoptosis
(C), and cleaved-caspase 3 expression (D,E) in granulosa cells after treatment
with 0, 0.2, 0.8, and 4 ng/mL TNF- for 96 h. The data expressed as the
mean SD. Bar = 20 m. n = 9. **, p 0.01,
vs. untreated group.
4. Discussion
It has been reported that TNF- in the ovaries is released from
macrophages, granulosa cells, and oocytes [2, 3, 21] and acts as an important
intraovarian regulator of steroidogenesis, follicular development, and atresia
[1, 8, 11, 12, 22, 23]. The ability of TNF- to promote follicular
development or atresia is dependent on the stage of follicular maturation.
TNF- is deemed to have a selective effect on progesterone secretion by
granulosa cells in all types of follicles before ovulation in chickens [24]. It
exerts a stimulatory effect at low doses but is inhibitory at high doses,
especially in the cells of preantral and antral follicles [17, 18]. In this
study, our results demonstrated that 0.2 ng/mL TNF- significantly
promoted granulosa cell proliferation, while 4 ng/mL TNF- notably
inhibited cell growth, which was possibly attributable to high apoptosis. Whether
TNF- exerts this biphasic effect on granulosa cells before the
development of antral follicle and luteal cells needs further investigation.
Sufficient E2 and inhibin are necessary for follicle development, ovulation, and
cyclic secretion of pituitary hormones at different phases of the ovarian cycle
while the granulosa cells in the preovulatory follicle just have the function of
synthesizing E2 and inhibin [25, 26]. It has been reported that TNF-
inhibits P450 aromatase catalytic activity and inhibin secretion in a
dose-dependent manner in granulosa cells [15, 27, 28, 29]. Our results showed biphasic
effects of TNF- on the expression of P450 aromatase and inhibin and the
secretion of E2 and inhibin A in granulosa cells, and the inhibitory effect of
the high dose may be attributable to apoptosis, as granulosa cell apoptosis is
recognized to play a key role in follicular atresia [30, 31, 32]. However, other
inducible repressors, such as cAMP-responsive element binding modulator (CREM),
which participates in the LH-triggered downregulation of aromatase and
-inhibin genes [15], probably also contribute to endocrine inhibition,
as transcriptional downregulation of P450 aromatase and - and
-subunits of inhibin were observed in our study.
The multiple biological functions of TNF- are mainly mediated by two
types of receptors, TNFR1 (p55/p60) and TNFR2 (p75/p80), which have homologous
extracellular domains but notably different intracellular domains for signaling.
TNFR1 contains an intracellular death domain essential for cytotoxicity and
apoptosis, while TNFR2 activation leads to cell survival, growth and
differentiation [33, 34, 35]. It has been verified that TNF- and its
receptors are expressed in follicles at different stages from primordial to
preovulatory follicles. Furthermore, the binding rate of TNF- to TNFR2
is 20 times rapider than TNFR1 when the receptors are isolated and purified, but
TNFR1 binds soluble TNF- with a much higher affinity than TNFR2 [34].
These findings may, to some extent, help to explain the biphasic effect of
TNF- on human granulosa cells. At a low dose, TNF-
preferentially binds to TNFR2, thus promoting cell growth, while at a high dose,
TNF- may bind to both TNFR1 and TNFR2 simultaneously, leading to
apoptosis.
Women with PCOS exhibit some metabolic abnormalities, such as obesity, insulin
resistance, type 2 diabetes mellitus [36, 37], which, to some extent, may lead to
systemically higher inflammatory cells and cytokines including TNF- in
peripheral blood and greater number of macrophages and lymphocytes immersed in
ovary as compared with healthy women [38]. Interestingly, high serum
TNF- level was also observed in lean PCOS patients [39]. Therefore,
chronic inflammation is common in PCOS patients even though the exact
pathogenesis of PCOS is not completely understood. Some studies have reported
that monoclonal anti-TNF- antibodies via intraovarian injection
improves follicular development and oocyte meiotic maturation, minimizes local
inflammatory response, decreases granulosa cell apoptosis, and improves viability
in mouse [17, 40, 41], which indicates that TNF- and TNFR1 play
essential roles in abnormal reproductive endocrine function in the ovaries. In
this study, consistent with previous reports, we observed higher levels of
TNF- in FF in the group of PCOS patients than in the control group.
Thus, it is possible that high levels of TNF- in FF and TNFR1 in
follicular cells partly contribute to the pathogenesis of PCOS (Fig. 6).
Furthermore, TNF- and TNFR1 may be promising therapeutic targets for
PCOS.
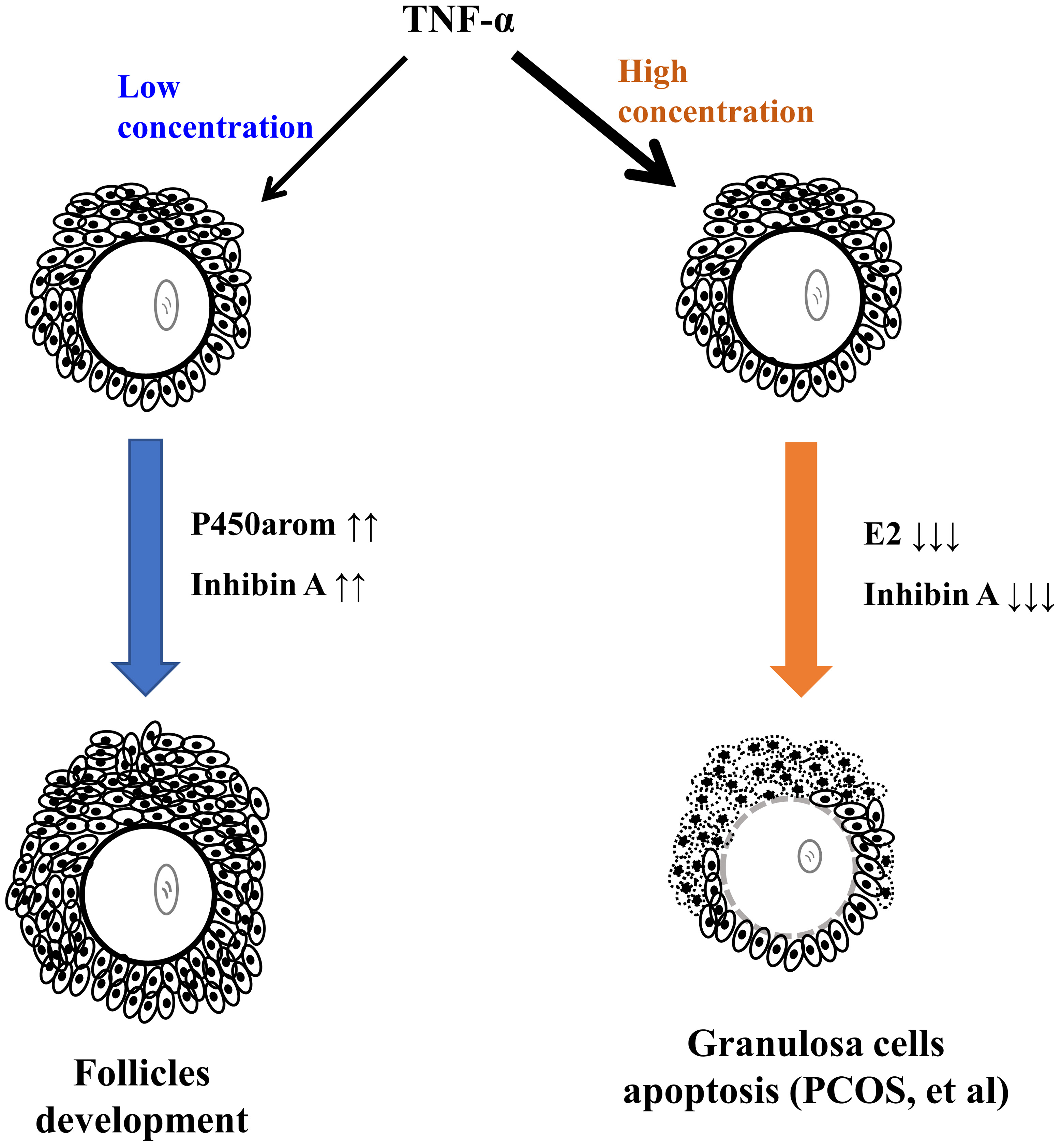 Fig. 6.
Fig. 6.
The proposed model of TNF- exerting a biphasic effect on
ovarian endocrine and follicular development.
5. Conclusions
In summary, the results demonstrate that TNF- exerts a biphasic effect
on ovarian endocrine and follicular development.
Author Contributions
DQF and JL conceived and designed the study; HYL, DKX and WHW performed the
experiments; DQF, XHT and BL analyzed the data and wrote the manuscript.
Ethics Approval and Consent to Participate
The project was approved by the Ethics Committee of China-Japan Friendship
Hospital (approval number: 2020-28-k20).
Acknowledgment
Not applicable.
Funding
This work was supported by the National Key R&D Program of China
(2018YFC1003900).
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5. Fig. 6.
Fig. 6.





