1 College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Department of Obstetrics and Gynecology, Fujian Maternity and Child Health Hospital, Fujian Medical University, 350000 Fuzhou, Fujian, China
2 Fujian Key Laboratory of Women and Children's Critical Diseases Research, Fujian Maternity and Child Health Hospital, 350000 Fuzhou, Fujian, China
Academic Editors: Anna Myriam Perrone and Michael H. Dahan
Abstract
Background: Upregulating tumor cell targeting antigens and improving
the cytotoxicity of chimeric antigen receptor T cell (CAR-T) are expected to
facilitate better treatment efficacy for solid cancers represented by ovarian
cancer. Methods: Killer cell lectin-like receptor subfamily K member 1
ligands (NKG2DL) are the target antigens for ovarian cancer. NKG2D-CAR-T cells
were constructed for NKG2D ligand on the ovarian cancer cell surface. We used
flow cytometry to evaluate the expression of NKG2DL on SKOV3 (a human ovarian
cancer adenocarcinoma cell line). Innovatively, when combined with romidepsin to
treat ovarian cancer cell SKOV3, to evaluate the killing ability of the combined
strategy, we verified the cytotoxicity of CAR-T cells by lactate dehydrogenase
(LDH) release test and determined the secretion of cytokines by enzyme-linked
immuno sorbent assay (ELISA). Results: The results of flow cytometry
showed effective activation of the NKG2D-CAR-T cells, and romidepsin treatment
resulted in increased expression of NKG2DL on the surface of SKOV3. Cytotoxicity
test showed that romidepsin could enhance the killing ability of NKG2D-CAR-T
cells against ovarian cancer cells by regulating their NKG2DL expression (p
Keywords
- CAR-T cells
- ovarian cancer
- NKG2DL
- Romidepsin
- cytotoxicity
Cellular immunotherapy exerts crucial application in tumor therapy, and chimeric antigen receptor T cells (CAR-T cells), which comprise adoptive cellular immunotherapy, through exogenous modification, recognize tumor target antigens in a manner independent of the major histocompatibility complex (MHC) molecules. These design further stimulate T cells to exert cytotoxic effects, thereby achieving direct killing of tumor cells. In the treatment of hematological malignancy, CAR-T cells show good anti-tumor efficacy. For B-cell tumors, anti-CD19-CAR-T cells are approved by the Food and Drug Administration (FDA), and patients exhibit clinical cure [1]. However, in solid tumors represented by ovarian cancer, the benefits of CAR-T cell therapy remain greatly limited. The main reason is due to the obvious solid tumor heterogeneity, which is specifically reflected in the complex cell types and the diverse gene phenotypes of the same cell type, thereby resulting in substantial differences in tumor antigens expressed by various tumor cells. It is difficult to identify single antigen marker on the surface of all or most of the tumor cells. Therefore, CAR-T cells against a single target are ineffective for solid tumor treatment [2]. Designing the application scheme for CAR-T cells contingent on the enhancement of tumor antigen expression in these cells is critical to improve treatment.
Among gynecological malignancies, ovarian cancer is the leading cause of mortality. Recurrence, metastases and drug resistance are the main characteristics of advanced ovarian cancer. CAR-T cell therapy in ovarian cancer has been the constant focus in the field of immunotherapy [3]. As early as the beginning of this century, CAR-T cells targeting folate receptors have been utilized to treat patients with advanced ovarian cancer, however, the clinical responses are not obvious [4]. For several years, mannose-specific lectin CEA (CEA), mMucin-16 (MUC16), mesothelin (MSLN), receptor protein-tyrosine kinase (HER-2), epithelial cell adhesion molecule (EpCAM), urokinase plasminogen activator surface receptor (uPAR) and tumor necrosis factor ligand superfamily member 7 (CD70) have been used as targets for CAR-T cells to treat ovarian cancer [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. Notably, a target family, killer cell lectin-like receptor subfamily K member 1 ligand, NKG2DL, was identified in reference to the activation principle of NK cells. The basic principle of natural killer cell (NK cell) activation is the balance or coordination between activated and inhibited receptors. Among them, NKG2D is an important receptor for NK cell activation. As a ligand family of the NKG2D receptor, NKG2DL usually is not found in normal cells but it is specifically expressed in several solid malignant tumors, including ovarian cancer, glioma, and colon cancer. NKG2DL-NKG2D binding is involved in promoting NK cell activation, thereby exerting specific killing effects [15]. NKG2DL family includes several ligands, unlike MHC-1 molecules, mainly comprising class I peptide-related sequences A/B (MICA/MICB) of the major histocompatibility complex and UL16-binding protein 1-6 (ULBP1-6) members [16, 17]. However, due to the heterogeneity of ovarian cancer and other solid tumors, the tumor surface antigen expression on these cells is non-uniform. Therefore, enhancing target antigen expression on the surface of tumor cells is beneficial for assisting CAR-T cells to capture tumor cells and exert killing effects.
Romidepsin is a type I histone deacetylase inhibitor (HDACi), which can regulate the balance between histone acetylation and deacetylation, thereby affecting the regulation of gene expression and the cellular epigenetics [18]. In acute lymphoblastic leukemia and non-Hodgkin’s lymphoma, romidepsin can significantly up-regulate the expression of MICA/MICB and ULBP1-4, thereby enhancing the killing ability of NK cells with the help of IL-2 [19]. In ovarian cancer, romidepsin can causes DNA damage and can enhance the anti-tumor effects of cisplatin in the cell apoptosis mechanism [20]. In cell autophagy, different mechanism from apoptosis, romidepsin acts as the role of a mediator to inhibit autophagy in resistant ovarian cancer cells [21]. However, whether romidepsin can enhance NKG2DL expression on the surface of ovarian cancer cells remains elusive. It is worth evaluating whether increasing antigen expression on the surface of ovarian cancer can enhance the killing ability of CAR-T cells. Therefore, using NKG2DL as the target antigen to construct CAR-T cells targeting NKG2DL, we verified cytotoxicity of CAR-T cells can be promoted under the action of romidepsin.
The human ovarian serous cystadenocarcinoma cell line, SKOV3, was preserved in our laboratory after sub-culturing and freezing following short tandem repeat (STR) identification. RPMI-1640 (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA) comprised the cell culture medium and 1% cyanine streptomycin double antibody (Hyclone, Logan, UT, USA) was added to prevent culture contamination. Trypsin was used to digest cells (Hyclone, Logan, UT, USA) and subculture on the following day. SKOV3 cells were incubated with romidepsin (Biyuntian Biology, Nanjing, China) at 50 ng/mL. After culturing, NKG2DL expression was identified by flow cytometric analysis. Primary T lymphocytes were derived from mononuclear cells (PBMCs) from the peripheral blood of healthy adult volunteers. Informed consent was obtained from all volunteers participating in this study and the design received ethical approval from the Fujian maternal and child health hospital. After density gradient centrifugation with Percoll (Solarbio, Beijing, China), peripheral blood samples were incubated in media with high concentration fetal bovine serum (20%). After the cells adhered, these were resuspended in the culture medium, and T lymphocytes were activated and amplified using cluster differentiation-3 (CD-3) and cluster differentiation-8 (CD-8) monoclonal antibodies (Tongli Haiyuan biology, Beijing, China) and IL-7 and IL-15 cytokines (Peprotech, Newark, NJ, USA). All cells were incubated in sterile cell incubators at 37 °C with 5% CO2.
Anti-NKG2DL-CAR (also named as NKG2D-CAR) structure uses the basic framework of
the third generation CAR. NKG2D was selected as the extracellular domain, and its
affinity with NKG2DL was used for complete antigen recognition. The framework of
NKG2D-CAR was NKG2D+CD8
Flow cytometry (BD, Newark, NJ, USA) was used to assess NKG2DL
expression in ovarian cancer cells treated with romidepsin. After centrifugation,
the cells were washed twice in PBS and fluorescent antibody MICA/MICB-PE (BD, Newark, NJ,
USA) was added at a concentration of 10
The killing ability of CAR-T cells was tested by a target killing assay. The ratio of effector T cells to tumor cells was 5:1 or 10:1, respectively. The effector cells comprised control T cells and NKG2D-CAR-T cells. The ovarian cancer cell line, SKOV3, was treated with romidepsin for 24 hours (referred to as Ro-SKOV3). The control, high control, high control blank, low control, background blank, and sample blank holes were set following the instructions of the LDH kit (Tongren chemical, Tokyo, Japan), and three multiple holes were set. Other conditions were consistent with those of the sample hole. The sample holes were set at the effective target killing ratio of 5:1. They were divided into control T+SKOV3, NKG2D-CAR-T+SKOV3, control T+Ro-SKOV3, and NKG2D-CAR-T+Ro-SKOV3 cell groups, respectively. The effective target ratio of 10:1 was set for the same grouping. Three multiple holes were set for each of the eight groups of sample holes.
The effective target killing experiment was set up with the ratio of 5:1 and
10:1, respectively, to detect the release of IFN-
GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) was used for all
statistical analyses. The data distribution was determined by F-test; data
following normal distribution were tested for significant differences by
independent sample t-test, and those following a nonnormal distribution were
evaluated by the Mann-Whitney-U test. Data were expressed as mean
The extracellular recognition fragment targeting NKG2DL-CAR-T cells is derived
from NKG2D. When constructing CAR-T cells, the CAR-T cell framework was selected,
that is, except for CD3
 Fig. 1.
Fig. 1.NKG2D-CAR T cells were constructed and activated. (A) NKG2D was selected as the extracellular recognition region of CAR T cells. (B) Retrovirus vector packaging CAR fusion protein fragment. (C) The original T cells did not express CD25 or CD69. After culture with CD3 and CD28 monoclonal antibodies, IL-7 and IL-15 cytokines and CAR T retrovirus transduction, T cells were activated effectively.
SKOV3 cells were cultured by conventional techniques. Romidepsin at a final concentration of 50 ng/mL in PBS was added to the SKOV3 cell culture medium and the cells were treated for 24 hours. Digested cells were subjected to flow cytometry. The control cells were SKOV3 cells cultured in PBS of the same concentration. Flow cytometry analysis showed that the rate of MICA/B expression in SKOV3 cells not treated with romidepsin was 52.9%, with average fluorescence intensity (MFI) of 538. The expression rate of MICA/B in SKOV3 cells treated with romidepsin for 24 hours was 84.5%, with an MFI of 806. These results are shown in Fig. 2A.
 Fig. 2.
Fig. 2.Romidepsin promotes cytotoxicity of NKG2D-CAR by increasing
MICA/B expression on cell surface. (A) Romidepsin increased MICA/B expression on
SKOV3 of ovarian cancer cells. The left showed that MICA/B expression in SKOV3
cells without romidepsin treatment; The right showed that MICA/B expression in
SKOV3 cells treated with romidepsin for 24 h. (B) Co-incubation of effector and
target cells to evaluate the cytotoxicity of CAR T cells on ovarian cancer cells
Control T cells and NKG2D-CAR T cells were co-incubated with SKOV3 and
romidepsin-treated SKOV3 at an effect target ratio of 5:1 and 10:1, respectively,
and cell lysis rates of different groups were observed (* p
After isolation of the T lymphocytes from peripheral blood samples, these were
amplified and activated in vitro and transduced with control and CAR retroviral
constructs. Flow cytometry was performed to determine whether these were
activated to obtain effector cells. SKOV3 cells and romidepsin treated SKOV3
cells (Ro-SKOV3) were the target. The effective target killing experiments were
set at 5:1 or 10:1. The results showed that the cell lysis rate of control T
cells+SKOV3 was (21.43
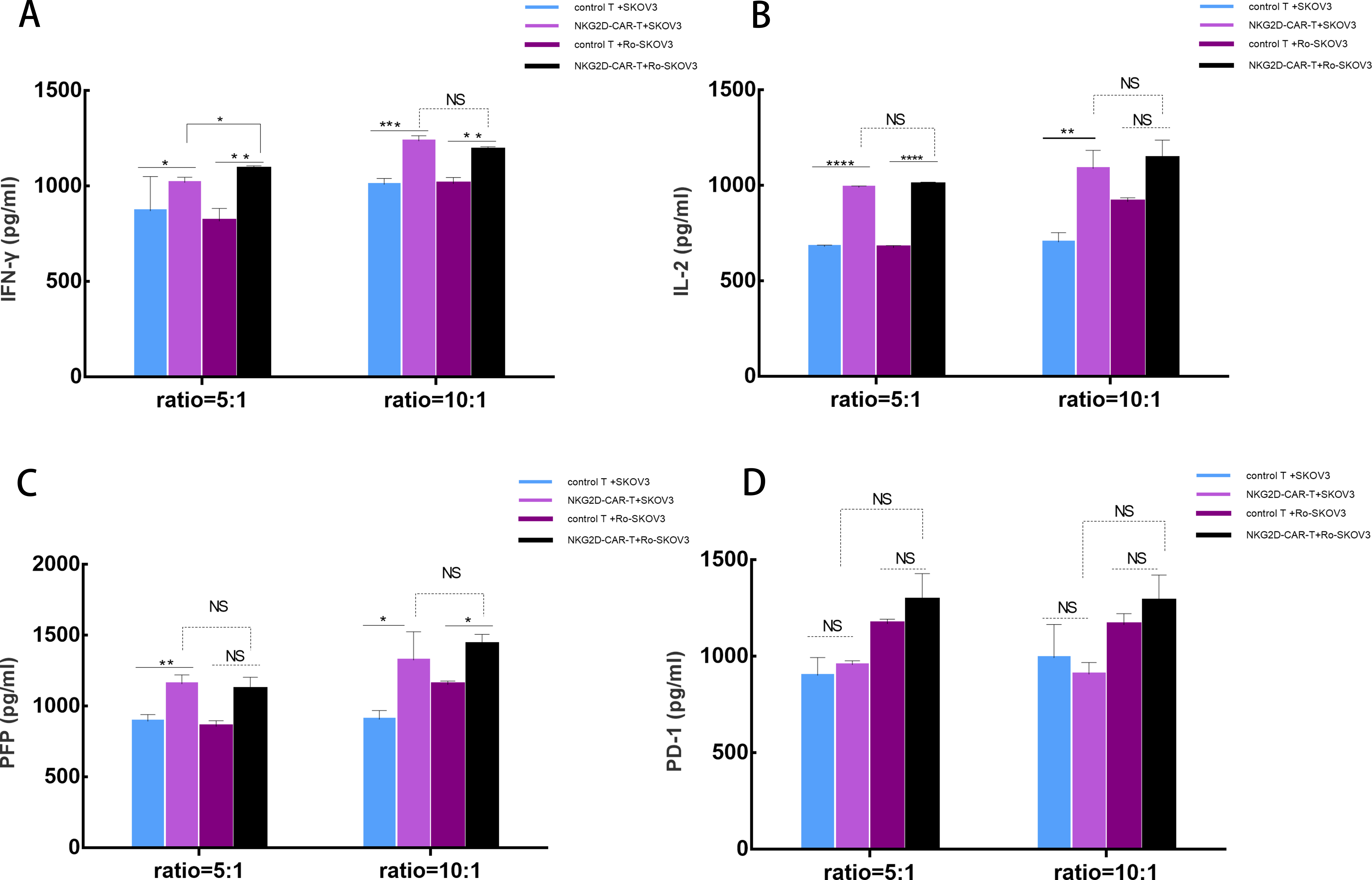
Ovarian cancer cells and T cells were co-incubated in 10:1 and 5:1 effect target
ratios, respectively. The cell supernatants from each group were analyzed for
cytokine secretion by ELISA. IFN-
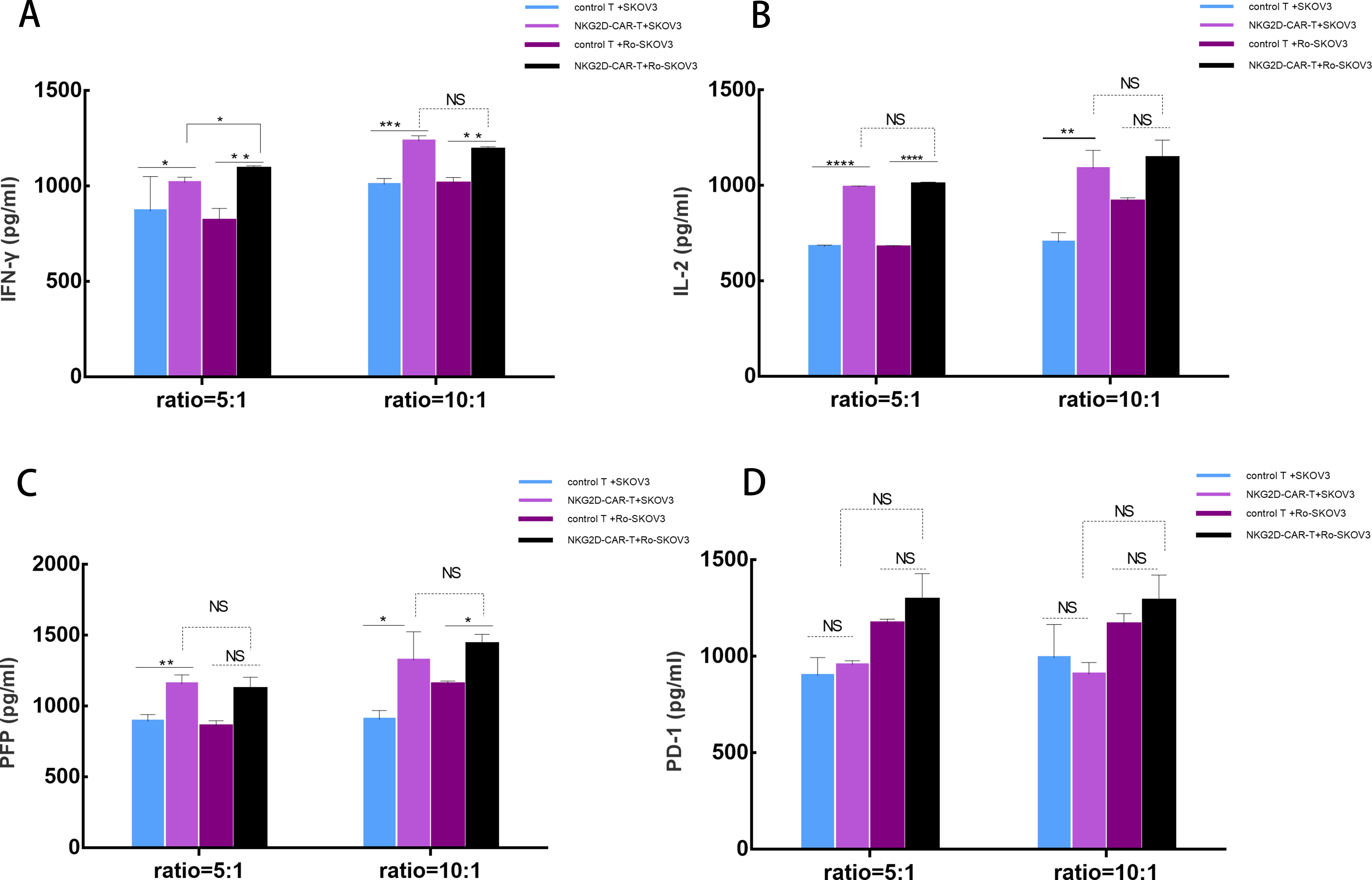 Fig. 3.
Fig. 3.Secretion of cytokines, Pore-forming protein (PFP) and programmed
cell death protein 1 (PD-1) by NKG2D-CAR T cells with cytotoxic effects. (A) Compared with control T cells, IFN-
In this study, CAR-T cells targeting NKG2DL were successfully constructed. According to the activation principle of NKG2D for NK cells, the intracellular domain of the third generation CAR-T cell was reformed, and hematopoietic cell signal transducer (DAP10) was used as the intracellular activation domain. After confirming the activation of T cells, romidepsin was found to promote the killing effects of targeted NKG2DL-CAR-T cells against ovarian cancer cells by increasing the expression of MICA/MICB on the surface of ovarian cancer cells. However, romidepsin treatment in ovarian cancer cells showed not obvious significance in cytokine secretion. The significance of the study addressed the problem of poor application of CAR-T cells in solid tumors represented by ovarian cancer. By enhancing the expression of tumor antigen, the number of immune synapses formed by CAR and tumor antigen increased significantly, and the killing ability of CAR-T cells against ovarian cancer was enhanced.
The effects of CAR-T cells in solid tumors are generally limited especially in
clinic. The current research mainly focuses on immune checkpoint blockade
combined with CAR-T cells to influence the immunosuppressive microenvironment
[22]. However, reiterating the essence of immune synapse formation, increasing
the number of target antigens, promoting the number of infiltrating immune cells,
and increasing the formation of immune synapses are crucial to improving the
therapeutic effects of T cells [23]. Romidepsin is a relatively safe histone
deacetylase inhibitor (HDACi) in clinical settings. Its anti-tumor ability has
been verified in hematological tumors. However, no clear conclusion on the
effects of romidepsin in ovarian cancer is known [24]. In this study, romidepsin
was used to enhance the expression of the target antigen, which was suitable and
clinically safe. In the clinic, ‘cytokine storm’ seriously limits the
clinical safety of CAR-T cells, and CAR-NK cells have gradually become a research
hotspot in cellular immunotherapy [25]. Romidepsin in ovarian cancer cells
resulted in increased expression of NKG2DL and enhanced cytotoxicity of CAR-T
cells. The killing effects of T cells showed near saturation at 10:1 condition.
The practical significance for clinical practice is that relatively few CAR-T
cells can be used to achieve a high anti-tumor effect, which may be efficacious
in reducing the ‘cytokine storm’ caused by T cell reinfusion in vitro.
Thus, it could serve as a method to reduce the side effects of CAR-T. Cytokines
are a double-edged sword, whereby they may cause ‘cytokine storm’ but
are also the main indicators of routine evaluation of CAR-T cell function. At the
cell culture stage, cytokines that induce differentiated T cells in vitro also
affect the function of immune effector cells. For example, IL-2 tends to promote
the expansion of effector T cells, while IL-7 and IL-15 promote the expansion of
primitive T cells, which is conducive to prolonging the time limit of CAR-T cell
functions [26]. IFN-
Immune checkpoint blockade has been extensively performed to assist CAR-T cells
to infiltrate into tumor tissues. For example, the CRISPR/Cas9 system
specifically blocks the endogenous PD-1 pathway, thereby CAR-T cells from
allogeneic sources have better function [29]. Moreover, the combination of
semi-synthetic shark V
In general, we propose an idea to affect the killing ability of CAR-T cells by regulating the expression of tumor cell target antigens. The strategy of combining existing drugs with clinically proven safety is recommended. In the future, the two-pronged combined immune checkpoint blocking approach may serve as a basis for CAR-T cells.
Our study suggests that upregulating the expression of antigens of ovarian cancer cells can enhance the cytotoxicity of CAR-T cells. Targeting NKG2DL by NKG2D-NKG2DL binding in CAR-T cell design is promising when combined with romidepsin. Further, whether any antitumor mechanism under romidepsin in cellular epigenetics and the association between immune cell therapy and epigenetics are still need to be studied in our project.
LW and PMS designed the experimental study. LW, XTL and LRY performed the experimental operation. LW, XTL and LRY analyzed the data. LW, XTL and LRY wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study is cellular experimental study. All procedures in this article and further studies in our project are performed in accordance with the 1964 Helsinki declaration. The ethic code number of this study is 2022KYLLRD03063 in Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University.
This manuscript was prepared with the assistance of BULLET EDITS LIMITED, an English-language scientific editing company.
This study was supported by grants to Natural Science Foundation of Fujian Province (2021J05077), Science and Technology Project of Fujian Provincial Health Commission (2020GGB014).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.