Academic Editor: Qian Zhong
Background: Endometriosis is one of the common benign
gynecological diseases among reproductive aged women, which almost lead to pelvic
pain, infertility and menstrual disorders. There is no blood test available for
the diagnosis of endometriosis. MUC17 has been revealed to play a role
in a variety of cancers, but the role of MUC17 single nucleotide
polymorphisms (SNPs) in endometriosis susceptibility remains unclear.
Methods: In the present study, genotyping for four MUC17
polymorphisms in 117 endometriosis patients and 118 female
control participants was undertaken using the Agena Mass ARRAY. An unconditional
logistic regression model was used to estimate the role of MUC17 gene
polymorphisms in endometriosis. Results: Bioinformatics analysis showed
that rs6966570 could be relevant to the transcription factor binding sites of
proteins bound and was related to expression quantitative trait Loci (eQTL) and
Motifs. Rs10246021 affected eQTL and Motifs. Nevertheless, there was no
significant difference in the frequency of mutation of MUC17 gene
between the case group and the control group (p
Endometriosis is defined as the presence of functional endometrial glands and stroma outside the uterine cavity with features of an array of symptoms including dysmenorrhea, infertility, excessive menstrual pain, pelvic pain with defecation and chronic pelvic pain, affecting 5–10% of women of reproductive age [1, 2]. The most common locations for the ectopic endometrial implants are the ovaries, the uterosacral ligaments and the posterior cul-de-sac [3]. Diagnosis for endometriosis is solely made through surgery as no consistent biomarkers for disease diagnosis exist. The global average time of delayed diagnosis of endometriosis is 10 years [4], while it is 13 years in Chinese women [5]. Surgery combined with hormone drugs are commonly used to treat endometriosis. But the current treatments are mainly aimed at target symptoms but not the underlying mechanisms of disease. Furthermore, the overall rate of recurrence 5 years after the operation is up to 50%. The theory of transplantation for the etiology of this disease is widespread. However, the exact pathogenesis of endometriosis is still obscure [6]. With the advent of genome-wide association studies (GWAS), some loci related to endometriosis have been revealed [7, 8], which implies that gene mutations may serve a crucial purpose in the occurrence of endometriosis. Mucins are a group of high-molecular-weight glycoprotein, which can be divided into transmembrane and secretory categories. They affect the occurrence of cancer by regulating inflammation, cell adhesion or apoptosis, and can even supervise the damage and repair of epithelial cells [9, 10, 11], which is being recognized by clinical research institutes as a target for the treatment of inflammation and cancer [12, 13].
The MUC17 gene is located in the q22.1 region of the chromosome, whose full-length open reading frame transcribes 13 exons [14]. This protein has two EGF-like domains, promoting cell proliferation, migration and restraining cell apoptosis by ErbB2 mechanism [15, 16]. Previous studies have demonstrated that MUC17 is critical for the progression of breast cancer, colon cancer, cholangiocarcinoma, and pancreatic ductal adenocarcinoma [17, 18]. Particularly, evidence showed that MUC17 expression is elevated in epithelial ovarian cancer patients [19], patients with endometriosis have a higher risk of developing ovarian cancer [20]. Hence, we hypothesized that MUC17 may be the same risk factor in endometriosis as in ovarian cancer. Moreover, a study of small sample size in Taiwan province found that the MUC17 mutation was associated with the development of endometriosis. There was only one case reported so far. Therefore, we carried out a study on women in central China to ascertain the correlation between genetic variations of MUC17 and endometriosis among population in this area.
In the present case-control study, 117 endometriosis patients
were enrolled at The Third Affiliated Hospital of Zhengzhou University between
October 2018 and December 2019, and they were all diagnosed with
histopathological examination. Participants
in the controlled group were selected from unrelated patients who underwent
laparoscopy or laparoscopic surgery and excluded endometriosis and adenomyosis
due to hydrosalpinx, simple ovarian cyst or teratoma over the same period. All
participants were native populations of Henan and surrounding provinces and not
related. Those with genetic disorders in the family history, gynecological benign
or malignant neoplasm, and who have taken any hormone drugs within three months
were excluded. There were no statistical differences in the mean (
Combining with previously published reports, and a minor allele frequency of greater than 5% in the East Asian population, we selected rs11979706, rs10246021, rs6966570, and rs4729655 from gnomAD databases (https://macarthurlab.org/2018/10/17/gnomad-v2-1/). Genomic DNA was isolated by Genomic DNA Purification Kit (BioTeke, Biotechnology, Beijing, China) in accordance with the manufacturer’s instructions. The DNA concentration and purity were determine dusing a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). We performed primers designing with Mass ARRAY Assay Design 3.1 Software (Agena, San Diego, California, USA). Agena Mass ARRAY RS1000 was utilized for SNP genotyping [21], and the data were analyzed using Mass ARRAY Typer 4.0 software (Agena Bioscience Inc, San Diego, California, USA). In this study, the rate of success in genotyping was above 95%. In order to confirm the robustness of the technology, we randomly chose 10% of samples for genotyping in duplicate.
To confirm the effect of MUC17 SNPs on allele-specific transcriptional regulation and chromatin structure, we used RegulomeDB [22] and HaploReg V4 [23].
The
Although RegulomeDB lacks sufficient evidence to prove that four loci affect transcription factor binding (Table 1), HaploReg predicted that rs6966570 was likely to affect Proteins bound and was related to eQTL and Motifs. Additionally, rs10246021 may influence eQTL and Motifs. The evidence of rs4729655 affecting EQTL is also available. Additionally, rs11979706 mutation can give rise to mistranslation.
| SNP | Gene | chromosome | Position | RegulomeDB | HaploReg |
| rs4729655 | MUC17 | 7q22.1 | 101058170 | 4 | Selected eQTL |
| rs6966570 | MUC17 | 7q22.1 | 101051777 | 4 | Proteinsbound, Motifs changed, Selected eQTL hits |
| rs10246021 | MUC17 | 7q22.1 | 101049399 | 5 | Motifs changed, Selected eQTL hits |
| rs11979706 | MUC17 | 7q22.1 | 101038078 | 5 | DNase, missense |
| SNP, single nucleotide polymorphism; eQTL, expression quantitative trait loci; 4, TF binging + DNase peak; 5, TF binging or DNase peakHWE. | |||||
The duplicate genotyping results were of 100% concordance. Firstly, as shown in
Table 2, the distribution of all the genotypes was in the HWE (p
| Allele | allele frequencies | HWE | MAF | OR | (95% CI) | p | ||
| Case, N (%) | Control, N (%) | |||||||
| rs11979706 | G | 184 (78.6) | 194 (82.2) | 0.42 | 0.19 | 0.83 | (0.53–1.30) | 0.427 |
| C | 50 (21.4) | 42 (17.8) | ||||||
| rs10246021 | G | 186 (79.5) | 196 (83.0) | 0.83 | 0.19 | 0.82 | (0.51–1.32) | 0.410 |
| T | 48 (20.5) | 40 (17.0) | ||||||
| rs6966570 | C | 184 (78.7) | 195 (82.6) | 0.68 | 0.20 | 0.80 | (0.51–1.27) | 0.355 |
| T | 50 (21.3) | 41 (17.4) | ||||||
| rs4729655 | C | 153 (65.9) | 152 (64.7) | 0.19 | 0.35 | 1.04 | (0.99–1.09) | 0.848 |
| T | 79 (34.1) | 82 (35.3) | ||||||
| SNP, single nucleotide polymorphism; OR, odds ratio; 95% CI, 95% confidence
interval; HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequencies.
p | ||||||||
| SNP | Model | Genotype | Case, N (%) | Control, N (%) | OR (95% CI) | p |
| rs11979706 | Codominant | GG | 72 (61.5) | 82 (69.5) | 1 | 0.348 |
| GC | 40 (34.2) | 30 (25.4) | 0.61 (0.28–1.34) | |||
| CC | 5 (4.3) | 6 (5.1) | 1.09 (0.58–2.02) | |||
| Dominant | GG | 72 (61.5) | 82 (69.5) | 1 | 0.242 | |
| GC + CC | 45 (38.5) | 36 (30.5) | 0.72 (0.42–1.24) | |||
| Recessive | GG + GC | 112 (95.7) | 112 (94.9) | 1 | 0.639 | |
| CC | 5 (4.3) | 6 (5.1) | 1.34 (0.39–4.60) | |||
| Log-additive | — | — | — | 0.83 (0.53–1.29) | 0.421 | |
| rs10246021 | Codominant | GG | 74 (63.2) | 82 (69.5) | 1 | 0.672 |
| GT | 38 (32.5) | 32 (27.1) | 0.85 (0.37–1.96) | |||
| TT | 5 (4.3) | 4 (3.4) | 0.90 (0.45–1.78) | |||
| Dominant | GG | 74 (63.2) | 82 (69.5) | 1 | 0.368 | |
| GT + TT | 43 (36.8) | 36 (30.5) | 0.77 (0.45–1.34) | |||
| Recessive | GG + GT | 112 (95.7) | 114 (96.6) | 1 | 0.858 | |
| TT | 5 (4.3) | 4 (3.4) | 0.88 (0.22–3.41) | |||
| Log-additive | — | — | — | 0.82 (0.52–1.29) | 0.392 | |
| rs6966570 | Codominant | CC | 73 (62.4) | 81 (68.7) | 1 | 0.654 |
| CT | 38 (32.5) | 33 (27.9) | 0.81 (0.42–1.57) | |||
| TT | 6 (5.1) | 4 (3.4) | 0.98 (0.43–2.119) | |||
| Dominant | CC | 73 (62.4) | 80 (67.8) | 1 | 0.374 | |
| CT + TT | 44 (37.6) | 38 (32.2) | 0.78 (0.45–1.34) | |||
| Recessive | CC + CT | 111 (94.9) | 114 (96.6) | 1 | 0.609 | |
| TT | 6 (5.1) | 4 (3.4) | 0.71 (0.19–2.61) | |||
| Log-additive | — | — | — | 0.80 (0.51–1.26) | 0.341 | |
| rs4729655 | Codominant | CC | 55 (47.4) | 50 (42.7) | 1 | 0.542 |
| TC | 43 (37.1) | 52 (44.5) | 1.37 (0.78–2.41) | |||
| TT | 18 (15.5) | 15 (12.8) | 0.95 (0.64–1.42) | |||
| Dominant | CC | 55 (47.4) | 50 (42.7) | 1 | 0.502 | |
| TC + TT | 61 (52.6) | 67 (57.3) | 1.19 (0.71–2.01) | |||
| Recessive | CC + TC | 98 (84.5) | 102 (87.2) | 1 | 0.570 | |
| TT | 18 (15.5) | 15 (12.8) | 0.80 (0.38–1.69) | |||
| Log-additive | — | — | — | 1.06 (0.74–1.53) | 0.760 | |
| SNP, single nucleotide polymorphism; OR, odds ratio; 95% CI, 95% confidence
interval.
p | ||||||
 Fig. 1.
Fig. 1.The LD (linkage disequilibrium) among 4 SNPs of the MUC17 gene. Haplotype blocks for the 117 control subjects and 118 endometriosis patients were constructed according to the confidence interval approach using Haploview software. The values within each red square indicate the score of the related r2 measure for allelic association between SNPs. The greater the score correspond to higher degrees of LD, up to a maximum of 1.
| SNP | Freq | Haplotype | endometriosis % | control % | p |
| rs11979706/rs10246021/rs6966570/rs4729655 | 0.645 | GGCC | 65.7 | 65.4 | 0.816 |
| 0.185 | CTTT | 17.1 | 20.5 | 0.383 | |
| 0.153 | GGCT | 17.0 | 14.1 | 0.370 | |
| SNP, single nucleotide polymorphism.
p | |||||
Taking account into dysmenorrhea, infertility, as well as
CA125 levels associated with endometriosis
[26], we further investigated the role of the four SNPs of the MUC17
gene with regard to dysmenorrhea, infertility and serum CA125 levels in patients.
However, as shown in Table 5, none of the gene polymorphisms associations with
infertility and CA125 levels was discovered (p
| SNP | Allele /Genotype | CA125 value | Reproduction ability | Dysmenorrhea | |||||||||
| CA125 |
CA125 |
OR (95% CI) | p |
non-infertility, N | Infertility, N | OR (95% CI) | p |
non-dysmenorrhea, N | dysmenorrhea, N | OR (95% CI) | p | ||
| rs11979706 | C/G | 50/184 | 42/194 | 0.93 (0.41–2.12) | 0.869 | 37/137 | 5/49 | 0.39 (0.14–1.04) | 0.051 | 21/53 | 19/137 | 0.37 (0.18–0.74) | 0.030* |
| CC /GC/GG | 5/40/72 | 6/30/82 | 1.92 (0.45–8.22) | 0.661 | 6/26/56 | 0/5/22 | 0.51 (0.17–1.51) | 0.095 | 5/11/21 | 1/17/60 | 2.10 (0.54–8.14) | 0.035* | |
| 0.70 (0.26–1.89) | 0.00 (0.00–NA |
0.26 (0.08–0.79) | |||||||||||
| Dominant | 1.09 (0.37–3.18) | 0.863 | 0.41 (0.14–1.19) | 0.103 | 0.39 (0.17–0.91) | 0.031 | |||||||
| Recessive | 0.46 (0.06–3.31) | 0.447 | 0.46 (0.06–3.31) | 0.074 | 0.07 (0.01–0.72) | 0.024* | |||||||
| Log-additive | 0.94 (0.44–2.02) | 0.882 | 0.37 (0.14–1.06) | 0.046* | 0.40 (0.20–0.80) | 0.008* | |||||||
| rs10246021 | T/G | 48/186 | 40/196 | 0.91 (0.39–2.10) | 0.832 | 35/139 | 5/49 | 0.91 (0.39–2.10) | 0.072 | 19/55 | 19/137 | 0.43 (0.21–0.88) | 0.018* |
| TT/GT/GG | 5/38/74 | 4/32/82 | 2.15 (0.49–9.46) | 0.576 | 4/27/56 | 0/5/22 | 0.55 (0.12–2.9) | 0.133 | 3/13/21 | 1/17/60 | 1.36 (0.34–5.33) | 0.060 | |
| 0.64 (0.22–1.82) | 0.90 (0.27–2.9) | 0.34 (0.10–1.08) | |||||||||||
| Dominant | 1.09 (0.37–3.18) | 0.863 | 0.41 (0.14–1.19) | 0.103 | 0.39 (0.17–0.91) | 0.031 | |||||||
| Recessive | 0.38 (0.04–3.05) | 0.367 | 0.38 (0.04–3.05) | 0.153 | 0.14 (0.01–1.45) | 0.102 | |||||||
| Log-additive | 0.89 (0.40–1.96) | 0.781 | 0.40 (0.15–1.09) | 0.066 | 0.43 (0.21–0.90) | 0.024* | |||||||
| rs6966570 | T/C | 50/184 | 41/195 | 0.95 (0.41–2.21) | 0.916 | 36/138 | 5/49 | 0.38 (0.13–1.12) | 0.081 | 19/55 | 20/136 | 0.45 (0.22–0.92) | 0.026* |
| TT/CT/CC | 6/38/73 | 4/33/81 | 2.29 (0.52–10.02) | 0.535 | 4/28/55 | 0/5/22 | 0.45 (0.15–1.32) | 0.112 | 3/13/21 | 1/18/59 | 1.44 (0.37–5.65) | 0.592 | |
| 0.65 (0.23–1.84) | 0.00 (0.00–NA) | 0.34 (0.11–1.09) | |||||||||||
| Dominant | 1.17 (0.41–3.39) | 0.763 | 0.38 (0.13–1.12) | 0.080 | 0.42 (0.18–0.97) | 0.043* | |||||||
| Recessive | 0.38 (0.04–3.04) | 0.367 | 0.00 (0.00–NA) | 0.155 | 0.14 (0.01–1.45) | 0.103 | |||||||
| Log-additive | 0.94 (0.42–2.07) | 0.876 | 0.39(0.14–1.05) | 0.056 | 0.45 (0.22–0.93) | 0.032* | |||||||
| rs4729655 | TC | 6/111 | 4/114 | 1.43 (0.66–3.07) | 0.359 | 67/105 | 13/41 | 0.52 (0.26–1.04) | 0.062 | 32/40 | 46/110 | 0.48 (0.26–0.88) | 0.017* |
| TT/TC/CC | 18/43/55 | 15/52/50 | 1.24 (0.39–3.95) | 0.609 | 14/39/33 | 1/11/15 | 0.39 (0.13–1.14) | 0.204 | 8/16/12 | 6/34/38 | 1.42 (0.59–3.42) | 0.079 | |
| 1.30 (0.53–3.16) | 0.53 (0.22–1.28) | 0.48 (0.26–0.91) | |||||||||||
| Dominant | 1.63 (0.62–4.27) | 0.320 | 0.53 (0.22–1.28) | 0.158 | 0.53 (0.23–1.24) | 0.142 | |||||||
| Recessive | 1.34 (0.24–7.52) | 0.736 | 0.18(0.02–1.51) | 0.126 | 0.28 (0.09– 0.91) | 0.034* | |||||||
| Log-additive | 1.45 (0.70–3.00) | 0.314 | 0.49 (0.24–0.99) | 0.046* | 0.47 (0.25–0.88) | 0.017* | |||||||
| *Indicates statistical significance.
p | |||||||||||||
Of note, in the allele model, the C allele of rs11979706 (OR:
0.37; 95% CI: 0.18–0.74; p = 0.03), the T allele of rs10246021 (OR:
0.43; 95% CI: 0.21–0.88; p: 0.018), the T allele of rs6966570 (OR:
0.45; 95% CI: 0.22–0.92; p: 0.026), and the T allele of rs4729655 (OR:
0.48; 95% CI: 0.26–0.88; p: 0.017) reduced the risk of the occurrence
of dysmenorrhea in endometriosis (Table 5). Further logistic regression analysis
was carried out. After adjustment for age and BMI, we observed that the frequency
of the heterozygous variant of rs11979706 was a protective factor in patients
with endometriosis (Table 5). The Recessive model after adjustment for age showed
that the individuals with MUC17 rs11979706 CC genotype have a 93% lower
risk of dysmenorrheal than GC + GG genotype (OR: 0.07; 95% CI: 0.01–0.72;
p: 0.024). Meanwhile, individuals with rs4729655 TT genotype have a 72%
lower risk of dysmenorrheal than CT + CC genotype (OR: 0.28; 95% CI: 0.09–0.91;
p: 0.034). It is also worth noting that the “log-additive” could be an
extremely sensitive model in our case. In this model, all four genetic loci were
significantly associated with dysmenorrhea (p
As shown in Table 6, the four protective alleles haplotypes were available for analysis. Unsurprisingly, haplotype “CTTT” showed a lower frequency of dysmenorrhea in the endometriosis group (p = 0.008). On the contrary, patients with haplotype “GGCC” showed a significantly increased frequency of dysmenorrhea (p = 0.045) (Table 6, Fig. 2). Therefore, MUC17 haplotypes could be an indicator of dysmenorrhea in patients.
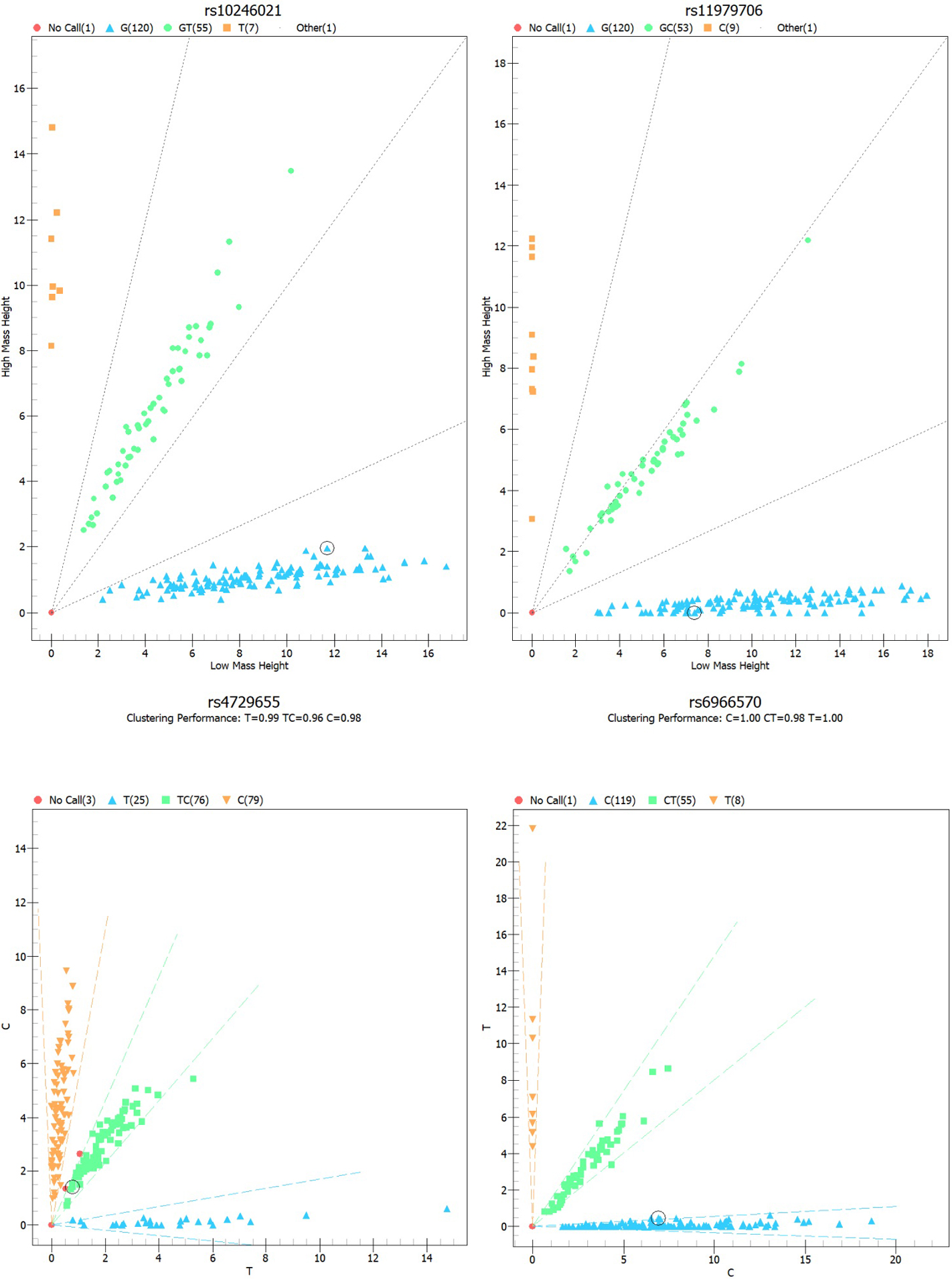 Fig. 2.
Fig. 2.The LD (linkage disequilibrium) among 4 SNPs of the MUC17 gene that associated with dysmenorrhea in endometriosis patients. Haplotype blocks for the 26 patients without infertility and 88 patients with infertility were constructed according to the confidence interval approach using Haploview software. The values within each red square indicate the score of the related r2 measure for allelic association between SNPs. The greater the score correspond to higher degrees of LD, up to a maximum of 1.
| SNP | Haplotype | Freq | Non-dysmenorrhea, % | Dysmenorrhea, % | p |
| rs11979706/rs10246021/rs6966570/rs4729655 | GGCC | 0.651 | 69.8 | 57.6 | 0.045* |
| CTTT | 0.169 | 12.6 | 27.0 | 0.008* | |
| GGCT | 0.167 | 17.6 | 15.4 | 0.631 | |
| *Indicates statistical significance; SNP, single nucleotide polymorphism.
p | |||||
Endometriosis is a common gynecological disease, and secondary dysmenorrhea is a
typical manifestation of endometriosis. In our study, 67.8% of the patients with
endometriosis had dysmenorrhea symptoms. Previous reports showed that
dysmenorrhea is caused by the local inflammatory response due to the ectopic
implantation of endometrial cells. This response leads to the release of
inflammatory factors, promoting uterine contraction, afterwards, triggering
dysmenorrhea. Yang B et al. [27] found MUC17 maintained the
MYH9-p53-RhoA regulatory feedback loop through the EGF-like domain
and downstream sequence, and then activated
p38 signal to transduce NF-
However, unfortunately, we did not discover that MUC17 (rs11979706, rs10246021, rs6966570, rs4729655) gene polymorphisms were associated with endometriosis in Central Plains Chinese women. Similar to our results, Yang C et al. [33] failed to show a correlation between rs4729655 polymorphism and the risk of endometriosis in Taiwan population either. However, they revealed the genetic variation of rs4729655 was associated with a lower risk of infertility, which was also reflected in the data we obtained, not obvious, though. As they research suggests, the rs4729655 could link to endometriosis-related infertility through regulate miR-4508 and miR-3158-3p expression. Moreover, rs11979706 also played a protector role in our study, which may work through the missense mutations and haplotype mechanisms above mentioned.
However, several limitations need consideration in our study. Endometriosis may be a disease caused by multiple factors and genes. Whether the interaction with other genes can affect the occurrence and development of endometriosis requires a lot of cellular and molecular biological evidence. Moreover, the sample size of our research is small, and whether this result can be necessarily generalizable to the whole Chinese population has yet to be confirmed by a larger cohort, multi-regional, and multi-ethnic joint research.
In conclusion, this study showed that the MUC17 gene polymorphism could not be a significant relevant factor to endometriosis in Central Plains Chinese women. Nonetheless, MUC17 SNP locis and haplotype could be potential targets of endometriosis dysmenorrhea and treatment in this population, and reference index for patients with endometriosis-induced infertility to choose in vitro fertilization (IVF). Future study should be in larger populations, in an attempt to clarify the relationship between MUC17 gene polymorphism and endometriosis, and establish the system of endometriosis cell lines research to perform functional tests to further understand the association between MUC17 locus mutation and endometriosis.
MQ—Conceptualization, Formal Analysis, Investigation, Methodology, Software, Resources, Writing - original draft; HZ—Conceptualization, Formal Analysis, Data Curation, Methodology, Resources, Writing - original draft; YX—Data Curation, Methodology, Resources, Software, Writing - review & editing. LY—Conceptualization, Formal Analysis, Investigation, Project administration, Writing - review & editing. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of The Third Affiliated Hospital of Zhengzhou University (Approval number: (2019) Medical ethics review NO.20).
Not applicable.
This work was supported by National Health and Family Planning Commission of Henan Province (Grant No. 2018020215).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
