1 Department of Endocrinology, Medical Faculty, Medical University – Sofia, 1431 Sofia, Bulgaria
Academic Editors: Ugo Indraccolo and Michael H. Dahan
Abstract
Background: Hyperprolactinemia is a common finding in women of reproductive age that could result from physiological factors, pathological conditions or the use of different medications. The therapeutic approach depends on the etiology of hyperprolactinemia, thus, the proper diagnosis is paramount. Case: Herein, we present a case of a young woman with pituitary incidentaloma and leiomyoma-associated hyperprolactinemia. The initial diagnosis was microprolactinoma, based on typical clinical features, increased prolactin levels, and pituitary adenoma. However, dopamine agonist treatment did not lead to any reduction of prolactin levels or clinical improvement. The patient was myomectomized because of uterine fibroid enlargement. After the surgery, the hyperprolactinemia resolved completely, while the pituitary adenoma did not show any changes. The concomitant development of pituitary adenoma and extrapituitary hyperprolactinemia might not be such a rare phenomenon, considering the high prevalence of pituitary incidentalomas. Conclusions: Currently, there are no specific tests that could distinguish pituitary from extrapituitary prolactin production. Our case report and the literature review show that leiomyoma-associated hyperprolactinemia should be considered in women of reproductive age with treatment-resistant prolactinoma and uterine fibroids above 5 cm. Further studies are needed to explore the underlying mechanisms and the possible regulators of the ectopic prolactin secretion.
Keywords
- Ectopic hyperprolactinemia
- Prolactinoma
- Myoma uteri
- Infertility
Hyperprolactinemia is a common hormonal disorder leading to galactorrhea, hypogonadism, infertility, and bone mineral density reduction. Plenty of physiological and pathological factors might be associated with hyperprolactinemia including pregnancy, breastfeeding, coitus, stress, hypothalamic-pituitary stalk damage, sellar or parasellar tumours, systemic renal or hepatic disorders, polycystic ovarian syndrome, hypothyroidism, and different pharmacological agents [1]. The thorough diagnostic approach in symptomatic patients with increased prolactin levels requires exclusion of potential secondary causes of hyperprolactinemia as well as pituitary imaging considering the possibility for pituitary lesions [2]. However, the pituitary incidentaloma might co-exist with secondary hyperprolactinemia leading to huge obstacles in the differential diagnosis. Moreover, ectopic intracranial prolactinomas originating from pharyngeal pituitary remnants as well as ectopic prolactin production by mesenchymal and other tumours have been described in the literature since 1970-ies [3, 4, 5, 6, 7, 8]. Leiomyoma-associated hyperprolactinemia has also been reported, though contradictory results have been obtained by immunostaining for prolactin in fibroid tissues [9, 10]. Nevertheless, the rapid normalization of prolactin after myomectomy in patients with intact pituitary supports the diagnosis [9, 10].
On the opposite, herein we present a more complicated case of a patient with pituitary microadenoma and leiomyoma-associated hyperprolactinemia. The diagnostic and therapeutic difficulties in such cases are briefly reviewed.
A 38-year-old Caucasian woman was referred to an Endocrinology department because of hyperprolactinemia treatment resistance. Since the age of 37 years, the patient complained of oligo- to amenorrhea and galactorrhea. As expected, moderate hyperprolactinemia (3242 mIU/L) had been found and therapy with cabergoline 0.5 mg/twice weekly had been started. Magnetic resonance tomography (MRT) scan of the pituitary had visualized an adenoma 4.7/4.4 mm, so a microprolactinoma had been suspected. However, the 6-month treatment with dopamine agonist was not related to any clinical improvement, and the control prolactin levels were still significantly raised (3552 mIU/L). The cabergoline dose was increased (0.5 mg/three times weekly) and the MRT follow-up showed a microadenoma reduction to 3.5/2.2 mm without any effect on prolactin levels. The patient was still amenorrheic with bilateral galactorrhea. She did not use any medications, had no previous gynecological interventions or pregnancies but wanted to conceive. Her past medical history was unremarkable except for two uterine fibroids. At the age of 30 she had been diagnosed for the first time with a fibroid 5.8 cm during transvaginal ultrasonography but she had been with regular menstruation. At the age of 37 a significant enlargement of the myoma was visualized by ultrasound and therefore MRT of the pelvis was performed showing two fibroids—2 cm and 8.8 cm large. A surgery was recommended but the patient postponed the procedure due to personal reasons. The subsequent transvaginal ultrasound at the age of 38 showed an enlargement up to approximately 9.9 cm and the operative treatment was strongly recommended again.
Meanwhile, the hormonal investigations at the Endocrinology department showed
repeatedly high prolactin levels despite the dopamine agonist dose
increase—prolactin 2781 mIU/L (reference range
At the age of 39 years the patient underwent a laparotomy. Two transmural and
subserous formations on the posterior wall and fundus uteri with diameter 7 cm
and 1 cm were extirpated and the leiomyomas were proven histologically. The
immunohistochemical study showed a weak focal expression of prolactin in the
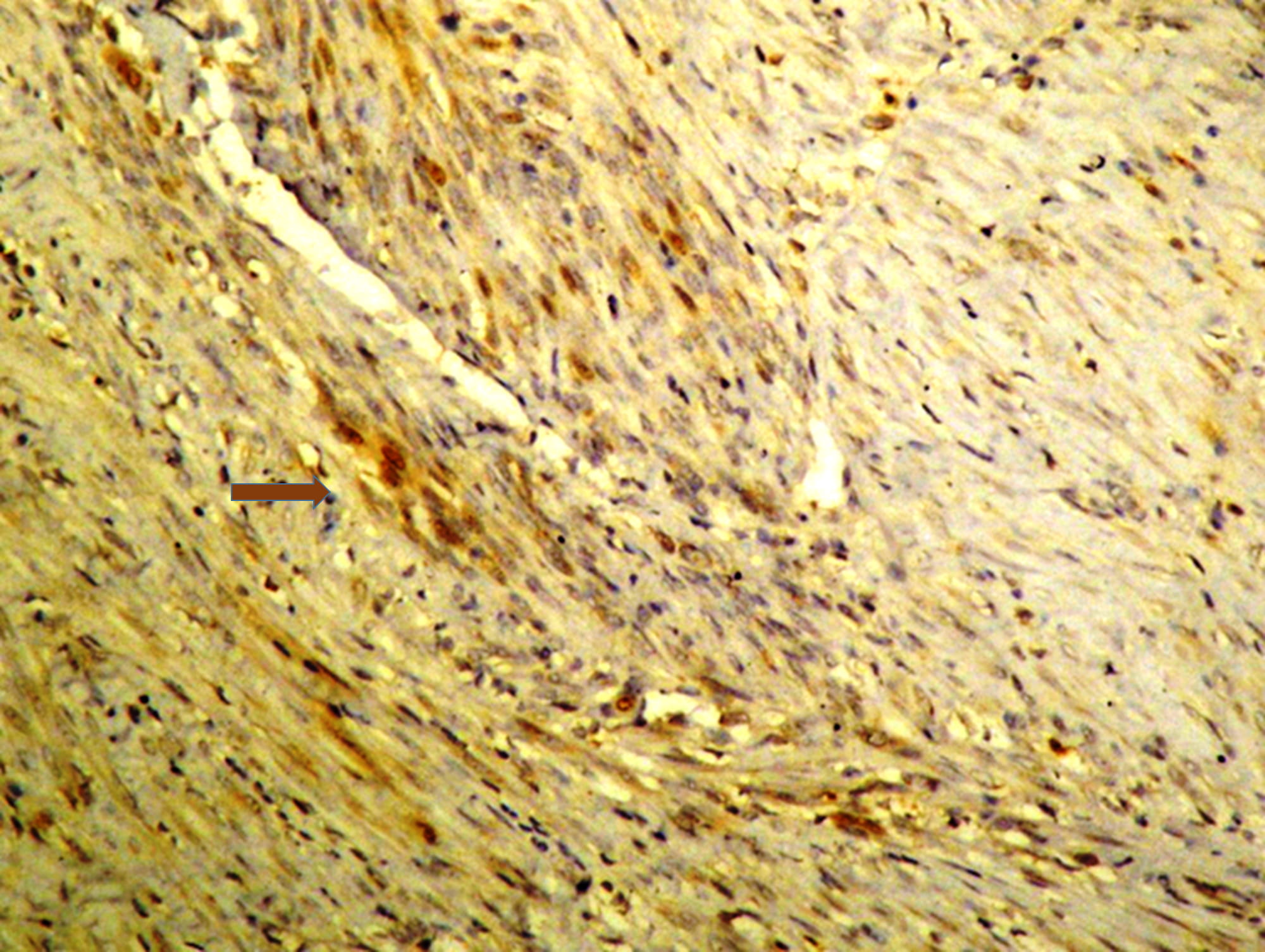
smooth muscle cells of the larger tumour (Fig. 1). No postoperative complications
emerged and the patient recovered relatively fast. Approximately one month after
the operation, the regular menstrual cycle was restored and galactorrhea
disappeared. Prolactin levels dropped and were maintained in the middle of the
normal referent ranges without any treatment for the next two years (215/316
mIU/L, reference range:
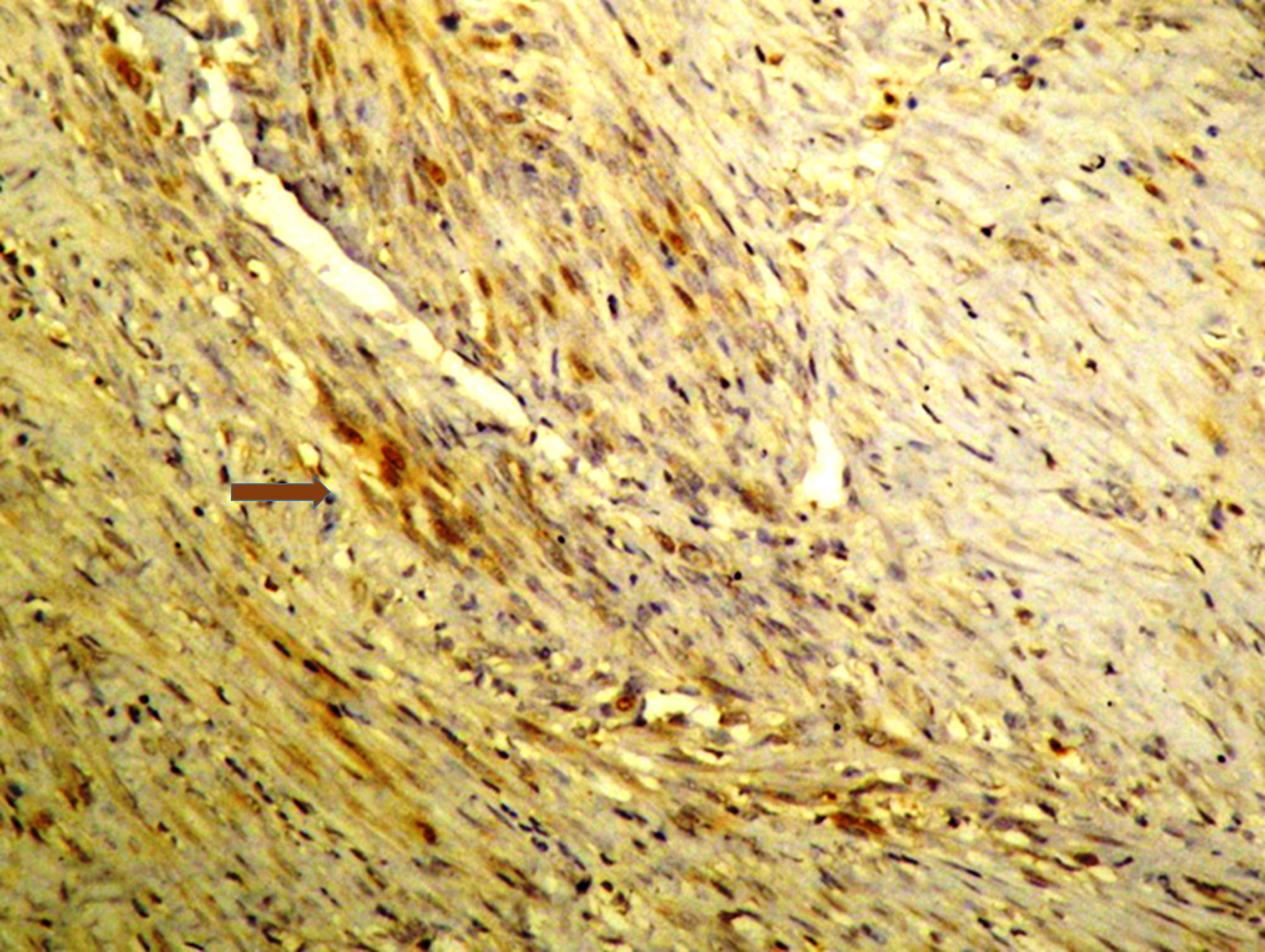 Fig. 1.
Fig. 1.
Immunohistochemical study of the leiomyoma showing a weak
expression of prolactin (
The present case describes a patient with extrapituitary hyperprolactinemia from a benign uterine tumour as well as a non-secreting pituitary microadenoma focusing on the difficulties in the differential diagnosis.
Pituitary lactotrophs are the main source of prolactin synthesis in the body but other tissues such as myometrium, endometrium, decidua, and breast, some types of immune cells and neurons might also produce prolactin [11, 12]. Despite similar immunological and biological features of the hormone, the extrapituitary prolactin-secreting cells might have different storage capacity and tissue-specific regulation by local autocrine and paracrine factors. Thus, the stimulators and inhibitors of prolactin release could differ significantly in the pituitary, uterine and tumour tissues [11, 12]. Uterine leiomyomas are capable to produce prolactin and a high prolactin gene expression has been described especially in some uterine fibroid subtypes [13, 14]. The clinical significance of the extrapituitary hormonal synthesis is obscure, although increased prolactin levels have been described in patients with leiomyomas compared to healthy women [15].
Only a few case reports in the literature presented patients with
leiomyoma-associated hyperprolactinemia (Table 1, Ref. [5, 6, 9, 10, 16, 17, 18, 19, 20, 21]). Common characteristics of all cases were the presence of normal
pituitary gland, large uterine fibroids (at least 5.5 cm), mild to moderate
prolactin increase with classic clinical symptoms, complete resistance to
dopamine agonist treatment, a rapid decrease of the hormonal concentrations after
tumour removal, and negative immunostaining for prolactin by an
immunohistochemical study of the leiomyomas [9, 16, 17, 18, 19]. Recently, Sachdev
et al. [10] found for the first time a prolactin-producing fibroid
tissue with focal but intense immunochemical staining for prolactin. Similarly,
Korytnaya et al. [7] described a prolactin-secreting perivascular
epithelioid cell tumour and detected strong prolactin staining in 5–10% of the
cells on immunohistochemistry. They calculated that the total volume of the 5%
prolactin-secreting cells in the 17
| Study | Age | Reproductive symptoms | Prolactin before uterine surgery [ng/mL] | Treatment with dopamine agonists | Uterine formations [size, imaging type] | Prolactin after uterine surgery [ng/mL] | Immuno-staining for prolactin |
| Other symptoms /diseases | Effect on the prolactin levels | Pituitary adenoma [size, imaging type] | |||||
| Ectopic leiomyoma-associated hyperprolactinemia with pituitary adenoma | |||||||
| Cordiano V [20], 2004 | 44 F | SA | 218 | Bromocriptine | 1 uterine fibroid [8 |
15.00 | N.A. |
| Secondary erythrocytosis | Resistance | Pituitary microadenoma [2 mm by MRT] | |||||
| Current case | 38 F | OA, G | 167 | Dostinex | 2 uterine fibroids [up to 9.9 cm by US] | 10.10 | Weak focal positive staining |
| Infertility | Resistance | Pituitary microadenoma [4.7 | |||||
| AITD | |||||||
| Ectopic leiomyoma-associated hyperprolactinemia without pituitary adenoma | |||||||
| Herzog AG [16], 2000 | 36 F | OA, G | 40 to 100 | Bromocriptine | 2 uterine fibroids [larger – 5.5 cm with subsequent increase by US] | Normal | N.A. |
| Migraine | Paradoxal rise | ||||||
| Joint pains | |||||||
| Sato et al. [17], 2018 | 45 F | SA, G | 74.6 | Bromocriptine | 1 uterine fibroid [9.0 cm by CT] | 0.80 | Negative |
| Dostinex | |||||||
| Paradoxal rise | |||||||
| Sendur et al. [18], 2019 | 25 F | SA, G | 150 | Bromocriptine | 1 uterine fibroid [6.0 |
3.43 | Negative |
| Dostinex | |||||||
| Resistance | |||||||
| Upreti et al. [19], 2020 | 41 F | SA, G | 94 to 277.3 | Dostinex | 1 uterine fibroid [8.8 |
1.08 | Negative |
| Infertility | Paradoxal rise | ||||||
| Headaches | |||||||
| Sachdev et al. [10], 2020 | 47 F | SA, G | 213 | Dostinex | 1 uterine fibroid [up to 13.9 |
8.70 | Intense focal positive staining |
| Resistance | |||||||
| Barry et al. [9], 2021 | 37 F | OA, G | 46.53 to 87.23 | Dostinex | 1 uterine fibroid [15.2 |
2.49 | Negative |
| Infertility | Paradoxal rise | ||||||
| Ectopic hyperprolactinemia associated with other uterine formations without pituitary adenoma | |||||||
| O’Meara et al. [21], 2009 | 35F | G, abnormal bleeding | 85.60 | Not treated | 1 uterine tumor resembling ovarian sex cord tumor–recurrent, malignant [9.9 |
N.A. | Negative |
| Simsir et al. [5], 2012 | 34 F | SA, G | 151.89 to |
Bromocriptine | 1 uterine formation - low-grade malignant mesenchymal tumor [15 |
0.30 | Negative |
| Dostinex | |||||||
| Paradoxal rise | |||||||
| Dimitriadis et al. [6], 2020 | 46 F | SA | 223 to 546 | Dostinex | 1 uterine tumor resembling ovarian sex cord tumor–recurrent, malignant [11 cm by CT] | 3.00 | Negative |
| Paradoxal rise | |||||||
| SA, secondary amenorrhea; OA, oligoamenorrhea; G, galactorrhea; AITD, autoimmune
thyroid disease; US, ultrasound scan; MRT, magnetic resonance tomography scan;
CT, computer tomography scan; F, female; N.A., not available. Prolactin levels are presented in ng/mL ( | |||||||
Several mechanisms have been suggested to explain leiomyoma-associated hyperprolactinemia. The increased secretion of prolactin by uterine fibroids might result from local dysregulation and activated hormonal synthesis. On the other hand, tumour secretion of currently unknown prolactin-stimulating factors might also be a plausible explanation considering the contradictory immunohistochemical results. Other possible causes for the development of hyperprolactinemia include a presence of ectopic pituitary tissue, prolactin secretion from concomitant lymphocyte infiltration in the tumour, as well as a mass effect of the huge leiomyomas on the surrounding tissues with stress-associated prolactin increase [5, 11, 16, 17, 18]. Interestingly, the beginning of the clinical complaints in our patient coincided with the enlargement of the uterine fibroid above 5.8 cm. Thus, we could assume that a certain minimal threshold for tumour volume is needed so that local prolactin secretion could exert systemic effects.
On the other hand, the increased local prolactin secretion in the leiomyomas might be one of the factors responsible for their rapid enlargement. Prolactin has been shown to stimulate the growth and fibrotic differentiation of smooth muscle cell lines by signal transducer and activator of transcription 5 (STAT5) as well as mitogen-activated protein kinase (MAPK) dependent mechanisms [22, 23]. Since leiomyomas might express dopamine receptor type 2, treatment with cabergoline has been tried in women with benign uterine fibroids with promising results on volume shrinkage and reduction of symptoms [23, 24]. However, in all described patients with leiomyoma-associated hyperprolactinemia the dopamine agonist treatment was ineffective (Table 1, Ref. [5, 6, 9, 10, 16, 17, 18, 19, 20, 21]). Thus, the development of systemic hyperprolactinemia in women with leiomyomas might be a sign of complete autonomization of prolactin secretion potentiating the tumour growth and resistant to medical treatment.
The proper diagnosis of extrapituitary hyperprolactinemia is difficult in patients with normal pituitary, but the same diagnosis might become a real challenge in the presence of a concomitant pituitary incidentaloma. According to the common clinical practice the prolactinoma diagnosis should be based on the presence of sustained hyperprolactinemia and a pituitary adenoma in the absence of other possible causes [2]. Our patient met all the criteria, and her pituitary adenoma had shown an initial reduction of the tumour size with 25% on dopamine agonist treatment. The patient had clear indications for myomectomy considering the tumour enlargement and the planning of fertility. Therefore, the possible therapeutic options regarding hyperprolactinemia, e.g., increase of cabergoline doses or transsphenoidal adenomectomy were postponed. To the best of our knowledge, there is only one published case of leiomyoma-associated hyperprolactinemia in a patient with a pituitary microadenoma described by Cordiano et al. [20]. Their patient had been treated ten years with bromocriptine up to 30 mg/d without any decline of the prolactin levels. She was hysterectomized because of recurrent abdominal pain and suspected myomatous erythrocytosis syndrome. After the surgery her prolactin levels dropped to normal ranges and hematological abnormalities resolved [20]. Blood count abnormalities have not been found in the other described patients, including our case.
The concomitant development of pituitary adenoma and extrapituitary hyperprolactinemia might not be such a rare phenomenon, considering the high prevalence of the microincidentalomas on MRT (10–38%) [25]. Approximately 10% of patients with microprolactinomas are resistant to cabergoline treatment, while some non-functioning adenomas might respond to dopamine agonist treatment with shrinkage up to 62% [26].
Current diagnostic tests are not able to distinguish the pituitary from extrapituitary hyperprolactinemia. For instance, we could not exclude the concomitant presence of prolactinoma suppressed by dopamine agonist treatment and leiomyoma-associated hyperprolactinemia in our patient. Ectopic prolactin secretion should be considered in young women with “treatment-resistant prolactinoma” and uterine fibroids above 5 cm. Further scientific efforts are needed to reveal the proper pathophysiological mechanisms. New diagnostic markers are also needed to distinguish pituitary from extrapituitary prolactin secretion.
In conclusion, the presented case report emphasizes on leiomyoma-associated hyperprolactinemia as an important part of the differential diagnosis in women of reproductive age with pituitary adenoma and hyperprolactinemia resistant to dopamine agonist treatment.
RR, TK, AE and SZ participated in the review of the literature and interpretation of the case report. RI performed the pathological investigations. RR, TK and RI wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Patient’s consent was obtained for publishing the case report.
The authors express their sincere gratitude to the patient herself who has personally read and approved the manuscript.
This research received no external funding.
The authors declare no conflict of interest. RR is serving as one of the Guest editors of this journal. We declare that RR had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to UI and MHD.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.