1 Department of Ultrasound, the Second Affiliated Hospital, Xi'an Jiaotong University, 710004 Xi'an, Shaanxi, China
2 Department of Obstetrics and Gynecology, the Second Affiliated Hospital, Xi'an Jiaotong University, 710004 Xi'an, Shaanxi, China
†These authors contributed equally.
Academic Editor: Paolo Ivo Cavoretto
Abstract
Background: It reported that serum Elabela level was decreased in preeclampsia patients. However, there is no researcher done the study on the correlations between plasma Elabela and kidney function, blood pressure, blood glucose and lipid in preeclampsia patients. Our objective is determined whether plasma Elabela could be a marker for the severity and kidney function of preeclampsia. Methods: 72 pregnant women were enrolled in this study and divided into healthy group (n = 37), mild preeclampsia group (n = 20) and severe preeclampsia group (n = 15). The level of plasma Elabela was detected using ELISA. Results: Plasma Elabela was reduced in preeclampsia patients compared to healthy group, and severe preeclampsia patients had the lower level of Elabela and poorer kidney function. The level of plasma Elabela was negatively correlated with those of uric acid (UA), creatinine (Cre), cystatin C (CysC), systolic blood pressure (SBP) and diastolic blood pressure (DBP). Conclusions: Lower concentration of plasma Elabela correlated with worse kidney function, and higher blood pressures in preeclampsia patients.
Keywords
- biomarker
- blood pressure
- Elabela
- kidney function
- preeclampsia
Elabela, also called Apela/Toddler, was first identified in zebrafish embryos in 2013 [1]. It is expressed in adult heart, blood vessels, stem cell, prostate and kidney [1, 2, 3, 4]. Elabela is also a hormone related with pregnancy. It can be secreted by embryos, fetus or placenta, and enter into maternal peripheral blood via the ways of paracrine, endocrine or autocrine [3]. The receptor of Elabela is apelin receptor (APJ). APJ is a G-protein-coupled receptor with seven-transmembrane structure [5]. Elabela/APJ signals involve in heart development, embryonic vasculogenesis/angiogenesis, and early placental development [5], also contribute to embryonic stem cells self-renewing, bone formation, development and homeostasis. Moreover, Elabela can lower blood pressure by relaxing the aorta vessels; inhibit renal remodeling and has anti-renal fibrosis function; alleviate food intake, and regulates water homeostasis [6].
Preeclampsia (PE) is a serious complication of pregnancy, usually happens during late 2nd and 3rd trimester. The main clinical manifestations are hypertension and proteinuria. It is caused by the poor perfusion of placenta [7]. The incidence of preeclampsia is 5–7%, and it occupies nearly 20% of pregnant-related deaths [8, 9]. Termination of pregnancy is the most effective treatment of preeclampsia. So, the diagnosis of preeclampsia as early as possible is of vital importance.
The role of Elabela in preeclampsia was recently reported by Ho et al. [3] that Elabela knock-out pregnant mice exhibited preeclampsia-like symptoms, including hypertension and proteinuria. Infusion of exogenous Elabela could normalize hypertension and proteinuria. Elabela was secreted by conceptus and placenta, and might act independently and earlier than the angiogenic factors (sFlt1, Vegf, Plgf) in the pathogenesis of preeclampsia. Moreover, human placenta was also examined. Elabela was primarily expressed in villous cytotrophoblasts and syncytiotrophoblasts of placental tissue, and Elabela could contribute to trophoblast invasion and remodeling spiral artery to prevent the development of preeclampsia during human pregnancy.
Although Elabela expression in human preeclampsia has been reported by some articles, it still remains controversial. Panaitescu et al. [10] reported plasma Elabela concentrations in early-onset preeclampsia patients were lower than those in late-onset preeclampsia patients and no difference with normal pregnant women. However, some researchers reported serum Elabela level was remarkably decreased in preeclampsia patients compared to normal pregnancy. In addition, placental Elabela and its receptor APJ were accordingly down-regulated [11, 12, 13]. In umbilical arteries and veins of newborns, Elabela concentration was also decreased [13]. Whereas, Pritchard et al. [14] and Villie et al. [15] found the levels of placenta and plasma Elabela in preeclampsia patients had no difference with those of normal pregnant women.
So, in our study, we measured the level of plasma Elabela in healthy controls and mild, severe preeclampsia patients, examined the kidney function of preeclampsia patients, and analyzed the correlations between plasma Elabela and kidney function, blood pressure, blood glucose and lipid, finally determined whether plasma Elabela could be a marker for the severity and kidney function of preeclampsia.
The study enrolled 35 pregnant women who diagnosed for preeclampsia from May
2019 to October 2019 at the second affiliated hospital, Xi’an Jiaotong
University. The inclusion criteria were as follow: (1) Preeclampsia diagnosed and
categorized according to ACOG (the American College of Obstetrics and Gynecology)
practice bulletin [16]; (2) age
37 age- and gestation weeks-matched pregnant volunteers were enrolled to serve as healthy controls. The inclusion criteria: natural conception, single pregnancy, first pregnancy, no systemic disease, no complications during pregnancy, and no tumor disease.
Pregnant women were excluded from the study if they (1) pregnant after
in vitro fertilization (IVF), multiple pregnancies, chromosomal
abnormalities of pregnant women and fetus, (2) complicated with primary renal
system disease, (3) had liver disease, heart failure, stroke, rheumatic disease,
autoimmune disease, or malignant tumors, (4) Body Mass Index (BMI)
The study was approved by the ethics committee of the second affiliated hospital, Xi’an Jiaotong University. The study protocol was explained to all patients, and each participant provided written informed consent.
According to the inclusion and exclusion criteria, 72 late pregnant women were enrolled in our study. The plasmas were collected from healthy pregnant women, mild and severe preeclampsia patients, and the level of plasma Elabela was detected using enzyme linked immunosorbent assay (ELISA). The serums were examined for kidney functions (blood urea nitrogen (BUN), creatinine (Cre), glomerular filtration rate (GFR), uric acid (UA), cystatin C (CysC)), and also for glucose and lipid metabolism (fasting plasma glucose (FPG), total cholesterol (TCHO), triglycerides (TG), high-density lipoproteincholesterol (HDL-C), low-density lipoproteincholesterol (LDL-C)) using biochemical analyzer. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded for every subject. The correlations between plasma Elabela and the above clinical parameters were analyzed.
Venous blood samples were collected in Ethylene Diamine Tetraacetic Acid (EDTA) anticoagulation tubes from healthy controls and preeclampsia patients. The blood samples were centrifuged at 3000 rmp/min for 15 min, and the plasmas were stored at –80 °C until use. The level of human Elabela was detected using ELISA kit (Laibio, Shanghai, China) according to manufacturer’s protocol. Each sample was measured in duplicate and the mean was calculated.
Venous blood samples were collected in procoagulant tubes from healthy controls and preeclampsia patients, the following parameters: BUN, Cre, GFR, UA, CysC, FPG, TCHO, TG, HDL-C and LDL-C were detected by fully automatic biochemical analyser (BX-4000, sysmex corporation, Japan). The correlations between the concentration of Elabela and the levels of the above parameters were also analyzed.
The results are presented as the mean
A total of 72 women were included in our study. Table 1 showed the clinical
characteristics of the enrolled cases. The mean ages of mild (n = 20) and severe
(n = 15) preeclampsia patients were 29.41
| Groups | Numbers (n) | Ages (y) | Weeks of pregnancy (w) | SBP (mmHg) | DBP (mmHg) |
| Healthy pregnant | 37 | 31.01 |
35 |
119.9 |
76.73 |
| Mild preeclampsia | 20 | 29.41 |
36 |
147.3 |
100.4 |
| Severe preeclampsia | 15 | 29.11 |
35 |
170.0 |
118.3 |
p values were from the one-way analysis of variance (ANOVA). SBP, systolic blood pressure; DBP, diastolic blood pressure. | |||||
The level of plasma Elabela was decreased in mild (4.146
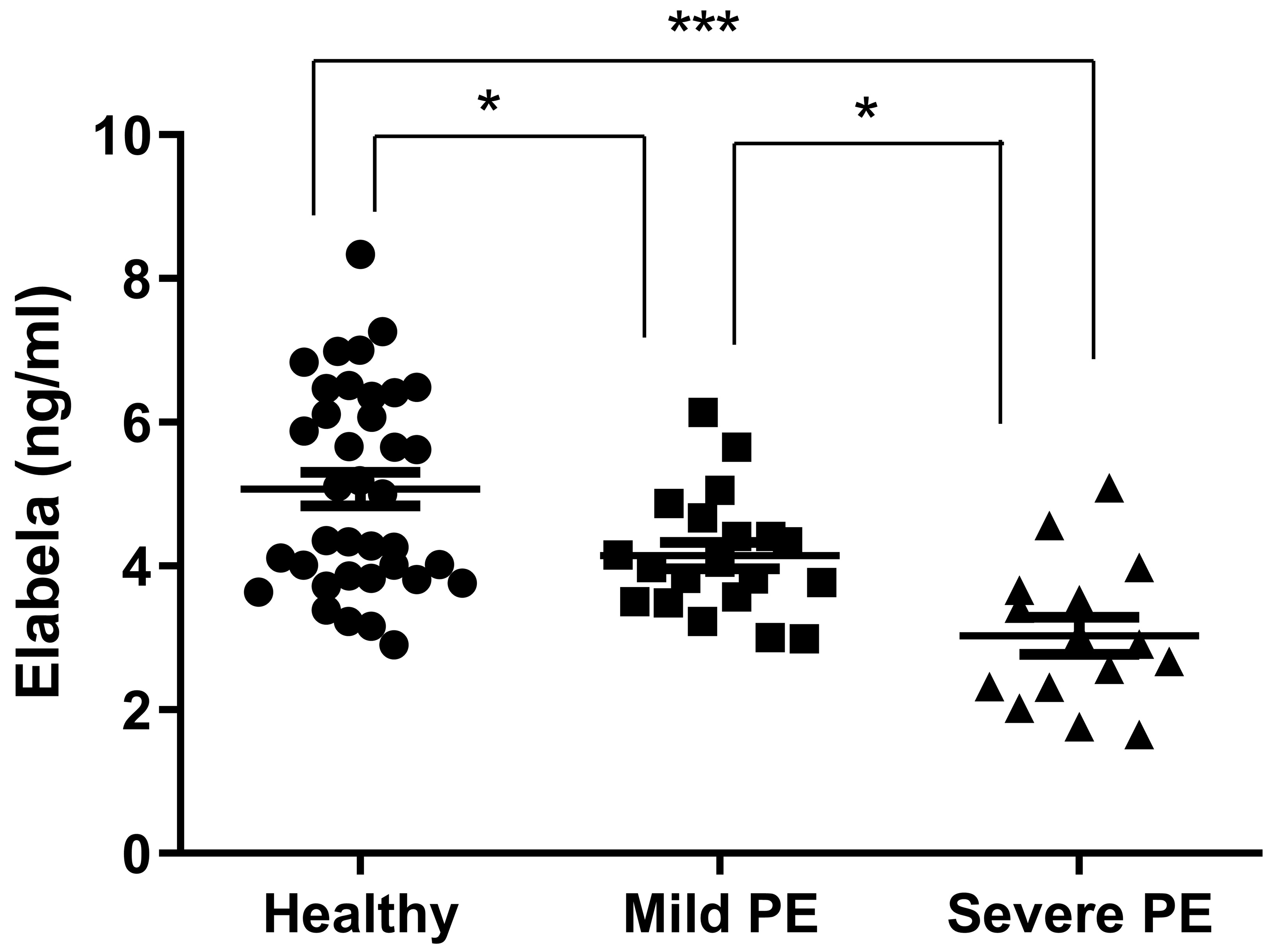 Fig. 1.
Fig. 1.The detection of plasma Elabela levels in mild and severe
preeclampsia patients and healthy controls. The plasmas of healthy controls (n =
37), mild (n = 20) and severe (n = 15) preeclampsia patients were collected, and
the level of Elabela was detected using ELISA. PE, preeclampsia. ** p
Because preeclampsia patients might manifest kidney dysfunction, we then detected the kidney function of mild and severe preeclampsia patients. The levels of UA, Cre and CysC were up-regulated in preeclampsia patients than healthy controls. Among those, the levels of severe preeclampsia patients were highest. However, GFR was decreased in mild and severe preeclampsia patients than healthy controls (Fig. 2). These results indicated that the more severe symptoms of preeclampsia, the worse function of kidney.
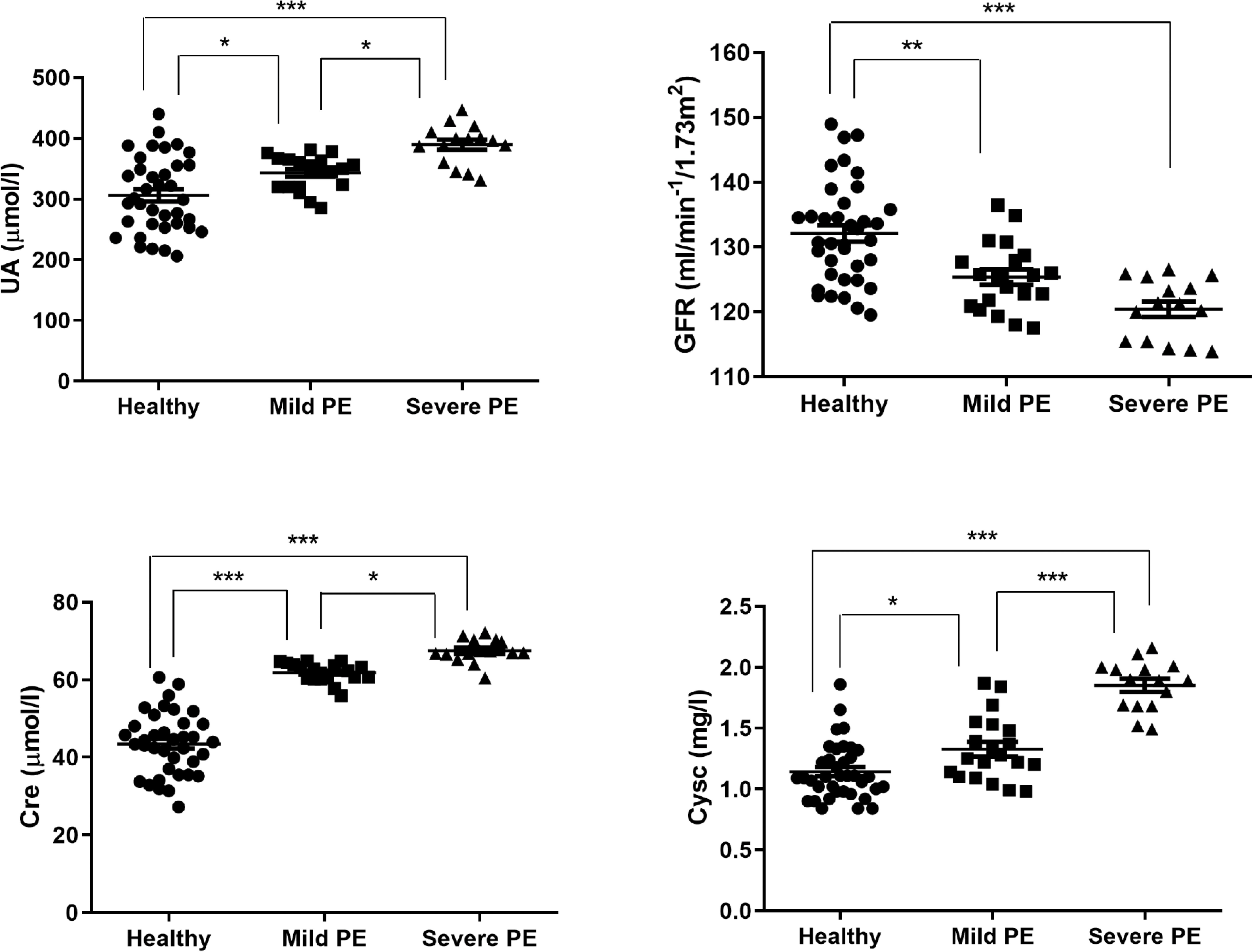 Fig. 2.
Fig. 2.The detection of UA, GFR, Cre, CysC levels in mild and severe
preeclampsia patients and healthy controls. The serums of healthy controls (n =
37), mild (n = 20) and severe (n = 15) preeclampsia patients were collected, and
the levels of UA, GFR, Cre, CysC were detected using fully automatic biochemical
analyser. UA, uric acid; GFR, glomerular filtration rate; Cre, creatinine; CysC,
cystatin C; PE, preeclampsia. * p
We then analyzed the correlation between the level of plasma Elabela and renal function, blood pressure, glucose and lipid metabolism. The level of plasma Elabela was negatively correlated with those of UA, Cre, CysC, SBP and DBP. However, it was positively correlated with that of GFR. Moreover, there were no correlations between the level of plasma Elabela and BUN, FPG, TCHO, TG, HDL-C, LDL-C (Fig. 3). These results indicated that the lower level of plasma Elabela, the worse function of kidney, thus the more severe symptoms of preeclampsia.
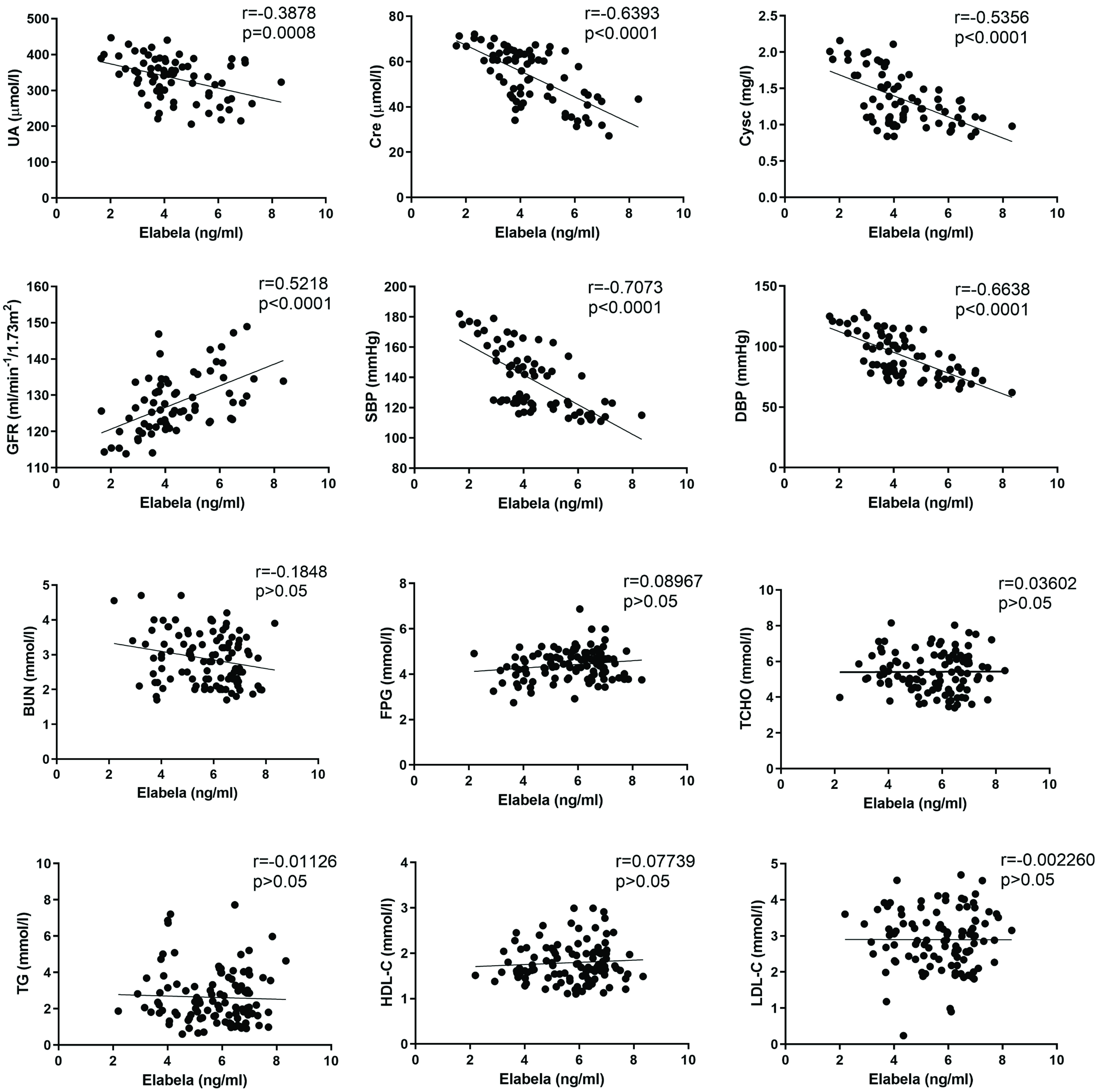 Fig. 3.
Fig. 3.The correlation analysis between plasma Elabela level and kidney function (UA, GFR, Cre, CysC, BUN), blood pressure (SBP, DBP), glucose (FPG), lipid metabolism (TCHO, TG, HDL-C, LDL-C). UA, uric acid; GFR, glomerular filtration rate; Cre, creatinine; CysC, cystatin C; BUN, blood urea nitrogen; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TCHO, total cholesterol; TG, triglycerides; HDL-C, high-density lipoproteincholesterol; LDL-C, low-density lipoproteincholesterol.
Since Ho et al. [3] reported Elabela deficiency promoted preeclampsia in mice, several research groups examined the level of Elabela in human preeclampsia, and the results were controversial. The different Elabela levels from different research groups might be due to different samples used (maternal plasma or serum, umbilical artery and vein), or because Elabela is a small peptide hormone which can be quickly degraded by protease, the improper sample collection and preservation might cause disparate results [5]. In our studies, we found that the level of plasma Elabela was decreased in preeclampsia patients, and the level was lower in severe preeclampsia patients compared to that in mild preeclampsia ones. Our results were in line with Ho et al. [3], Zhou et al. [11], Hao et al. [12] and Deniz et al. [13], indicating that Elabela might provide a protective role in human preeclampsia, the decreased Elabela aggravated the severity of preeclampsia.
The clinical manifestations of preeclampsia patients are arteriole spasm induced increased vascular resistance, and then the reduced organic blood flow. The kidney is the most affected organ. It shows that glomerular spasm, insufficient blood supply, kidney tubules injury, and then the decreased function of kidney, electrolyte and acid-base disturbance, even kidney failure, eventually threat the safety of mother and fetus. Clinically, Cre, UA, CysC and GRF are used to evaluate kidney function [17, 18]. In our studies, compared to normal pregnancy, the levels of UA, Cre, CysC were increased, and GFR was decreased in preeclampsia patients, indicating the kidney function was affected in preeclampsia patients. In addition, severe preeclampsia patients had the poorer kidney function than mild preeclampsia patients.
Elabela, highly expressed in the kidney, has been proved to have protective roles on kidney. Chen et al. [19] reported that the level of Elabela was markedly reduced in murine acute kidney injury (AKI). In vitro experiments showed that overexpression of Elabela in cultured renal tubular cells could suppress AKI-induced inflammation, DNA damage and apoptosis. Moreover, Elabela could promote the viability of renal tubular cells. Elabela also suppressed DNA damage response (DDR) in adriamycin-stimulated renal tubular cells. In vivo studies showed that Elabela treatments inhibited AKI-induced morphologic changes, inflammation and fibrosis. A recent study by Lu et al. [20] revealed that in chronic kidney disease patients, serum Elabela levels decreased with decreasing eGFR. In our studies, we found that the level of Elabela was negatively correlated with that of UA, Cre, CysC, and positively correlated with GFR in preeclampsia patients, indicating that human Elabela could be a predictive indicator for kidney function in preeclampsia patients. In addition, human Elabela might be a promising reagent in the protection of kidney function. However, whether Elabela is more specific and sensitive than UA, Cre, CysC and GFR in the prediction of kidney function still needs further exploration.
Elabela, as a new ligand of APJ, also played an important role in the regulation of blood pressure. Elabela could bind to APJ and weaken angiotensin II (AngII) ability of contracting blood vessel via down-regulating the expression of FoxM1 and angiotensin-converting enzyme (ACE), thus decrease the blood pressure [21]. Elabela could also reduce high-salt-diet-induced hypertension in rats [22]. Yang et al. [4] reported that the level of Elabela was markedly decreased in pulmonary arterial hypertension (PAH) patients and rats, exogenous administration of Elabela could attenuate the elevation of right ventricular systolic pressure and myocardial hypertrophy, as well as pulmonary vascular remodeling, thus ameliorated PAH in rats. In our studies, the level of Elabela was reduced, while SBP and DBP were increased in preeclampsia patients, indicating Elabela might also participate in the regulation of blood pressure in the pregnant women.
In addition, Elabela could suppress food intake via activation of anorexigenic
neuropeptide arginine vasopressin (AVP) and corticotropin-releasing hormone (CRH)
neurons, and increase c-Fos expression and [Ca
The level of Elabela was decreased and negatively correlated with the severity of preeclampsia. Preeclampsia patients had kidney dysfunction. Decreased level of Elabela was correlated with poorer kidney function in preeclampsia patients. Elabela could be a marker for predicting the severity and kidney function of preeclampsia patients in the future.
The data are available from the corresponding author on reasonable request.
JFW and YHQ designed and supervised the study. YMH, MYM performed the experiment. YMH, YHQ analyzed the data. YMH and MYM made statistical analysis. YMH and YHQ wrote the manuscript. YHQ, JFW revised the manuscript. All authors read and contributed to the manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the second affiliated hospital, Xi’an Jiaotong University (approval number: 2021214).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research was funded by the Health Commission of Shaanxi province under grant [number 2018D061] and Key R & D projects of Shaanxi Province, grant [number 2021SF-134, 2021SF-001, 2020SF-057].
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



