1 Department of Biology, Saarland University, 66123 Saarbrücken, Germany
2 Theoretical Medicine and Biosciences, Medical Faculty, Saarland University, 66421 Homburg, Germany
3 Dynamics of Fluids, Experimental Physics, Saarland University, 66123 Saarbrücken, Germany
Abstract
The article provides a comprehensive overview of biological membrane lipid composition and distribution and ion transport processes, focusing particularly on red blood cells (RBCs). It begins with a historical perspective, detailing the introduction of the terms ‘cell’ and ‘membrane’ in biological sciences, and the development of the fluid-mosaic model of membrane structure. Early findings on ion transport highlighted the non-equilibrium distribution of Na+ and K+ across cell membranes, leading to the discovery of the Na+/K+ pump. The article delves into the lipid composition of RBC membranes, emphasising the roles of various lipids, including cardiolipin, and the concept of lipid rafts. These rafts, enriched with sphingolipids and cholesterol, play crucial roles in cellular processes. Variations in RBC shapes are discussed, with biophysical theories explaining transformations and pathological conditions affecting RBC morphology, such as sickle cell anaemia. Na+ and K+ transporters in RBC membranes are explored, highlighting the almost ubiquitous presence of the Na+/K+ pump (absent in Carnivora RBCs) and various ion channels, including the Gárdos and Piezo1 channels. The article notes species-specific differences in ion transport mechanisms and the activation or suppression of transporters during RBC maturation. The mechanism of residual ion transport is examined, questioning whether a Na+(K+)/H+ antiporter exists in the human RBC membrane. Residual ion fluxes are mediated by this antiporter, influenced by the fatty acid composition of the RBC membrane. The outlook section underscores the need for further research to fully understand the complexities of RBC membrane structure and function, suggesting that many questions remain unanswered despite significant advances.
Keywords
- red blood cell
- membrane lipid composition
- lipid rafts
- red blood cell shapes
- red blood cell deformability
- residual (leak) ion transport
This publication addresses various open questions in red blood cell (RBC) membrane research. Its main goal is to assist young scientists in this field by providing insights into past research and considerations for future experiments and theoretical calculations. The content was presented at the 25th Meeting of the European Red Cell Society (ERCS) in April 2024 on Ameland, The Netherlands. The talk was dedicated to the memory of Prof. Dr. Joseph F. Hoffman (USA) and Prof. Dr. h.c. Herrmann Passow (Germany), who passed away on May 19, 2022, and November 21, 2023, respectively, both at the age of 98 years. These eminent scientists significantly contributed to our understanding of the RBC membrane’s structure and function.
First of all, it is interesting to know who first used the words “cell” and “membrane”. The term “cell” was introduced by the English researcher Robert Hooke in 1665 [1], when he described the cellular structure of cork observed through a microscope. Identifying the first use of the term “membrane” is more challenging. To the best of our knowledge, it was the Swiss botany professor Carl Wilhelm von Nägeli [2] who introduced the term “membrane” in 1855 while working on osmosis in plant cells. Before and even after that time, the term “plasmalemma” was commonly used to describe the boundary of a biological cell.
In 1899, Overton [3] described the cell membrane as a structure of unknown components with holes permeable to water. In 1925, Gorter and Grendel [4] were the first to propose that a lipid bilayer forms a biological membrane. Significant progress was made between 1935 and 1943, when Danielli et al. [5] and Davson et al. [6] proposed a lipid bilayer with proteins on both membrane surfaces. Robertson [7] expanded on this in 1981 by introducing sugar elements on one surface of the membrane.
Our current understanding of biological membranes is based on the “fluid-mosaic model” developed by Singer and Nicolson in 1972 [8]. This model posits that membrane lipids are in a fluid-crystalline state and distributed relatively homogeneously within the membrane, with proteins inserted like islands in a mosaic. However, at least three extensions to the fluid-mosaic model should be considered: (i) Lipids in general also exist in crystalline states, with phase transition temperatures higher than the membrane’s surrounding temperature, leading to both fluid-crystalline and crystalline domains (e.g., [9]). (ii) Lipids and proteins are distributed asymmetrically, not only laterally but also transversally [10, 11]. (iii) In addition to forming a bilayer structure, lipids in biological membranes can also exist in non-bilayer structures [12].
Understanding the historical views and ideas about ion transport across biological membranes is also very interesting. The first significant findings were published by Abderhalden in 1897 [13]. Using chemical methods, he discovered that in human RBCs, the extracellular Na+ concentration is higher than the intracellular Na+ concentration, while the opposite is true for K+ – with a higher K+ concentration inside the cell than outside. It is worth noting that a non-equilibrium distribution of Na+ and K+ was observed even earlier. In 1894, Zaleski [14] attributed these findings to Carl Schmidt’s work from the 1850s.
At the end of the 19th century and later, physiologists believed that the cell membrane must be impermeable to ions (e.g., Gürber [15, 16]). However, in 1923, van Slyke et al. [17] described the high permeability of the RBC membrane to Cl–. In 1936, Fenn and Cobb [18] discovered that muscle cell membranes are permeable to Na+ and K+. Using radioactive isotopes, Cohn and Cohn [19], Dean et al. [20], and Eisenmann et al. [21] demonstrated between 1939 and 1941 that the RBC membrane is permeable to Na+ and K+. These findings led to the hypothesis of the Na+/K+ pump, developed by Dean [22] and Krogh [23] between 1941 and 1946. In 1957, Skou [24] identified the Na+/K+-ATPase in crab nerves as the enzymatic basis for the Na+/K+ pump, an achievement for which he received the Nobel Prize in Chemistry in 1997. Subsequent characterisation of ion transport via the pump was carried out by Glynn (e.g., [25]), Sachs (e.g., [26]), and Hoffman and Kregenow (e.g., [27]). In 1952, Hodgkin and Huxley [28] provided a quantitative description of the action potential through alterations in membrane permeability for monovalent cations, earning them the Nobel Prize in Physiology or Medicine in 1963. Initially, these permeability changes were thought to involve a carrier mechanism. However, in 1955, Hodgkin and Keynes [29] proposed the existence of membrane pores (channels) for ion movement.
From 1965 to 1978, there was significant debate about the existence of ion channels. Ultimately, Hille [30] and Armstrong [31] identified ion channels with two key features: (i) a selectivity filter and (ii) an opening mechanism (gate). In 1976, Neher and Sakmann [32] developed the “patch-clamp” technique to investigate single ion channels, a breakthrough that earned them the Nobel Prize in Physiology or Medicine in 1991. In the years following the invention of the patch-clamp technique, also RBCs were investigated extensively by this method [33, 34].
When investigating the function of lipids in biological membranes, particularly in RBC membranes, only five or six classes are typically considered. These include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), sphingomyelin (SM), and cholesterol. However, other lipids have been studied to a much lesser extent. These include lysolipids (e.g., lysoPC), glycolipids (cerebrosides and gangliosides), and especially cardiolipin. Cardiolipin, characterised by two phosphate groups and four fatty acids, is primarily located in the inner mitochondrial membrane. It is believed to be crucial for the optimal activity of several mitochondrial proteins and is also associated with various diseases, such as neuronal dysfunction [35, 36]. Although the role of cardiolipin in the RBC membrane is not fully understood, it may be significant for the activity of certain membrane proteins through direct interaction (see section 6 on membrane protein-lipid interactions). Additionally, cardiolipin might play a role in maintaining the balance of reactive oxygen species.
Nelson [37] and Wessels and Veerkamp [38] described the phospholipid head group
composition (PC, SM, PE, PS, and others) of RBC membranes from various species
(rat, dog, horse, guinea pig, rabbit, cat, human, pig, cow, sheep). The RBC
membrane lipid composition of RBCs of different mammalian species including PI
has been summarised by Kotyk and Janácek [39]. It was found that the
variability of PE and PS among these species is relatively low. In contrast, the
PC and SM content vary significantly. For example, dog and rat RBC membranes have
low SM (10.8% and 12.8%, respectively) and high PC content (46.9% and 47.6%,
respectively), while cow and sheep RBC membranes have high SM content (46.2% and
51.0%, respectively) and no PC. When comparing the PI content in the RBC
membrane across species, significant differences are observed. To current
knowledge, RBCs from horses have the lowest PI content (0.3%), while RBCs from
cats have the highest (7.4%) [39]. The head group composition of the lipids in
the RBC membrane in mice does not differ significantly from that in rats [40].
However, the lipid head group composition of camel RBCs shows significant
differences compared to human RBCs. Specifically, camel RBCs have a much lower PC
content but a much higher PE, with PS and SM levels nearly identical to those in
human RBCs [41]. Additionally, other studies have shown that compared to sheep
and goats, camels have significantly higher PC, SM, and cholesterol levels in
their RBC membrane (e.g., [42]). The cholesterol content of RBCs across various
mammalian species generally does not differ significantly, maintaining a total
phospholipid/cholesterol molar ratio close to 1, except in camel RBCs, where the
ratio is slightly higher [42, 43]. Nelson investigated also the fatty acid
composition of the phospholipids in the human RBC membrane, as reported in [44].
The main phospholipids (PC, PE, PS, SM, PI, lysoPC) contain various fatty acids.
In brackets, the trivial names are provided: 16:0 (palmitic acid), 16:1
(palmitoleic acid), 18:0 (stearic acid), 18:1 (oleic acid), 18:2 (linoleic acid),
20:0 (arachidic acid), 20:3 (eicosatrinoic acid), 20:4 (arachidonic acid), 22:0
(behenic acid), 22:1 (erucic acid), 22:4 (docosatetraenoic acid), 22:5
(docosapentaenoic acid), and 22:6 (docosahexaenoic acid). Other authors investigated the fatty
acid composition of the membrane lipids of RBCs from different mammalian species (rats, rabbits,
guinea pigs, sheep, cows, pigs, dogs, and cats) [39]. They also identified the presence of the
fatty acids 12:0 (lauric acid, only in sheep, 14:0 (myristic acid, only in sheep and rats), and 18:3 (
| Lipid | Human inner (%) | Human outer (%) | Bovine inner (%) | Bovine outer (%) |
| Phosphatidylcholine | 14 | 44 | - | - |
| Sphingomyelin | 10 | 44 | - | 100 |
| Phosphatidylethanolamine | 48 | 12 | 67 | - |
| Phosphatidylserine | 28 | - | 33 | - |
It should be mentioned that also new data on the fatty acid composition of RBC membrane lipids based on modern or sensitivity-improved measuring techniques (e.g., gas chromatography) are available. In most cases, authors are investigating the effect of diets on the fatty acid composition (examples for RBCs of cats and rats see [45, 46]), or the effect of diseases on the fatty acid composition (example for RBCs of dogs, see [47]). Analysing the transversal distribution of lipids in the RBC membrane reveals interesting differences between species. Table 1 (Ref. [48]) shows the asymmetrical transversal distribution of SM, PC, PE, PS in human and bovine RBC membranes. Notably, it is intriguing that the outer leaflet of the bovine RBC membrane is composed entirely of sphingomyelin.
The diverse lipid compositions of RBC membranes of various species suggest their significance in RBC flexibility (see section 5). Additionally, the phase transition temperatures of these lipids, determined by both their head groups and fatty acid content, vary relative to body temperature, indicating not all lipids maintain a fluid-crystalline state. It is important to note that the fluidity of the lipid bilayer and consecutively of the cell membrane is determined by its lipid composition. However, these findings suggest the formation of lipid microdomains (lipid rafts) within biological membranes since a random distribution of lipids in both fluid-crystalline and crystalline states appears energetically unfavourable. Lipid rafts, characterised by their detergent-insolubility, are enriched with sphingolipids and cholesterol. Sphingolipids, such as SM and glycosphingolipids, exist in a crystalline phase with saturated fatty acids, forming distinct phases within the plasma membrane of eukaryotic cells. Rafts also harbour glycosylphosphatidylinositol (GPI)-anchored proteins, with raft diameters typically ranging from 10 nm to 200 nm and containing up to 50 GPI-anchored proteins. Larger sub-micrometric domains with diameters between 300 nm and 500 nm have also been reported. The existence of lipid rafts was first suggested by Simons and Ikonen in 1997 [49], and the concept has since been further developed by researchers such as Sharma et al. [50], Carquin et al. [51, 52], and Conrad et al. [53]. Currently, at least 150 different human GPI-anchored proteins have been identified [54]. Examples of GPI-anchored proteins in the human RBC membrane include protectin (CD59) and Complement Decay Accelerating Factor (CD55) [54]. Rafts represent dynamic structures, with lipids and proteins residing within them for periods ranging from seconds to minutes. Identifying rafts in living cells remains a contentious subject. Future research should prioritise understanding their physiological functions and properties, including interactions with the cytoskeleton and protein receptors, crucial for signal transduction. Notably, Minetti’s group [55, 56] has significantly contributed to our comprehension of rafts in RBC membranes. An important finding regarding the RBC membrane was described by Conrad et al. [53]. Using fluorescence and confocal microscopy, three distinct lipid domains were identified: (i) cholesterol-enriched domains associated with high curvature areas of the RBC, (ii) ganglioside GM1/PC/cholesterol-enriched domains present in low curvature areas, and (iii) SM/PC/cholesterol-enriched domains also present in low curvature areas. Cholesterol- and SM-enriched domains in the RBC membrane have also been reported by other studies [51, 52]. Additionally, the molecular organisation within these different domains has been described in more detail [57].
Another intriguing aspect is the presence of plasmalogens in the RBC membrane. Plasmalogens are a subclass of glycerophospholipids characterised by a vinylether bond at the sn-1 position and polyunsaturated fatty acid at the sn-2 position. While their role in biomembranes is still under discussion, it is believed that plasmalogens contribute to the physical and chemical properties of the membrane and act as antioxidants, protecting unsaturated fatty acids and lipoproteins from oxidative stress [58, 59]. Plasmalogens have been identified in neuronal, immune, and cardiovascular cells [59] and are also present in the RBC membrane [60, 61]. Therefore, in future, it seems of importance to investigate their role in the RBC membranes. In addition, the biophysical properties of plasmalogens and their implications for certain diseases, such as Alzheimer’s disease, should be considered. For more details, we refer to the reviews by Honsho and Fujiki [62, 63].
Finally, very recent research showed significant lipid remodelling during reticulocyte maturation and the RBC ageing process beyond [64]. Lipid analysis showed that cholesterol and SM increase, while PC and PS decrease as reticulocytes mature into RBCs. Specific phospholipid subclasses change during the ageing of RBCs, with some approaching the composition of plasma lipoproteins. Furthermore, VPS13A, a lipid transport protein, is present in reticulocytes and decreases with RBC maturation, potentially playing a role in lipid exchange. The findings challenge the traditional view that RBC membrane maturation is solely linked to membrane skeleton assembly, suggesting a more complex process involving lipid remodelling [64]. The concrete molecular regulation of lipid remodelling during RBC aging is among the unsolved problems in RBC membrane research.
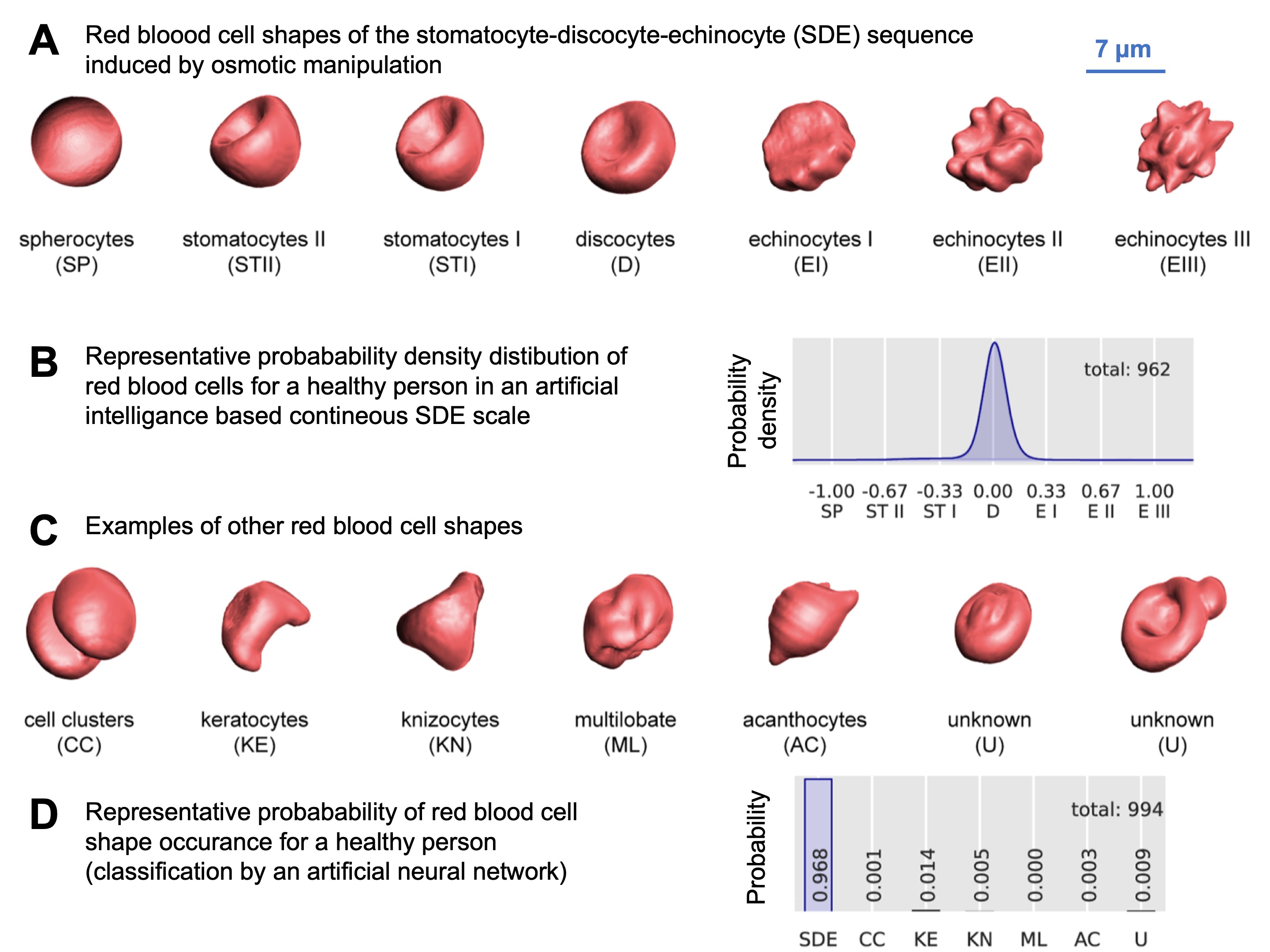
Under physiological conditions, human RBCs typically have a biconcave shape and are referred to as discocytes. When subjected to volume changes, such as alterations in the osmolarity of the surrounding medium as performed for Fig. 1A (Ref. [65]), the RBCs undergo a series of shape transformations in the sequence stomatocyte-discocyte-echinocytes (SDE). The normal distribution of these cell shapes under physiological conditions is illustrated in Fig. 1B. Experimentally, other methods can also induce similar RBC shape changes, as documented in the literature (e.g., [66]). Various biophysical theories have been proposed to explain the mechanisms behind these shape transformations. Additionally, unusual or pathological RBC shapes (for selected examples see Fig. 1C) can occur under physiological conditions, though at a very low frequency, as depicted in Fig. 1D.
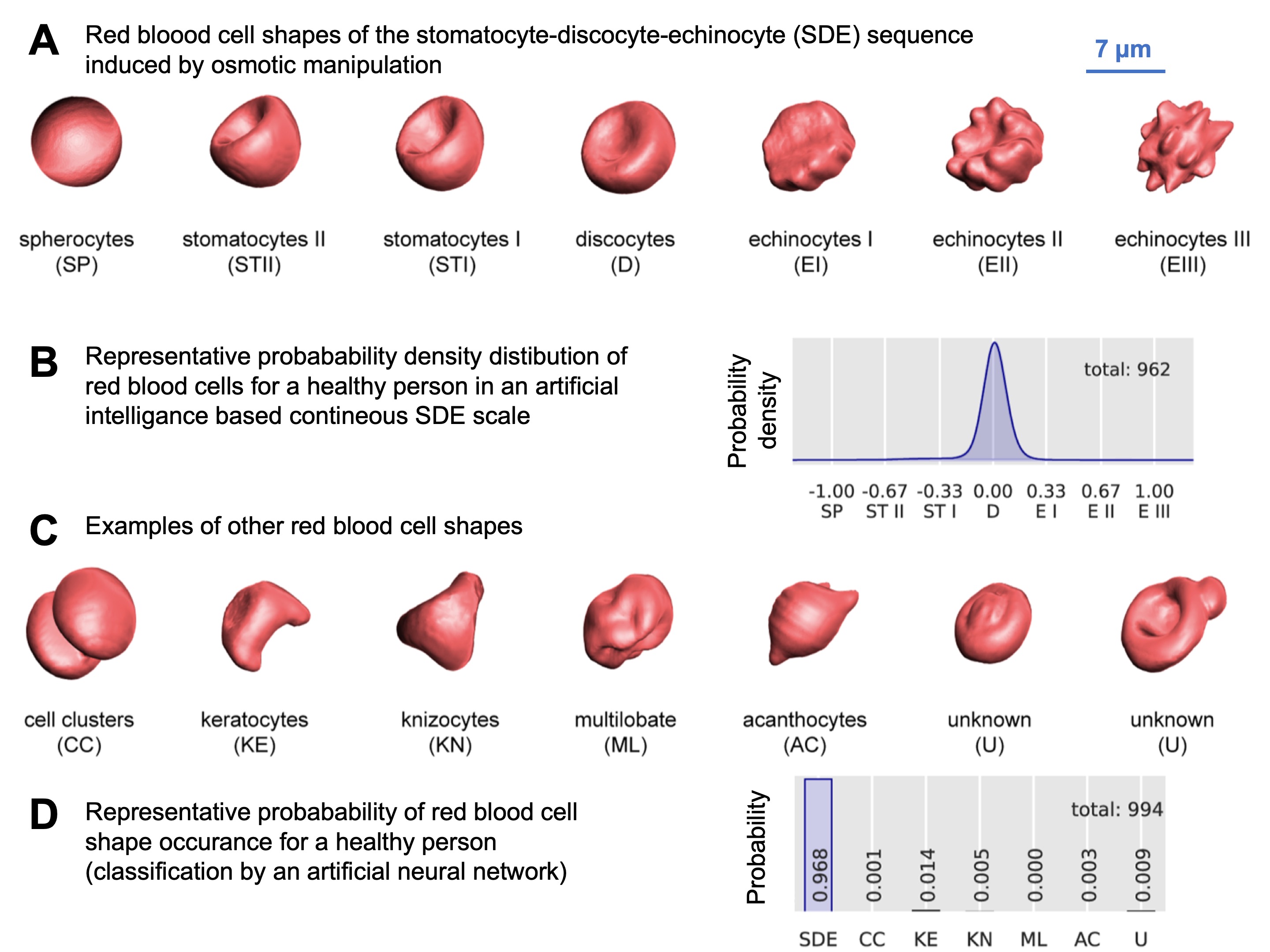 Fig. 1.
Fig. 1.
Overview of red blood cell (RBC) shapes. (A) represents 3D-rendered RBC from confocal microscopy recordings in the stomatocyte-discocyte-echinocyte (SDE) sequence. These cell shapes were induced by osmotic manipulation. Recently, the SDE sequence was proposed to be matched on an analogue scale from -1 to +1 [65]. (B) shows the distribution of the occurrence of the RBCs of the SDE sequence in a blood sample from a healthy person. (C) shows 3D images of examples of other cell shapes not covered by the SDE sequence. In blood samples of healthy donors such shapes occur very rarely. (D) indicates this probability compared to the RBCs of the SDE sequence. Reproduced with permission from Simionato et al, PLoS Computational Biology; published by PLoS, 2021 [65].
Furthermore, pathological RBC shapes are characteristic of specific diseases and have even given their names to some of them [67], such as drepanocytes (sickle cells), which are associated with sickle cell disease. This condition is explained by the replacement of normal haemoglobin (mainly HbA) with abnormal haemoglobin (HbS), which alters the interaction between haemoglobin molecules and the inner membrane surface of RBCs. Keratocytes and schistocytes often appear after cardiac or vascular surgery, while dacrocytes are associated with thalassemia, leukaemia, toxicity, and haemolytic anaemias. Codocytes are indicative of hypochromic anaemias. Acanthocytes are linked to a group of rare hereditary neurodegenerative disorders known as neuroacanthocytosis syndromes [68]. Elliptocytes, which are prominent in various anaemias like hereditary elliptocytosis, are also of significant interest.
Camel RBCs exclusively adopt an ellipsoid shape, termed ovalocytes. Bessis’ atlas comprehensively covers these shapes [69]. Notably, human elliptocytes lack the increased resistance to osmotic haemolysis observed in camel cells. Camels can lose significant body weight (30–40%) and rapidly consume vast amounts of water (up to 200 litres), leading to considerable water uptake of the RBCs resulting in their volume expansion. Camel RBCs can increase their volume to a much larger extent compared with human RBCs [70]. Possible explanations of such differences are still a matter of debate.
The deformability is one of the most, if not the most important property of the RBC. It is crucial for their ability to pass through narrow capillaries without rupturing, which is essential for maintaining efficient blood flow [71]. This flexibility varies significantly among different mammalian species, reflecting adaptations to their unique environmental challenges and physiological requirements. RBC deformability is influenced by several factors, including the structural integrity of the cell membrane — such as the lipid composition (see section 3, above) and the membrane proteins (their abundance, interaction and activity), the organization of the cytoskeleton, and the viscosity of the cytoplasm. However, the exact relation and contributions of all mentioned parameters is among the unsolved problems in RBC membrane research [72]. Additionally, there is a bio-molecular signaling component, namely the interplay between mechano-sensitive ion channels, such as Piezo1 and the Gárdos channel (see section 6, below). The activation of the mechano-sensitive channel allows Ca2+ to enter the RBC, which activates the Gárdos channel resulting primarily in the loss of K+, followed by Cl– and water, finally leading to a volume decrease [73]. Nevertheless, the biconcave shape of RBCs in most mammals (see section 4, above) is a key feature that enhances their flexibility, allowing them to undergo significant deformation while traversing capillaries [74]. Structural properties of the RBC membrane are subject to variation across species, depending on their environmental and physiological needs. Camels have evolved RBCs with unique characteristics [70] to survive in the extreme conditions of the desert (see sections 3 and 4, above). For comparative studies of RBC deformability see, e.g., Amin and Sirs [75], Nemeth et al. [76], and Plasenzotti et al. [77]. There is a wide range of methods to investigate the deformability of RBCs [78]. It includes micropipette aspiration [79], atomic force spectroscopy [80], optical tweezers [81], ektacytometry [82], and microfluidic assays [83], just to name a few.
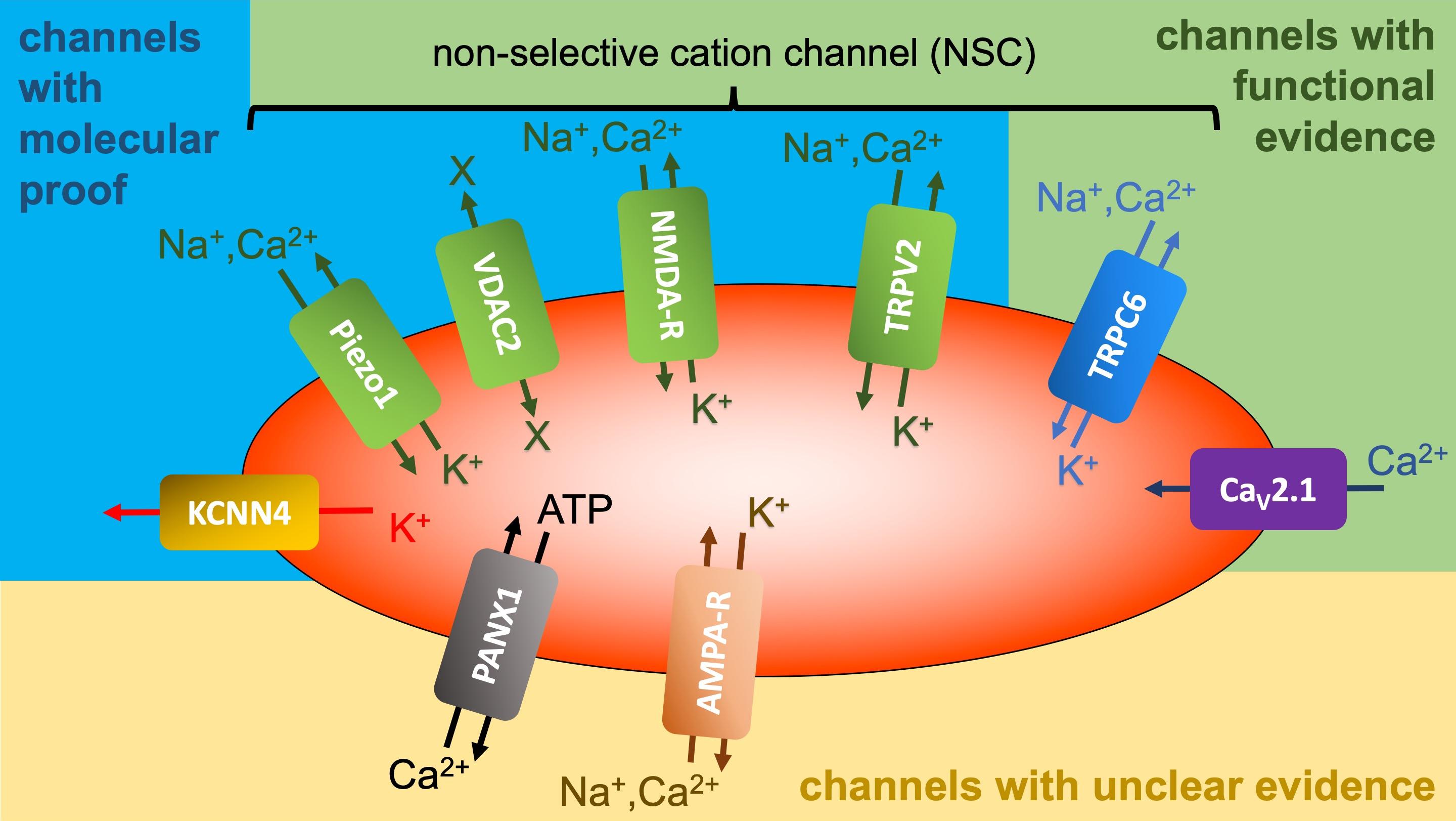
A wide array of ion transporters, particularly for Na+ and K+, are now recognized in the RBC membrane. Nearly all cells possess a Na+/K+ pump crucial for establishing the Na+ and K+ gradient across the cell membrane. Interestingly, mature RBCs of dogs and cats lack this pump, relying on alternative mechanisms for maintaining Na+ and K+ gradients (details see next paragraph (iii)) [84, 85, 86]. Besides the pump, the RBC membrane harbours various carriers, including the Na+-K+-2Cl– symporter (NKCC), K+-Cl– symporter (KCC), Na+-dependent amino acid transporters, Na+(Mn+)/Mg2+ antiporter, Na+/Li+ antiporter, Na+/H+ antiporter (NHE1), band 3 (anion transporter), which can act as NaCO3–/Cl– antiporter, and Na+(K+)/H+ antiporter (e.g., [87]). Further details on the Na+(K+)/H+ antiporter are provided below (see section 7). The presence of ion channels adds complexity to the picture. Initially, only the Ca2+-activated K+ channel (Gárdos channel) was known [88], which years later was identified as KCNN4 [89]. This was followed by the description of the non-selective, voltage-dependent cation (NSVDC) channel in human RBCs [90, 91, 92]. Fig. 2 (Ref. [88, 89, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104]) illustrates the current state of knowledge. The Gárdos channel and the Piezo1 channel have both been confirmed with molecular evidence [105]. It is highly probable that Piezo1 accounts for most of the measurements previously attributed to the NSVDC channel [93, 94, 106]. However, there are additionally non-selective cation channels in the RBC membrane, such as VDAC2 – Voltage-Dependent Anion Channel type 2 [95], NMDA-R – N-Methyl-D-Aspartate Receptor [96], TRPV2 - Transient Receptor Potential Channel of Vanilloid type 2 [97, 98], which may also account for specific previous NSVDC channel recordings. These channels have been unequivocally confirmed on the molecular level. However, there are also channels where standard molecular techniques, such as Western blots or proteomic mass spectrometry, fail or yield inconsistent results. This can be attributed to the detection limits of the methods combined with a low copy number of the channels per cell [105]. Despite these challenges, the effects of the channel openings can still be observed by applying specific agonists or antagonists or by a specific activation of a signalling cascade and measuring an expected consecutive cellular response. We refer to these as channels described mainly based on functional evidence. Channels in this category include TRPC6 – Transient Receptor Potential Channel of Canonical type 6 [99, 100] and CaV2.1 – Voltage-Dependent Calcium Channel 2.1 [101, 102]. In contrast, there are isolated, episodic reports of ion channels in RBCs [103, 104] that have not been confirmed by other research groups; we categorize these as channels with unclear evidence. Further investigations are required to determine the actual repertoire of ion channels in the RBC membrane as well as their genesis and physiological functions [107].
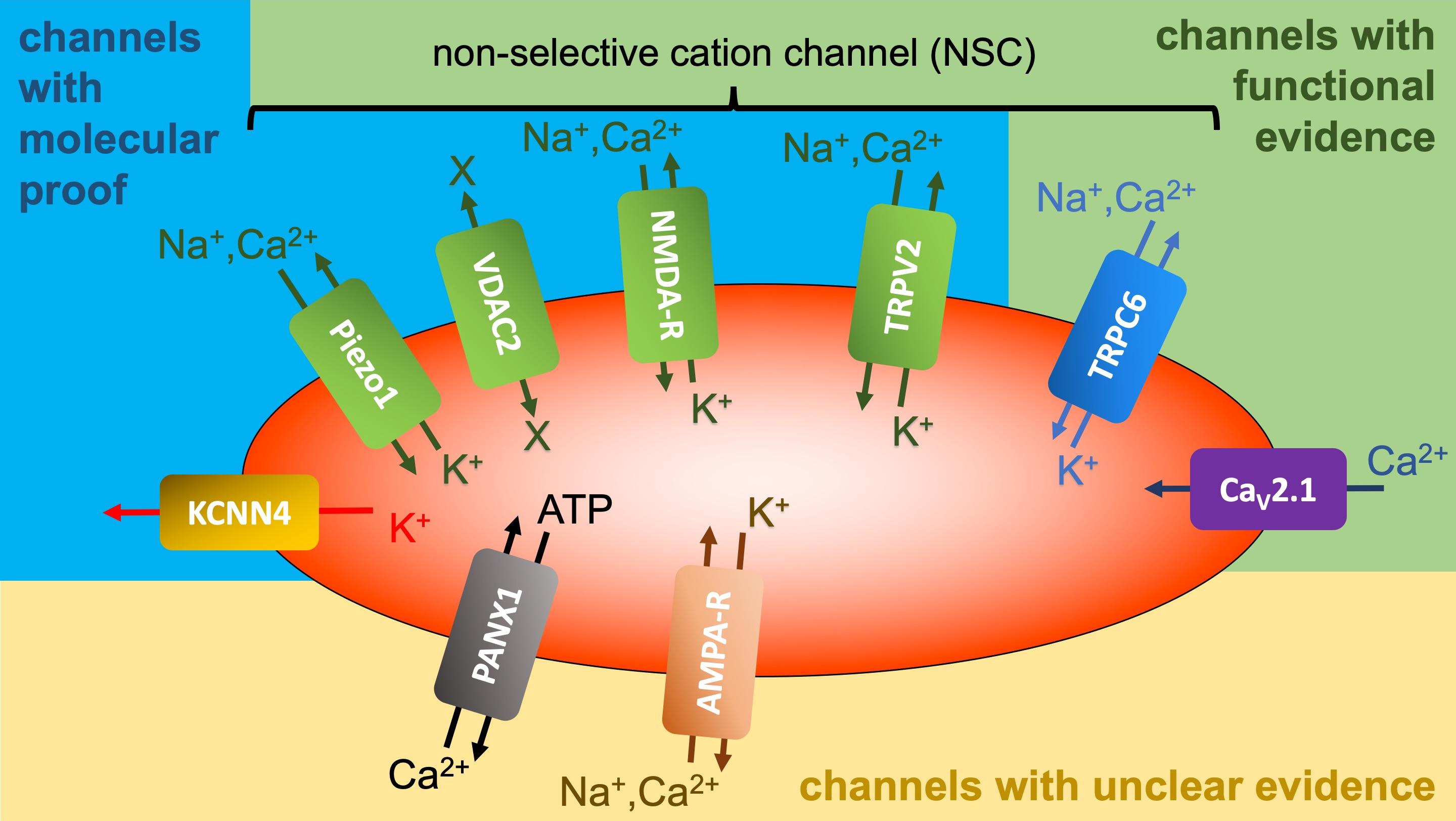 Fig. 2.
Fig. 2.
Overview of ion channels reported to be present in RBCs. Gárdos Channel (KCNN4) [88, 89]; piezo1 [93, 94]; voltage-dependent anion
channel type 2 (VDAC2) [95]; n-methyl-d-aspartate receptor (NMDA-R) [96]; transient receptor potential channel of vanilloid type 2 (TRPV2)
[97, 98]; transient receptor potential channel of canonical type 6 (TRPC6)
[99, 100]; CaV2.1, voltage-dependent Ca2+ Channel 2.1 [101, 102]; pannexin 1 (PANX1) [103];
Another intriguing aspect is the variation in ion transport pathways among RBCs of different species and their modulation during RBC maturation. For instance, (i) KCC is present in young but silent in mature human RBCs [108]. However, in mature RBCs it can be activated by different manoeuvres [109]. KCC is present in low K+-type (LK) sheep RBCs but absent in high K+-type (HK) sheep RBCs [110, 111]. The K+ content in sheep RBCs is under genetic control, resulting in either low K+-containing (LK) or high K+-containing (HK, similar to RBCs of most mammalian species). For more details, we refer to [109, 112]. No differences in the lipid composition between these two RBC types have been observed [113]. (ii) Voltage-activated cation transport occurs in HK but not in LK sheep RBCs [114]. On the contrary, the low ionic strength (LIS) induced residual (leak) cation transport is present in LK but not HK sheep RBCs ([115], for LIS effect see section 7). (iii) Despite the absence of the Na+/K+ pump in mature dog and cat RBCs, these cells possess a Ca2+/Na+ antiporter [116, 117] absent in RBCs of other mammals, enabling Na+ gradient generation based on the Ca2+ gradient realised by the Ca2+ pump (which is present in all mammalian cells) [118]. Assuming the existence of NKCC in dog and cat RBCs, a K+ gradient is ultimately established. (iv) Notably, Ouabain, a Na+/K+ pump inhibitor, requires much higher concentrations for rat and mouse RBCs compared to human RBCs [119, 120]. (v) Approximately 10% of Japanese cows lack band 3 protein crucial for gas exchange in their RBC membrane, raising questions about their survival mechanism [121].
It has long been known that the activity of integral membrane proteins, including ion transporters, can be influenced by the lipid environment within the membrane. It is now broadly accepted that the function of membrane proteins can be affected by the head group or fatty acid (or both) of the surrounding lipids. In addition to such specific effects, which are not fully understood, also non-specific effects, influenced by the physical properties of membrane lipids, e.g., the structure and the fluidity of the membrane, play a role in the regulation of membrane proteins [122, 123, 124]. In general, the investigation of lipid-protein interaction is complicated since three different possibilities have to be taken into consideration, the role of (i) bulk lipids, (ii) boundary or annular lipids, representing the first shell of the membrane protein coat, and (iii) specifically bound lipids at the membrane protein surface. One example of such effects is the Na+/K+ pump affected by the lipid environment via both general (physical) and specific (chemical) interactions [125, 126]. In the case of the RBC membrane, specific effects of membrane lipids on transport proteins have been demonstrated. These findings stem from a study using different mammalian species, by reconstitution of purified proteins in lipid bilayer structures, e.g., liposomes, or by altering the lipid composition of RBCs through the use of a phospholipid exchange protein (PLEP) or using right-site-out membrane vesicles [127]. Furthermore, the activity of certain transport proteins has been shown to depend on the molar phospholipid/cholesterol ratio, which has been modified by incubating RBCs with lipid vesicles containing varying amounts of cholesterol (for details see [128]). Another aspect not yet fully explored is how the movement of membrane lipids, particularly their transmembrane movement, affects the activity of integral membrane proteins. As this issue lies beyond the scope of this paper, interested readers are referred to a variety of reviews (e.g., [129]). However, it is worth mentioning that SM does not translocate from the outer to the inner membrane leaflet. Consequently, sheep and cow RBCs, which exclusively contain SM in the outer membrane layer, do not require scramblases, as reported by Nguyen et al. [130].
While numerous ion transporters exist in the RBC membrane, our understanding of pathways for trace metal ions such as zinc, copper, cobalt, nickel, chromium, manganese, iron, and cadmium remains limited [131]. Identifying and characterizing specific transporters for these ions is imperative for future research. Additionally, elucidating the dynamics, including transporter movement during ion translocation, is essential alongside determining the 3D structure of membrane transport proteins post-crystallization.
The question remains whether electrodiffusion of a particular ion can occur in
the human RBC membrane when all specific pathways for this ion (pumps, carriers,
channels) are inhibited. It has long been assumed that the remaining residual
(leak) ion flow is attributable to simple electrodiffusion [132, 133, 134, 135, 136]. In several
publications, we have demonstrated that the observed residual fluxes of Na+
and K+ are mediated by a Na+(K+)/H+ antiporter (e.g., [87, 137, 138]). This notion stems from studies [87, 137, 138] of residual K+
efflux in low ionic strength (LIS) solutions. It has been established for some
time that the residual K+ efflux, specifically the (ouabain + bumetanide +
ethylene glycol-bis(
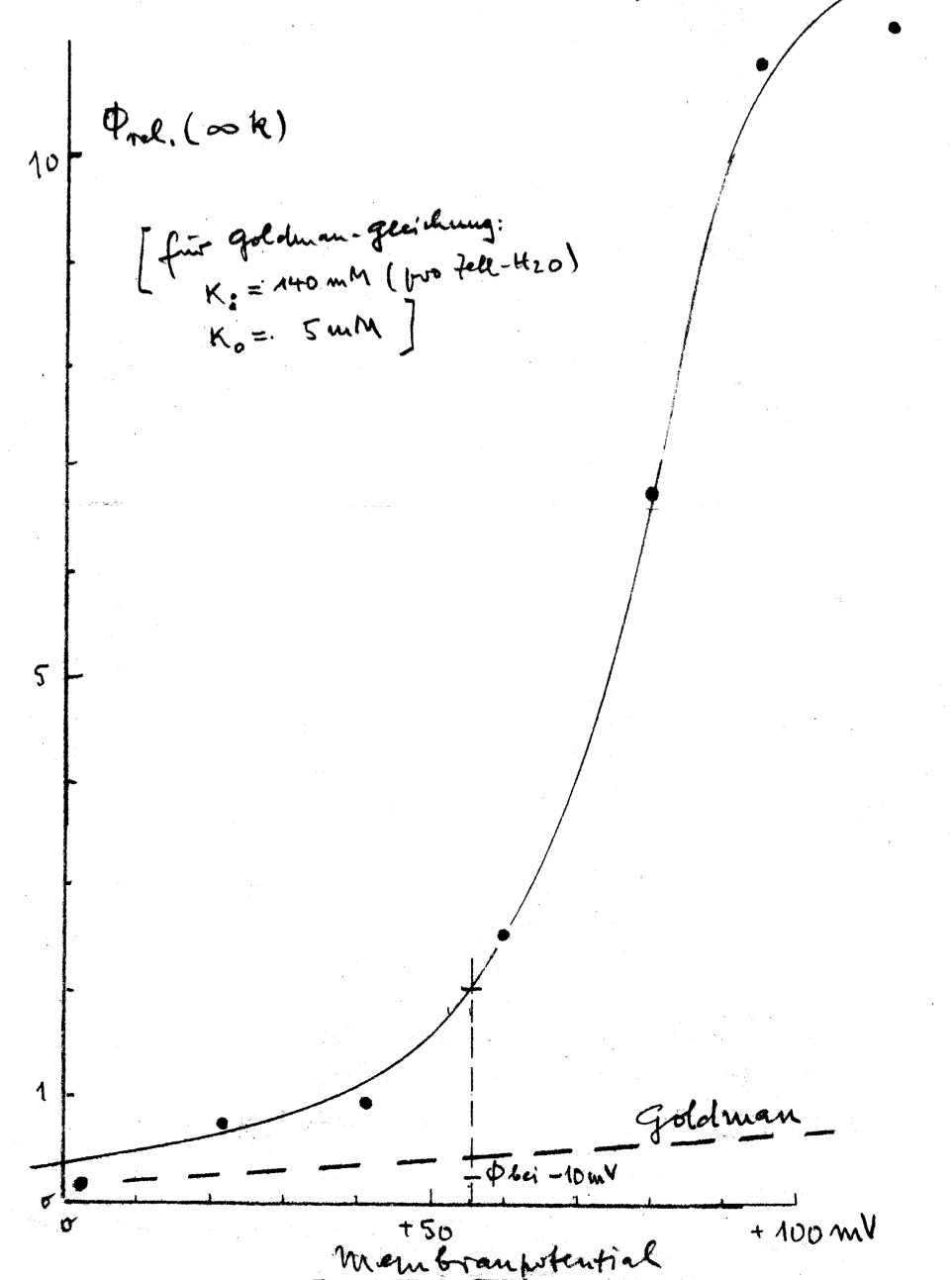
Experimental data illustrating this phenomenon are presented in Fig. 3. Theoretically, an increase in K+ efflux can be anticipated based on electrodiffusion mechanisms. This expectation arises from a shift of transmembrane potential, from approximately –12 mV in physiological solutions to roughly +50 mV in LIS solutions due to reduced NaCl concentration. However, the observed flux-increase surpassed predictions based on the Goldman flux equation:
with Jj – outward flux of the ion j, Pj – membrane permeability for ion j, Vm – transmembrane potential, cji – intracellular concentration of ion j, cjo – extracellular concentration of ion j, and zj – valence of ion j. F, R, and T – are the Faraday constant, gas constant, and absolute temperature, respectively. Thus, the Goldman flux equation (equation above) describes the electrodiffusion of an ion in dependence on the driving force, which includes the ion concentrations of both sides of the membrane and the transmembrane potential [140]. For an illustration of the effect, see Fig. 3.
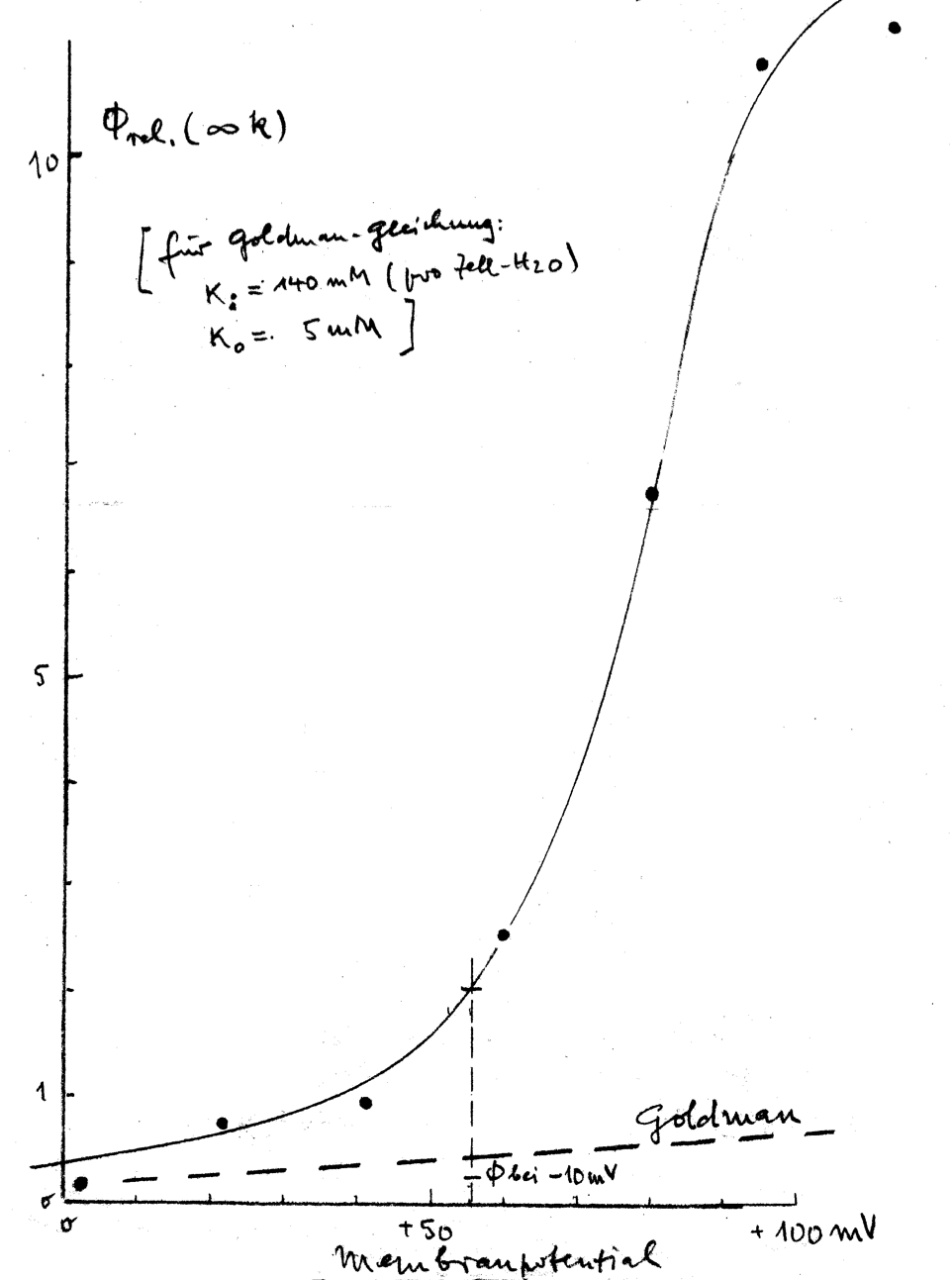 Fig. 3.
Fig. 3.
K+ efflux of human RBCs depending on the membrane potential, i.e., on the extracellular NaCl concentration of isotonic solution (the NaCl reduction was compensated by sucrose). The figure (hand drawing) represents one of the oldest data of K+ efflux in low ionic strength (LIS) solutions, probably obtained from experiments carried out before the 1960s and is a personal gift of H.J. Schatzmann (former Director of the Pharmacological Institute of the University Bern (Switzerland) to Ingolf Bernhardt.
Surprisingly, the K+ efflux in bovine and HK sheep RBCs remained largely unchanged in LIS solutions compared to physiological ones [141, 142, 143]. Thus, we conducted comprehensive measurements of all four residual fluxes for K+ and Na+ — efflux and influx for both — in human RBCs. Surprisingly, all four fluxes exhibited significant increases in solutions with decreasing ionic strength [137]. This phenomenon defies explanation solely through electrodiffusion, as it would necessitate two fluxes increasing and two decreasing under uniform changes of the driving force for all four. Various mechanisms were considered, with several possibilities eliminated based on arguments presented as early as 2003 [87]. The most plausible explanation for the observed effect was the involvement of a Na+(K+)/H+ antiporter [87, 137, 138]. A K+/H+ exchange has been also described in trout RBCs [144].
Despite accumulating evidence supporting this hypothesis, we unfortunately failed to demonstrate the presence of such a transporter in the RBC membrane at the molecular level. To date, 13 isoforms of Na+/H+ antiporters have been identified in biological membranes, with isoforms 1–9 relatively well-characterized [145]. While NHE1–NHE5 are typically found in cell membranes, NHE6–NHE9 have been primarily detected in organelle membranes, leading to the assumption that NHE6–NHE9 are absent from cell membranes [146]. However, recent evidence has suggested the presence of NHE9 in the plasma membrane of inner ear hair cell bundles [147].
Furthermore, NHE7 and NHE9 not only exchange Na+ but also K+ for H+. However, attempts to detect the presence of NHE7 in the human RBC membrane using mass spectrometry and fluorescent antibodies were unsuccessful. Our focus on NHE7 stemmed from limited knowledge about NHE9 at the time of our investigations. Future research should explore the hypothesis of NHE9’s presence in the human RBC membrane. Recent support for the presence of a Na+(K+)/H+ antiporter in the human RBC membrane comes from various other findings.
As already mentioned, unlike human RBCs, HK sheep and bovine RBCs did not exhibit increased residual K+ efflux in LIS solutions, despite experiencing similar changes of the transmembrane potential. Our research demonstrated that residual K+ efflux of RBCs of different species correlates with lipid composition of the RBC membrane, particularly the content of arachidonic acid. Furthermore, we observed an increased residual K+ flux in LIS solutions of new-born calf RBCs, which diminished over time (blood taken between one day and six weeks after birth of the calves), as their arachidonic acid content decreased (correlation coefficient between flux in LIS solution and arachidonic acid content of the RBC membrane of the calves: 0.951) [141, 143]. A recent study has shown that NHE9 activity is modulated by phosphatidylinositol-4,5-bisphosphate (PIP2), with potential implications for its interaction with arachidonic acid-enriched membranes [148]. This provides a plausible explanation for our earlier findings. In summary, residual transport of Na+ and K+ across the RBC membrane appears to be specific and mediated by a cation/proton antiporter in humans. Future research should aim to elucidate how monovalent cations traverse the RBC membrane when all known specific pathways (pumps, channels, carriers) are inhibited.
Although we have a substantial understanding of the structure and function of the RBC membrane, many questions remain unanswered. Notably, the RBC was almost always the first cell type studied when new methods for investigating biological cells were introduced. The only exception was the patch-clamp technique since RBCs are “designed” to pass through small capillaries, which made it difficult to create pipette geometry where RBCs could be “patched” and not just “sucked in the pipette” [149]. However, today, patch-clamp studies of channels in the RBC membrane are routine (e.g. [150]) and can even be performed using automated patch-clamp devices (e.g. [151]).
In this review we focussed on original findings, some dating back to the 1960s or 1970s, as we recognised that these data are often forgotten or unknown to younger scientists. Occasionally these measurements are repeated many years after the original investigations, yet the earlier work is often ignored and not cited in subsequent publications. Building on earlier investigations and employing modern techniques, it will be possible to address unsolved questions regarding RBC membranes. Some of these questions have been discussed in this paper.
EGTA, ethylene glycol-bis(
IB and LK made substantial contributions to the conception and design of the work. Both authors wrote the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
I. Bernhardt is thankful to H.J. Schatzmann (
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.