1 Department of Medical and Surgical Sciences, Division of Cardiology, “Magna Graecia" University, 88100 Catanzaro, Italy
2 Department of Experimental and Clinical Medicine, “Magna Graecia" University, 88100 Catanzaro, Italy
†These authors contributed equally.
Abstract
Acute coronary syndromes (ACSs) represent a significant global health challenge arising from atherosclerotic cardiovascular disease (ASCVD), with elevated low-density lipoprotein cholesterol (LDL-C) levels being a primary contributor. Despite standard statin therapy, individuals with ACS remain at high risk for recurrent cardiovascular events, particularly in the initial post-ACS period. Monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), such as evolocumab and alirocumab, offer a potential strategy to reduce LDL-C levels further and mitigate this residual risk. This review delves into the molecular mechanisms, effects on cholesterol metabolism, inflammatory modulation, and clinical outcomes associated with early administration of PCSK9 inhibitors following ACS.
Keywords
- acute coronary syndrome
- proprotein convertase subtilisin/kexin type 9
- low-density lipoprotein cholesterol
Acute coronary syndromes (ACSs) are an expression of atherosclerotic cardiovascular disease (ASCVD), ASCVD is one of the most prevalent causes of morbidity and mortality worldwide [1, 2].
Low-density lipoprotein cholesterol (LDL-C) is crucial for developing and progressing ASCVD and correlates with the risk of cardiovascular events [3, 4, 5]. Therefore, lowering LDL-C levels represents the central goal of the recent European Society of Cardiology/European Atherosclerosis Society and American Heart Association/American College of Cardiology guidelines for cardiovascular prevention [6, 7].
In patients who have suffered from an ACS, early plaque stabilization through
LDL-C reduction is important for preventing recurrent ischemic events, as
demonstrated by the substantial risk reduction of cardiovascular events among
patients receiving an early and intense statin therapy [8, 9]. Notably, LDL-C
targets vary according to the cardiovascular (CV) risk of the patient, with
patients at very high risk requiring an LDL-C reduction
Evolocumab and alirocumab are two humanized monoclonal antibodies (mAbs) that target and inhibit the protein responsible for the catabolism of the LDL-C receptor: proprotein convertase subtilisin/kexin type 9 (PCSK9) [14]. These drugs significantly reduce LDL-C levels and are currently indicated in primary and secondary prevention of ASCVD for patients with persistently elevated LDL-C levels despite high-intensity statin therapy and in cases of statin intolerance [15].
Recently, some studies have tested the use of PCSK9 inhibitors (PCSK9I) in the acute phase after ACS for an early reduction in LDL-C, demonstrating the safety, feasibility, and efficacy of this approach [16, 17, 18]. Furthermore, it has been shown that using these drugs in the first days after ACS allows remodeling of the atherosclerotic plaque, possibly stabilizing the non-culprit lesions [19].
Early management of dyslipidemia using PCSK9I mAbs in the acute phase of ACS patients may improve outcomes in this context, and this review aims to underline the molecular mechanisms and current clinical evidence of this strategy.
The PCSK9 enzyme, encoded by the PCSK9 gene situated on chromosome 1 in humans, plays a key role in the degradation of LDL receptors (LDL-R) [20, 21]. Formerly known as NARC-1 (neural apoptosis-regulated convertase-1), this protein consists of 692 amino acids with a molecular weight of 72 kDa and was first elucidated in 2003 within the cerebral tissues of individuals afflicted with familial hypercholesterolemia [22]. Upon LDL binding to its receptor, the resultant complex undergoes internalization into hepatic cells within vesicles, subsequently merging with lysosomes for LDL degradation, while the receptor is recycled to the cell surface [20, 21]. However, the presence of PCSK9 in the LDL+ receptor complex instigates the degradation of LDL-R within the lysosomes, thus attenuating the number of receptors on the hepatic cell membrane, consequently impairing LDL clearance [20, 21].
PCSK9 circulates in plasma with a wide range of concentrations (33–2988 ng/mL) in healthy individuals, with its levels rising in response to conditions such as hypoxia, inflammatory stimulation, hemodynamic shear stress, or exposure to reactive oxygen species (ROS) [23]. While the liver, kidney, and small intestine are the main sources of circulating PCSK9, it is also produced by vascular cells, including vascular smooth muscle cells (VSMCs), endothelial cells (ECs), and, to a lesser extent, macrophages.
The contribution of PCSK9 to the atherosclerotic process goes beyond regulating
LDL clearance, as it is directly involved in the process of vascular damage. A
study by Tang et al. [24] in apolipoprotein E knockout (apoE KO) mice
demonstrated that PCSK9 protein silencing led to less atherosclerotic burden than
in the controls. Notably, the lesions in the PCSK9-depleted group had a reduced
number of macrophages and decreased expression of vascular inflammation
regulators, with a downregulation in the toll-like receptor 4 and nuclear factor
kappa B (NF-
In the early phase of ACS, patients experience a surge in PCSK9 levels, which is
partially linked to the high prescription of statins in this context [28, 29].
Indeed, statins reduce intracellular cholesterol reservoirs by inhibiting its
endogenous production, which, in turn, stimulates PCSK9 transcription factors
(sterol regulatory element binding proteins [SREBP] and hepatocyte nuclear factor
1
Interestingly, several studies have also shown that a statin-independent mechanism contributes to the PCSK9 elevation in ACS. An analysis from the Ottawa Heart Genomics (OHGS) registry involving 45 individuals with acute myocardial infarction (AMI) revealed significantly elevated PCSK9 levels before initiating statin compared to 398 coronary artery disease (CAD) cases without myocardial infarction (MI). Similarly, in the Emory Cardiology Biobank study, PCSK9 levels were elevated in 74 individuals with AMI compared to the 273 individuals with CAD but no MI [31].
The molecular mechanism behind the surge in PCSK9 in patients who suffered from an ACS is unclear. However, evidence suggests that PCSK9 is associated with many pro-atherothrombotic states, including pro-inflammatory, pro-thrombotic, and endothelial pro-apoptotic states (Fig. 1).
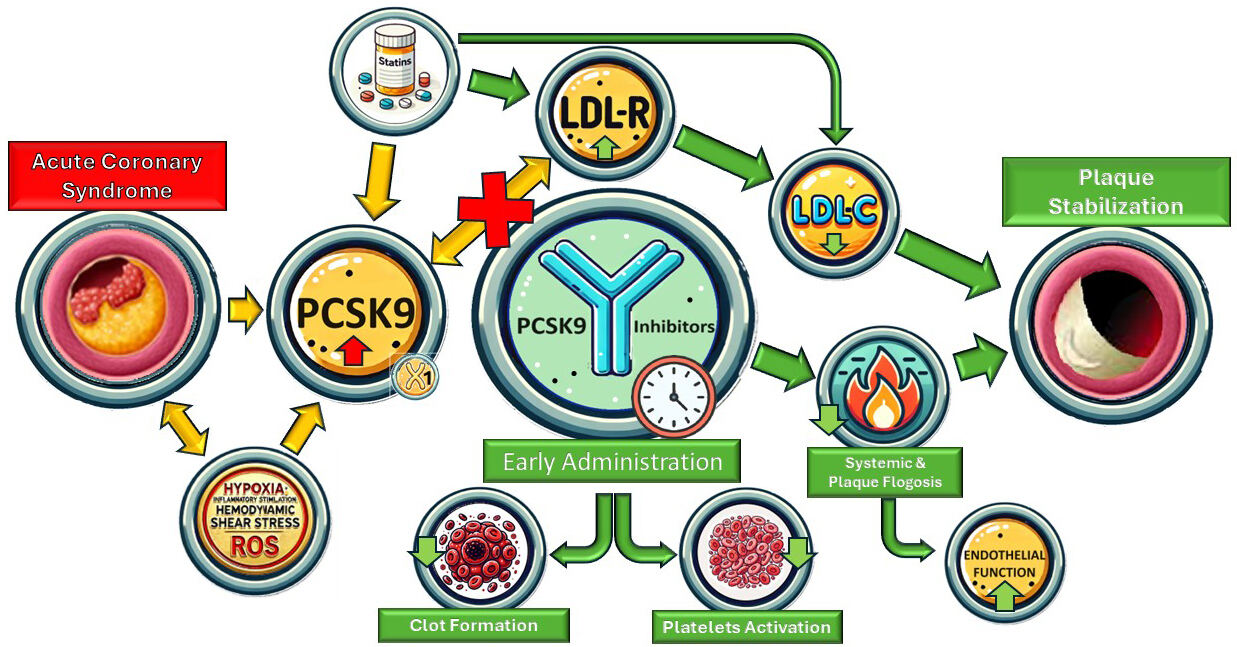 Fig. 1.
Fig. 1.
Summary figure. LDL-R, low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type 9; LDL-C, low-density lipoprotein cholesterol; ROS, reactive oxygen species.
Evidence suggests that activating the inflammatory process during ACS could
contribute to the increase in PCSK9 levels. The PEACE-Prospective AMI study
revealed a positive correlation between plasma PCSK9 levels and high-sensitivity
C-reactive protein (hs-CRP) levels, commonly used to assess systemic and
low-grade inflammation about cardiovascular risk [32]. Likewise, within a
substantial cohort of 2030 ACS patients undergoing coronary angiography in a
prospective Swiss study, PCSK9 levels increased 12–24 hours after ACS
presentation (374 + 149 vs. 323 + 134 ng/mL, p
Despite the correlation between PCSK9 and hs-CRP levels, a meta-analysis revealed that PCSK9Is have no significant effect on hs-CRP and, hence, no significant effect on systemic inflammation [35]. Similarly, secondary analyses from the EVOCATION trial (Evolocumab for prevention of microvascular dysfunction in patients undergoing percutaneous coronary intervention: the randomized, open-label EVOCATION trial), which was designed to assess the potential of PCSK9Is to reduce periprocedural microvascular resistance when administered six weeks before percutaneous coronary intervention (PCI), described no impact of PCSK9Is on hs-CRP, interleukin (IL)-6, and pentraxin-3 levels [36]. Overall, the interaction between systemic inflammation and PCSK9 remains elusive.
Even if PCSK9Is have failed to affect systemic inflammation markers
significantly, recent data suggest they have an anti-inflammatory effect within
atherosclerotic plaques. Hoogeveen et al. [37] demonstrated that using
alirocumab for 14 weeks significantly reduced arterial wall inflammation,
assessed by positron emission tomography/computed tomography (PET/CT), in 50 patients affected by CAD compared to controls, despite
no changes in systemic inflammatory markers. Conversely, Marfella et al.
[38] demonstrated, in a large translational study involving 645 patients who
underwent carotid endarterectomy, that the use of PCSK9Is significantly reduces
intraplaque expression of NLR family pyrin domain containing 3 (NLRP3) inflammasome and caspase-1 proteins, as well as
lowers levels of IL-1
Laboratory investigations have demonstrated the constitutive expression of PCSK9
in adult terminally differentiated rat cardiomyocytes [39, 40]. Notably, its
expression escalates under conditions of hypoxia, oxidized LDL exposure,
hypoxia/reoxygenation, and ischemia/reperfusion scenarios [39, 41]. Specifically,
exposure to hypoxia induces a significant upsurge in PCSK9 expression within
cardiomyocytes, a phenomenon mitigated by HIF-1
PCSK9 is expressed in the artery wall, particularly in endothelium cells, smooth muscle cells, and macrophages [44]. The endothelium acts as a selectively permeable barrier. However, shear stress on the artery wall alters cell shape and orientation, increasing macromolecule permeability, such as LDL, promoting PCSK9 production, and activating inflammatory pathways [45]. Endothelial dysfunction, which disrupts the balance between vasodilator and vasoconstrictor factors, is the initial step in the atherosclerotic process [46]. PCSK9Is improve CV outcomes by directly reducing LDL-C levels and impacting systemic inflammatory responses, oxidative stress on the artery wall, and nitric oxide production [47].
Several studies have demonstrated that PCSK9Is are associated with improvements in arterial stiffness concurrent with LDL-C reduction [48], potentially enhancing endothelial function. Maulucci et al. [49] demonstrated proportional improvement in endothelial function with decreased LDL-C levels. For instance, in patients with a previous MI that was treated with evolocumab (140 mg bi-weekly) alongside maximum tolerated statin and ezetimibe doses, the authors observed improved flow-mediated dilation (FMD) following the brachial artery vasoreactivity test after two months of treatment [49]. Similar results were found by Di Minno et al. [50] in familial hypercholesterolemia (FH) patients treated with evolocumab (140 mg bi-weekly), whereby a reduction in small dense LDL and changes in oxidation markers and endothelial function were observed.
In patients with AMI, results from a sub-study of the PACMAN-AMI trial suggest improvement in endothelial dysfunction one year after the acute event [51]. However, there was no additional direct improvement in FMD with alirocumab compared to rosuvastatin alone and no significant association between LDL-C reduction and FMD improvement in the PCSK9I group [51]. These findings underscore the need for further research on the effects of PCSK9Is on endothelial function across different patient populations and possible differences during the acute phase of AMI.
Elevated levels of PCSK9 have been correlated with heightened platelet reactivity and increased risk of ischemic major adverse cardiovascular events (MACEs). Laboratory studies support this hypothesis: the addition of hrPCSK9 to human-platelet-rich plasma samples significantly enhanced platelet aggregation induced by subthreshold concentrations of epinephrine (0.3 and 0.6 mM), reducing the lag time by 40% and increasing the area under the curve by 15% [52]. Furthermore, studies in mice have demonstrated that the absence of PCSK9 results in reduced arterial thrombus formation and stability, along with decreased platelet function. This evidence suggests that elevated PCSK9 levels may influence platelet reactivity, potentially predicting ischemic events in ACS patients undergoing PCI [52, 53, 54].
The effect of PCSK9 on platelet reactivity may be mediated by the CD36 receptor, which activates several pathways, such as Src kinase, mitogen-activated protein kinase (MAPK)- extracellular signal-regulated kinases (ERK)5, and c-Jun N-terminal kinases (JNK). These cascades increase the generation of ROS and thromboxane A2, ultimately intensifying platelet aggregability. In support of this hypothesis, Navarese et al. [55], in a recent analysis of 333 consecutive ACS patients treated with prasugrel or ticagrelor, showed a correlation between increased PCSK9 serum levels and platelet reactivity (r = 0.30; p = 0.004).
Notwithstanding these data, a mechanistic study conducted by Franchi et al. [56] has currently discarded the hypothesis of a significant impact of PCSK9 on platelet reactivity. In their study, Franchi et al. [56] examined the impact of evolocumab 420 mg on the pharmacodynamic profiles of clopidogrel in 84 ASCVD patients treated with clopidogrel. These patients were stratified into high platelet reactivity (HPR) and normal platelet reactivity (NPR) cohorts. The primary endpoint was P2Y12 reaction units (PRUs), assessed by Verify Now at 30 days. The study found that evolocumab significantly reduced LDL-C compared to the placebo at 14 and 30 days. At 14 days, the PRU levels were significantly lower in the evolocumab group compared to the placebo in the HPR cohort but not in the NPR cohort. At 30 days, there were no significant differences in PRUs in the HPR or NPR cohorts [56, 57].
Several studies have demonstrated that PCSK9 exhibits direct thrombotic and hypercoagulative activities, which promote the atherosclerotic process [47, 58]. Therefore, by providing antithrombotic activity, administering PCSK9Is may reduce CV events. Zhang et al. [59] showed that PCSK9 levels were positively associated with circulating fibrinogen in patients with stable CVD. Furthermore, by lowering the LDLR-related protein (LRP) levels, PCSK9 increases the plasma concentration of factor VIII (FVIII), which represents a critical element in the coagulation cascade [60].
The effects of PCSK9 on fibrinolysis are also linked to its correlation with plasminogen activator inhibitor-1 (PAI-1), which promotes the thrombotic process by inhibiting the tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (uPA) [61]. Levine et al. [61] demonstrated a direct correlation between PAI-1 levels and PCSK9 concentrations through RNA sequencing in vitro and in vivo in mice with hyperlipidemia. Additionally, the authors documented a positive correlation between PAI-1 and PCSK9 levels in patients with heart failure and preserved ejection fraction. After treatment with PCSK9Is in hypercholesterolemic patients, a statistically significant increase in PCSK9 levels and reduction in PAI-1 concentrations were observed, suggesting that PCSK9 modulation has varying effects on PAI-1 levels [61].
Basiak et al. [58] demonstrated that alirocumab (150 mg bi-weekly) used for 90 days in patients with isolated hypercholesterolemia is associated with significatively reduced PAI-1, factor VII, and fibrinogen, compared to the baseline and control group, and reduced von Willebrand factor (vWF) levels compared to the baseline.
Likozar et al. [62] randomized 100 stable post-MI patients with
uncontrolled LDL-C levels and elevated Lp(a) to receive either placebo or a
PCSK9I (alirocumab [150 mg bi-weekly] or evolocumab [140 mg bi-weekly]). All the
groups showed significantly increased PAI-1 levels and a borderline increase in
thrombin activatable fibrinolysis inhibitor (TAFI) levels in patients treated
with PCSK9Is (p = 0.062). Various factors, including previous statin
treatment for all patients in the study, could influence the PAI-1 concentration,
explaining the increased fibrinolytic parameter. However, the results highlighted
the correlation between LDL-C concentrations and PAI-1 (p = 0.049), as
well as TAFI (p
Overall, these findings suggest that PCSK9 may influence both primary and secondary hemostasis, either indirectly through its impact on LDL-C levels or directly by affecting hemostatic parameters. PCSK9Is may exhibit a multifaceted effect on fibrinolysis and coagulation, thereby suggesting potential future benefits.
Numerous studies utilizing intracoronary optical coherence tomography (OCT) have identified hallmark features of vulnerable plaque associated with adverse cardiovascular outcomes [63, 64]. They include the presence of a thin fibrous cap, large lipid pool, cholesterol crystals, spotty calcification, neovascularization, and potentially macrophage collections in culprit lesions and other sites within the vasculature of ACS patients [63, 64].
The phase 3, multicenter, double-blind HUYGENS (High-Resolution Assessment of
Coronary Plaques in a Global Evolocumab Randomized Study) [63] evaluated the
effect of evolocumab on plaque composition via serial OCT measures in patients
who suffered from an non-ST elevation myocardial infarction (NSTEMI). The lesions evaluated in this trial were non-culprit
plaques, which determined an angiographic stenosis of
The PACMAN-AMI trial (Effects of the PCSK9 Antibody Alirocumab on Coronary
Atherosclerosis in Patients with Acute Myocardial Infarction) showed a similar
effect of alirocumab on plaque composition. This is a double-blind,
placebo-controlled, randomized clinical trial that enrolled 300 patients who
suffered from MI [64]. Patients, in addition to statin therapy, were randomized
to receive biweekly subcutaneous alirocumab (150 mg;
n = 148) or placebo
(n = 152), starting from less than 24 hours from
urgent PCI of the culprit lesion, for 52 weeks. The study was powered for three
endpoints: change in percent atheroma volume (PAV) (primary endpoint, via intravascular ultrasound (IVUS)), change in maximum lipid
core burden index within 4 mm (secondary endpoint, via near-infrared spectroscopy (NIRS)), and change in
minimal FCT (secondary endpoint, via OCT). The results illustrated a greater
reduction in the mean PAV from baseline in the alirocumab group, compared with
the placebo group (–2.13% [95% CI, –2.53% to –1.73%] vs. –0.92% [95% CI,
–1.28% to –0.56%]; between-group difference, –1.21% [95% CI, –1.78% to
–0.65%]; p
In addition to stabilizing vulnerable plaques, long-term use of PCSK9Is has been
shown to reduce plaque burden compared to intensive statin treatment, with a
degree of benefit proportional to the extent of LDL-C lowering achieved. This
evidence comes from the GLAGOV (Global Assessment of Plaque Regression with a
PCSK9 Antibody as Measured by Intravascular Ultrasound) trial [65]. This
double-blind, randomized controlled trial included patients on a stable dose of
statins, with coronary stenosis
Overall, adding PCSK9Is to statin therapy could lead to an additional increase in fibrous cap thickness and reductions in lipid-rich plaque, even in the short term during the initial stages following ACS. However, the molecular mechanism behind this process has yet to be fully understood. A study conducted by Basiak et al. [66] in patients treated with PCSK9Is suggests that the therapy modulates the levels of pro-atherogenic cytokines, including osteopontin, osteoprotegerin, and metalloproteinase 9, which may contribute to the process of plaque stabilization. Moreover, prolonged administration of PCSK9Is may delay plaque progression and reduce plaque burden.
The impact of the early use of PCSK9Is after ACS on clinical outcomes has never
been investigated in large clinical trials. The largest phase 3 studies on
PCSK9Is excluded patients who suffered from an MI
| Study | Follow-up | Values (mg/dL) | Randomization | p-value | |
| Trankle et al. 2019 [16] | 14 days | Alirocumab 150 mg once | Placebo | ||
| (VCU-AlirocRT) | (Pts = 10) | (Pts = 10) | |||
| LDL | 28 (14–51) | 90 (75–131) | |||
| Nicholls et al. 2022 [63] | 350 days | Evolocumab 420 mg/mo | Placebo | ||
| (HUYGENS) | (Pts = 80) | (Pts = 81) | |||
| LDL | 28.1 |
87.2 |
|||
| HDL | 51.2 |
47.1 |
0.26 | ||
| TG | 114.8 |
133.5 |
0.13 | ||
| Okada et al. 2020 [72] | 28 days | Evolocumab 140 mg bi/weekly | Placebo | ||
| (Pts = 51) | (Pts = 49) | ||||
| LDL | –92.4 |
−44.8 |
|||
| Mehta et al. 2022 [69] | 42 days | Alirocumab 150 mg bi-weekly | Placebo | ||
| (EPIC STEMI) | (Pts = 38) | (Pts = 30) | |||
| LDL | 29 |
50.3 |
|||
| HDL | 42.2 (35.6–47.6) | 37.9 (33.3–47.6) | 0.29 | ||
| TG | 89.4 (72.6–123.9) | 105.3 (77.9–143.4) | 0.044 | ||
| Hao et al. 2022 [71] | 30 days | Evolocumab 140 mg bi-weekly | Placebo | ||
| (Pts = 68) | (Pts = 68) | ||||
| LDL | 22.04 |
48.7 |
|||
| HDL | 43.7 |
41.8 |
0.269 | ||
| TG | 96.5 |
93.8 |
0.749 | ||
| Wang et al. 2022 [73] | 30 days | Evolocumab 140 mg once | Placebo | ||
| (Pts = 35) | (Pts = 30) | ||||
| LDL | 56.1 |
77.7 |
0.18 | ||
| HDL | 36 |
32.5 |
0.32 | ||
| TG | 130.12 |
170.84 |
0.19 | ||
| Räber et al. 2022 [64] | 365 days | Alirocumab 150 mg bi-weekly | Placebo | ||
| (PACMAN-AMI) | (Pts = 126) | (Pts = 132) | |||
| LDL | 23.6 |
74.4 |
|||
| HDL | 48.3 |
45.0 |
|||
| TG | 94.2 |
126.0 |
|||
| Nicholls et al. 2016 [65] | 546 days | Evolocumab 420 monthly | Placebo | ||
| (GLAGOV) | (Pts = 484) | (Pts = 484) | |||
| LDL | 36.6 (34.5–38.8) | 93.0 (90.5–95.4) | |||
| HDL | 51.0 (49.8–52.1) | 47.1 (46.0–48.2) | |||
| TG | 105.1 (82.5–141.6) | 130.5 (100.3–177.2) | |||
| Koskinas et al. 2019 [17] | 56 days | Evolocumab 420 mg monthly | Placebo | ||
| (EVOPACS) | (Pts = 155) | (Pts = 153) | |||
| LDL | 30.5 |
79.7 |
|||
| HDL | 46.4 |
45.6 |
0.56 | ||
| TG | 117.8 |
127.6 |
0.25 | ||
| Vavuranakis et al. 2022 [70] | 30 days | Evolocumab 420 mg/mo | Placebo | ||
| (Pts = 39) | (Pts = 35) | ||||
| LDL | 34.7 |
61.8 |
|||
| HDL | 45.4 |
45.5 |
0.97 | ||
| TG | 87 (57–126) | 85 (69–134) | 0.55 | ||
| Luo et al. 2023 [74] | 30 days | PCSK9I monthly | Placebo | ||
| (Pts = 224) | (Pts = 259) | ||||
| LDL | 50.7 (33.3–64.2) | 82.4 (66.2–102.2) | |||
| HDL | 37.1 (32.4–44.3) | 37.9 (32.5–44.1) | 0.933 | ||
| TG | 136 (95–175.4) | 123.2 (93.9–174.5) | 0.233 | ||
Values are mean
The EVOPACS (evolocumab for early reduction of LDL in patients with acute
coronary syndrome) study [17] is an investigator-initiated, randomized,
double-blind, placebo-controlled trial including 308 patients hospitalized for
ACS with elevated LDL-C levels. Enrolled patients were randomly assigned to
receive either evolocumab 420 mg (n = 155) or placebo (n = 153) as early as
possible (within
The EPIC STEMI (effects of routine early treatment with PCSK9 inhibitors in
patients undergoing primary percutaneous coronary intervention for ST-segment
elevation myocardial infarction) trial [69] is an investigator-initiated
randomized, double-blind, placebo-controlled clinical study assessing the impact
of alirocumab on lowering lipid levels in ACS ST- elevation MI (STEMI) patients
undergoing PCI. Patients were randomized to alirocumab added to high-intensity
statin therapy irrespective of the baseline LDL-C level versus sham control. The
primary outcome was the percentage reduction in direct LDL-C up to 6 weeks. At a
median 45 days follow-up, alirocumab (150 mg bi-weekly), administered before PCI,
resulted in a significant reduction in LDL-C of 72.9% (from 2.97 mmol/L to 0.75
mmol/L) compared to a 48.1% reduction (from 2.87 mmol/L to 1.30 mmol/L) in the
sham-control group at 45 days (p
The EVACS (Evolocumab in Acute Coronary Syndrome; ClinicalTrials.gov, Unique
identifier: NCT03515304) trial [18] enrolled 57 patients with
non-ST-segment–elevation myocardial infarction and troponin I of
A pooled analysis [70] of patients enrolled in the EVACS I and in the EVACS II
trial (still ongoing), including 74 patients with an ACS diagnosis, who were
randomized to receive evolocumab or placebo within 24 hours of hospitalization,
revealed that the early use of PCSK9Is is not associated to a surge in Lp(a)
levels at 30 days, which occurred in the placebo group. In fact, at 30 days, the
placebo group showed a significant increase in Lp(a) to 82 nmol/L (24% median
percentage increase, p
In the Virginia Commonwealth University—Alirocumab Rapid Thrombus Resolution
(VCU-AlirocRT) trial [16], patients with NSTEMI, already receiving high-intensity
statin therapy, with LDL-C
The study by Hao et al. [71] in 2022 was a prospective, randomized,
controlled trial involving 136 patients diagnosed with extremely high-risk ACS.
Patients were randomly assigned to receive either evolocumab (within 48 hours
after PCI) or the placebo, in addition to standard statin therapy. During the
first month, the evolocumab group demonstrated a substantial reduction in LDL-C
levels, decreasing by –83.88%
The study by Okada et al. in 2020 [72] was a single-center, randomized,
controlled trial of 98 patients hospitalized for AMI designed to evaluate the
feasibility and safety of early initiation of evolocumab in patients undergoing
primary PCI. All patients also received pitavastatin (2 mg/day) and were
randomized to evolocumab 140 mg or placebo within 24 hours post-PCI and every 2
weeks [72]. The primary outcome was a change in LDL-C levels from baseline to 4
weeks. LDL-C levels decreased significantly after 4 weeks, with a reduction of
76.1% in the evolocumab group compared to 33.1% in the control group (mean
difference: –43.9%; 95% CI: –52.1 to –35.6%; p
Another small single-center, prospective, randomized, open-labeled trial
published by Nakamura and colleagues in 2020 [75] enrolled 38 patients diagnosed
with STEMI, of whom 17 received evolocumab, and 19 were in the non-evolocumab
group. In the non-evolocumab group, LDL-C levels decreased significantly from day
0 to day 3, followed by a temporary increase at day 5. In contrast, the
evolocumab group continuously decreased to 30.7
Secondary endpoints of the previously mentioned HUYGENS and PACMAN-AMI trial,
which were designed to evaluate plaque remodeling, include changes in LDL-C at
follow-up. In the HUYGENS [63], at 50 weeks of follow-up, LDL-C was significantly
reduced from 140.4 to 28.1 mg/dL in the evolocumab group, compared to a decrease
from 142.1 to 87.2 mg/dL in the placebo (between groups p
In a randomized study conducted in Shanxi Cardiovascular Disease Hospital in Taoyuan [73], a population of 65 patients with STEMI was divided into a routine pre-treatment group receiving high-intensity statin alone (40 mg atorvastatin or 20 mg rosuvastatin) and a combined treatment group receiving high-intensity statin plus PCSK9I (injection of evolocumab 140 mg). The primary endpoint was represented by the evaluation of the myocardial perfusion with a series of parameters, and between these, the corrected thrombolysis in myocardial infarction (TIMI) frame count (CTFC) was significantly lower, and TIMI myocardial perfusion grading (TMPG) was significantly improved both immediately after revascularization and in coronary angiography after one month. Nevertheless, combined treatment did not decrease the incidence of cardiovascular death, non-fatal MI, or target vessel revascularization, although, in the routine treatment group, there were two cases of plaque progression [73].
In a Chinese retrospective study [74], 483 patients were divided into a PCSK9I
group (n = 224) and a control group (n = 259). The PCSK9I group received statins
and short-term PCSK9I for 3 months, while the control group received only
statins. Composite endpoint events included cardiac death, recurrent MI, stroke,
unstable angina, heart failure hospitalization, and revascularization. Despite
the PCSK9I group having a lower mean age (p
It is necessary to underscore that the majority of the studies discussed above included patients already receiving statin therapy, while the benefits of PCSK9Is on clinical outcomes may differ for patients who are statin-intolerant and not undergoing statin therapy. For this reason, it would be important to conduct large-scale studies that include more patients not receiving statin treatment to validate PCSK9I benefits in this scenario. Similar information could be gathered from studies on Inclisiran, a small interfering RNA (siRNA) that suppresses the expression of PCSK9 messenger RNA (mRNA) in the liver. However, studies on clinical outcomes remain limited [14].
Despite intensive statin therapy, ACS patients remain at high risk for recurrent ischemic events, especially within the initial months. Beyond modulating LDL levels, PCSK9 is involved in multiple pathological processes that contribute to the development of ACS, including inflammation and thrombosis. Thus, prompt use of PCSK9 inhibitors may prevent early- ACS recurrences. These hypotheses are supported by the fast-occurring plaque-stabilizing effect associated with the use of these drugs. However, future studies with appropriate designs to test these hypotheses are needed.
SS, NS, SDR and DT contributed to the conception and design. ALu, ALa, AC, GC and ADC contributed to acquisition of data. SG, JI and SS contributed to analysis and interpretation of data. SG and JI have been involved in drafting the manuscript and reviewing it critically. ALa, ALu, AC, GC, NS, SS, ADC, SDR and DT participated in reviewing/editing of the manuscript. ALu, JI, AC and SG contributed to the creation of attached tables and figures. ALu, ALa, JI and SG contributed to conducting a literature review. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Salvatore De Rosa is serving as one of the Editorial Board members and Guest Editors of this journal. We declare that Salvatore De Rosa had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Manuel Martínez Sellés.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
