1 Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, 210029 Nanjing, Jiangsu, China
2 Department of Cardiology, The Third Affiliated Hospital of Soochow University, 213003 Changzhou, Jiangsu, China
3 Department of Ultrasound Imaging, Renmin Hospital, Wuhan University, 430060 Wuhan, Hubei, China
4 Department of Anesthesiology, The Second Affiliated Changzhou People's Hospital of Nanjing Medical University, 213000 Changzhou, Jiangsu, China
5 Department of Critical Care Medicine, The Third Affiliated Hospital of Soochow University, 213003 Changzhou, Jiangsu, China
†These authors contributed equally.
Academic Editor: Giuseppe Biondi-Zoccai
Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global public health emergency. As the number of confirmed cases increases, cardiovascular complications, such as myocardial injury and cardiac dysfunction, are evidenced. Takotsubo syndrome (TTS), which is common in the intensive care unit, is diagnosed among COVID-19 patients. There have been 68 more cases reports with over 119 patients since a COVID-19 patient with TTS was first reported on April 14, 2020. Angiotensin-converting enzyme 2 (ACE2), which is widely expressed in the lungs and heart, is the virus receptor. Nevertheless, randomized studies on COVID-19 related TTS are lacking, and the pathogenesis and pathophysiology are still unclear. Therefore, this review provides an overview of the potential pathogenesis, pathophysiology, clinical manifestations, diagnosis, and treatment strategy for TTS in the COVID-19 era based on current practices.
Keywords
- COVID-19
- diagnosis
- pathogenesis
- Takotsubo syndrome
- treatment
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global public health issue and has affected more than 200 countries worldwide. As of May 8, 2022, the World Health Organization had announced more than 513 million confirmed cases and more than 6,249,700 global deaths, and the number is still rising.
It has been reported that SARS-CoV-2 is more contagious than SARS-CoV and the Middle East respiratory syndrome coronavirus in 2003 and 2015, respectively. However, the mechanism of SARS-CoV-2 infection is similar to that of the traditional coronaviruses, that is, through angiotensin-converting enzyme 2 (ACE2) receptors, which is widely expressed in the lungs, heart, kidneys, and gut [1]. The localization of receptors correlates with presenting symptoms and organ dysfunction [2]. Recently, myocardial injury was observed among COVID-19 patients with a morbidity rate of 19.7%, and patients with cardiac injury exhibited a higher mortality rate of 51.2% [3]. There have been 68 cases reports with similar clinical symptoms in the COVID-19 era since a case of typical Takotsubo syndrome (TTS) triggered by SARS-CoV-2 was first reported on April 14, 2020 [4] (Supplementary Material 1). Acute onset of TTS in hospitalized patients is not uncommon, and more importantly, the risk of overall mortality was not trivial [5]. Awareness of the need to distinguish TTS in the COVID-19 era is exceedingly necessary. This review outlines the epidemiology, manifestations, pathogenesis, and therapeutic strategies of TTS triggered by the COVID-19 pandemic.
TTS is characterized by transient and reversible left ventricular (LV) systolic dysfunction, which is frequently preceded by emotional or physiological stress [6]. The term “Takotsubo” is derived from the specific appearance of a resemblance of an octopus pot at the LV end-diastolic phase. TTS mimics the clinical manifestations in patients with acute coronary syndrome (ACS) (e.g., acute chest pain, T-wave inversion, and ST-segment elevation) and must be differentiated from it [6]. Notably, TTS is not an acute myocardial injury caused by coronary artery stenosis or obstruction. However, its serious cardiac complications are similar to ACS, which increasingly receives more attention [6].
Since the first report of TTS in 1991, its prevalence has indicated an increasing trend in the past decades [7]. In relation to the increased awareness of TTS, the incidence of TTS increased from 315 cases in 2006 to 6230 cases in 2012 [8]. TTS could now be easily differentiated from ACS owing to the advancement of efficient diagnostic techniques such as invasive coronary angiography. The prevalence of TTS was reported to be approximately 2% in patients with suspected ACS (up to 10% in women), and 85%–90% of TTS patients are postmenopausal women [6, 9]. Nevertheless, the prevalence of TTS is still underestimated because of immature diagnostic criteria, such as the possibility of coexisting coronary artery disease (CAD).
TTS is often triggered by strong mental or physical stressors and has been described as ‘stress cardiomyopathy’. Both negative and positive emotions can trigger TTS [6, 10]. Males tend to be more affected by physical stressors, whereas women are more susceptible to emotional stressors [11]. Physical stressors include severe somatic diseases, such as acute respiratory failure, severe infection, and malignant tumors [12]. In addition, surgery, invasive tests, and exogenous drugs, such as glucocorticoids and catecholamines, may act as physical stressors for the onset of TTS [12].
Although few people develop TTS, acute psychological and physiological stressors are universal, which predispose individuals to be more susceptible to TTS. Abnormality in the autonomic nervous system and catecholamine sensitivity caused by mental and nervous system disorders (stroke, subarachnoid occurrence, epilepsy, etc.) and specific sensitive adrenergic receptor genotypes increase TTS susceptibility [13, 14, 15]. Moreover, special cardiac anatomical structures, such as dysplasia of the hypoplastic branches of the apical coronary arteries and LV outlet tract obstruction, may act as risk factors of TTS [16, 17]. Interestingly, the cardioprotective effect of estrogen weakens after menopause, potentially causing a higher incidence of the onset of TTS [18].
Although the exact pathogenesis of TTS is unclear, there is considerable
evidence that sympathetic activation might play a key role. In addition,
markedly elevated levels of catecholamines
may contribute to the onset of TTS [19, 20]. The distribution of cardiac
sympathetic nerve endings was gradually decreased from the basal ventricular
myocardium to the apical myocardium. The density and sensitivity of
Although enhanced sympathetic stimulation and excess catecholamine release play a critical role in TTS, the mechanism of atypical TTS with other regional ballooning patterns (midventricular, basal, etc.) is still unknown. The hypotheses of potential involvement of catecholamine toxicity, myocardial stunning, vasospasm, and microcirculatory dysfunction have been proposed.
A biopsy study found that catecholamine-induced myocarditis was observed in TTS, suggesting catecholamine toxicity in cardiomyocytes [23]. Catecholamine overload can induce cardiomyocyte injury via calcium overload, mitochondrial damage, and rupture of the mitochondrial respiratory chain [24, 25]. Meanwhile, an extremely high level of catecholamine can cause coronary vasospasm, which contributes to cardiac stunning [26, 27]. Ischemic cardiac stunning results in regional systolic dysfunction, which shows the atypical ballooning patterns [28]. TTS exhibits coronary microvascular dysfunction in addition to epicardial coronary vasospasm. Endomyocardial biopsies in TTS reveal apoptosis of microvascular endothelial cells [29]. In addition, adenosine can improve myocardial perfusion and ejection fraction, indirectly suggesting the role of microcirculatory dysfunction in TTS [30].
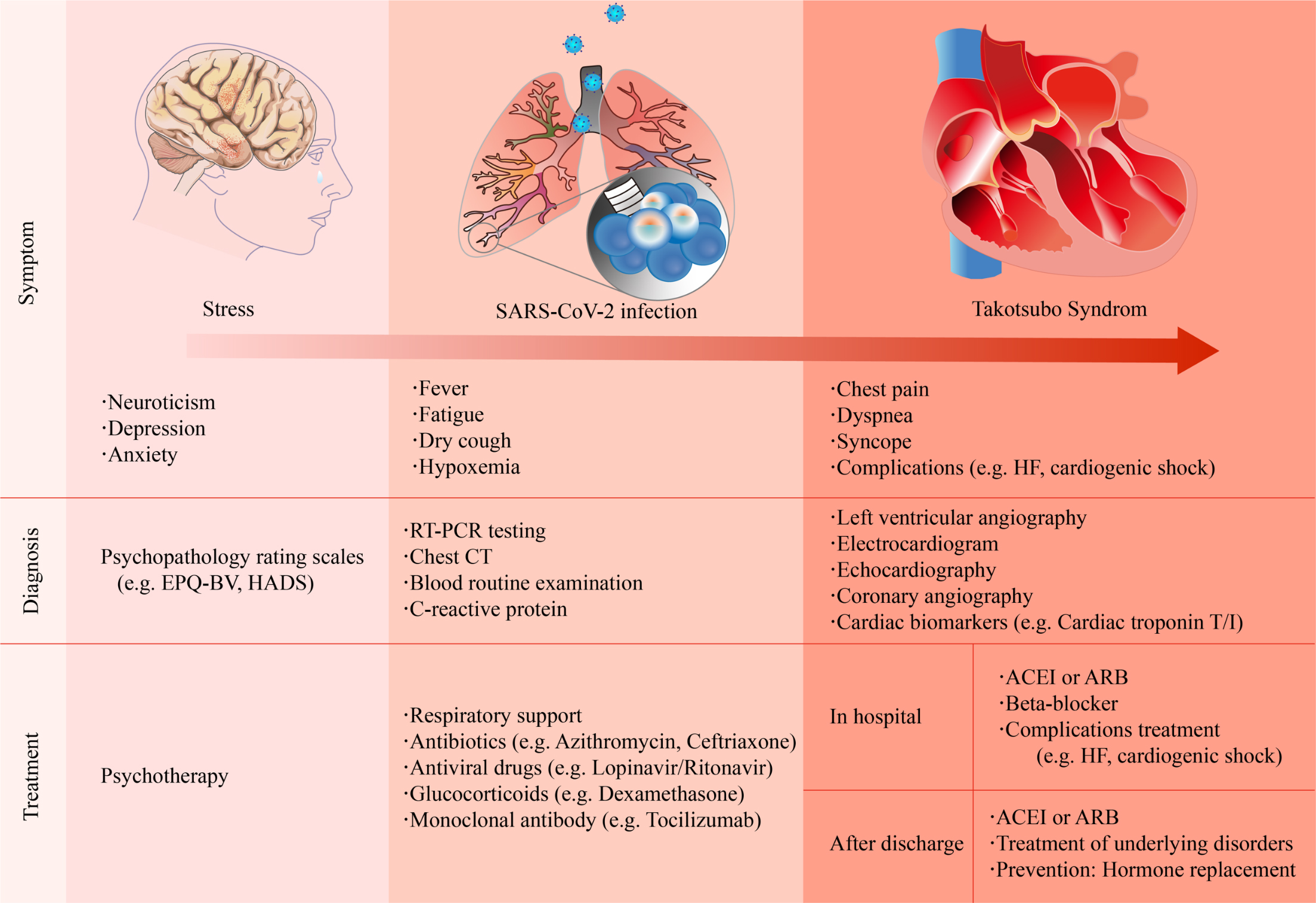
The clinical manifestations of COVID-19-related TTS are diverse (Fig. 1). Patients with mild symptoms may experience fatigue, fever, or dyspnea [31, 32], whereas some patients present with acute chest pain [4, 33]. Patients may present with tachycardia or even cardiogenic shock when the condition deteriorates [34]. The possible triggers of TTS usually resemble those of physical stress, such as intubation and mechanical ventilation, or emotional stress, including a relative’s death because of the COVID-19 pandemic.
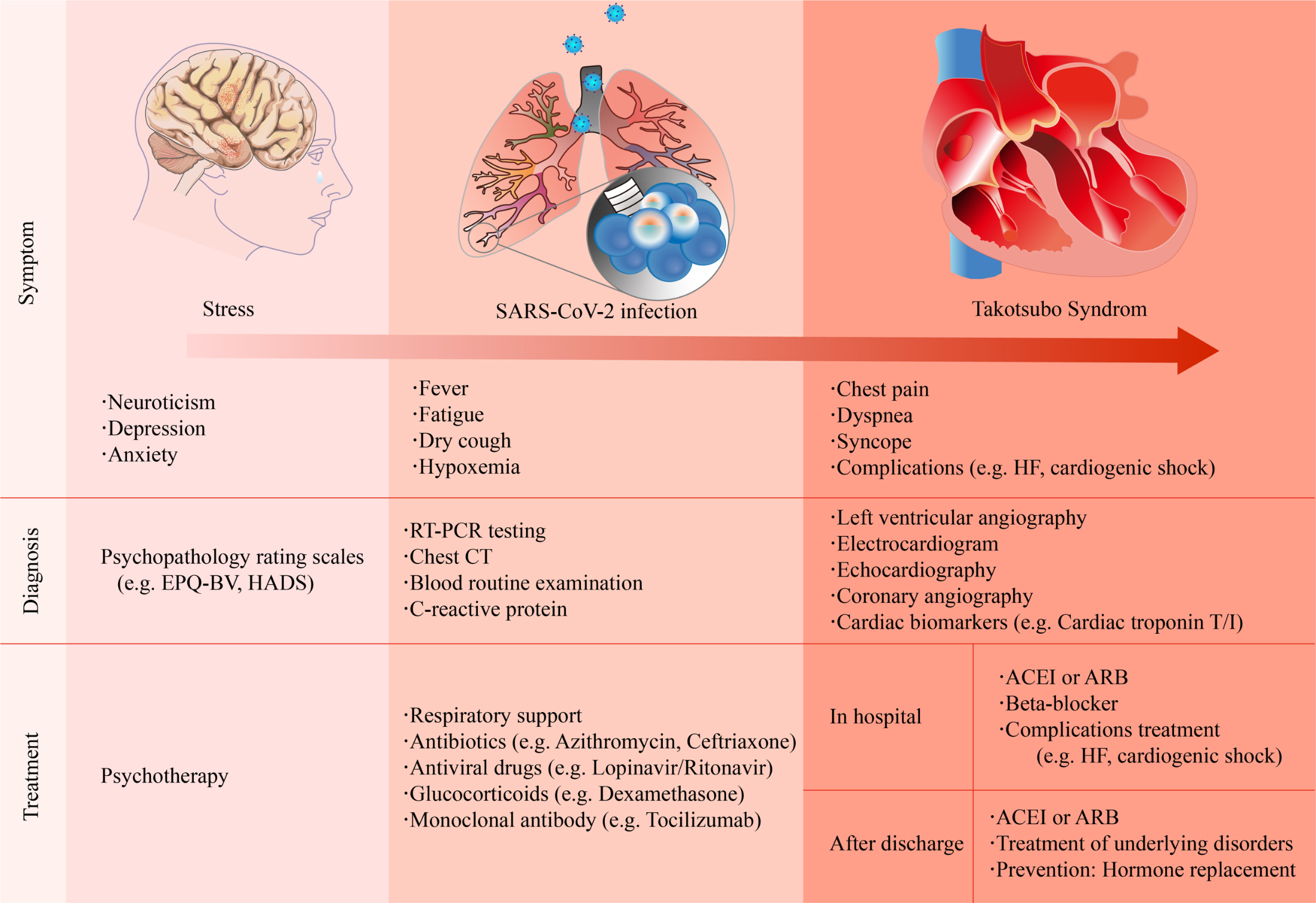 Fig. 1.
Fig. 1.Summary of the clinical symptoms, diagnosis, and treatment strategies of COVID-19-related Takotsubo syndrome. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CT, computed tomography; EPQ-BV, Eysenck personality questionnaire brief version; HADS, hospital anxiety and depression scale; HF, heart failure; RT-PCR, reverse transcription polymerase chain reaction.
The diagnosis of TTS is still a challenge because of the lack of specificity in clinical manifestations, electrocardiogram (ECG), and biomarkers. The most common ECG abnormality in TTS patients is ST-segment elevation, T-wave inversion, QTc prolongation or both [11, 35]. Most patients exhibit elevated cardiac levels of troponin, which is similar to those with an ACS. However, the peak values are relatively lower than ACS [35]. B-type natriuretic peptide (BNP), which reflects LV dysfunction, substantially increase in patients with TTS [36]. The upregulation of other potential biomarkers, such as interleukin (IL)-6 and IL-7 or microRNA-16 and microRNA-26a, is associated with stress-related disorders [37].
For suspected myocardial infarction with ST-segment elevation on ECG, emergency
coronary angiography (CAG) should be performed to rule out ACS [35]. Meanwhile,
an InterTAK diagnostic score can be considered for a non-ST-segment elevation on
ECG, with a score of
Among patients with COVID-19-related TTS, cardiac troponin T/I, and BNP elevated in all patients [40]. most of which were preceded by physical or emotional stress. However, left ventriculography is the gold standard for TTS.
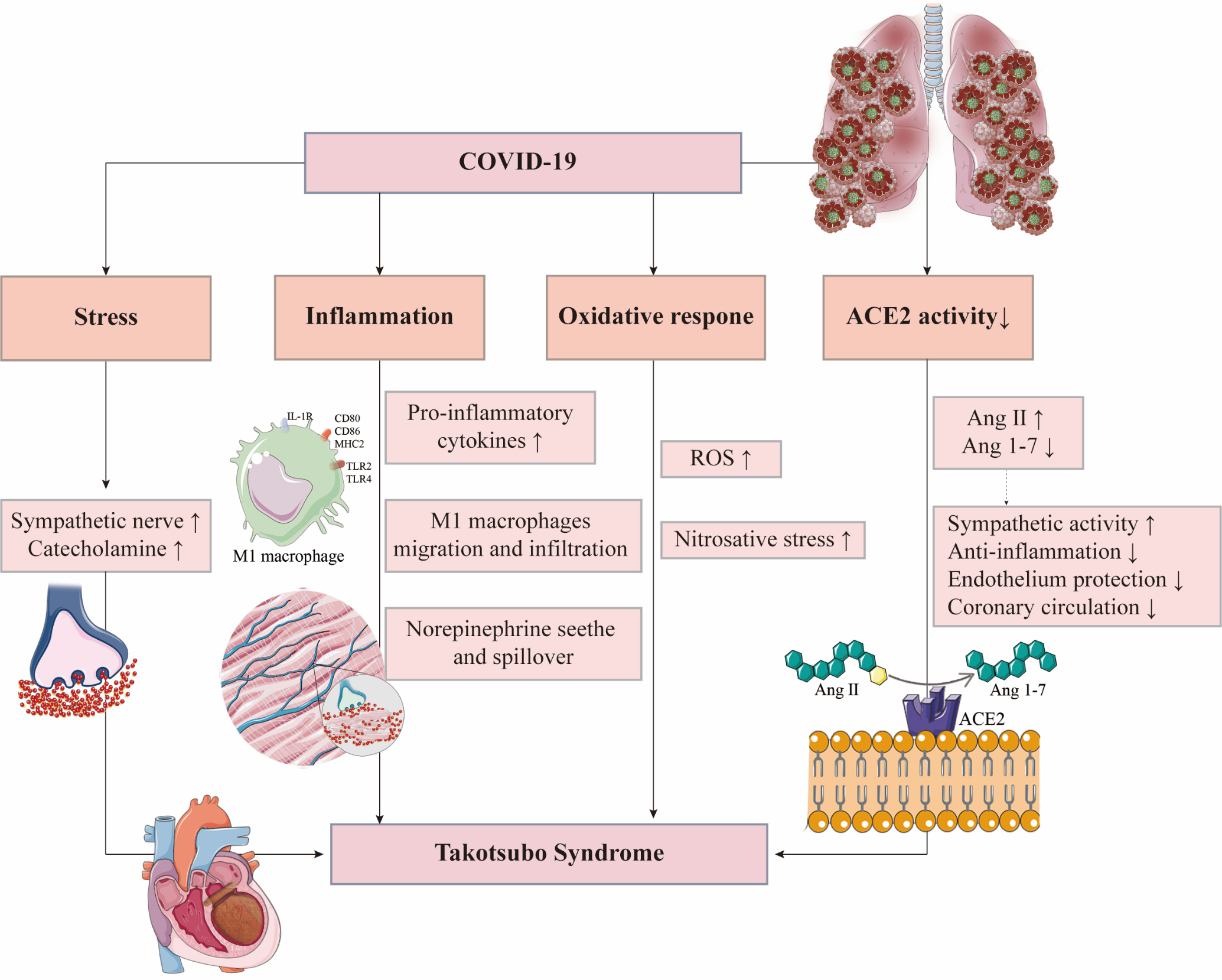
Until now, 68 TTS case reports in COVID-19 have been reported. More cases may be unreported given the incomplete diagnostic criteria of TTS. Therefore, exploring the pathogenesis of TTS in COVID-19 patients is of great significance (Fig. 2).
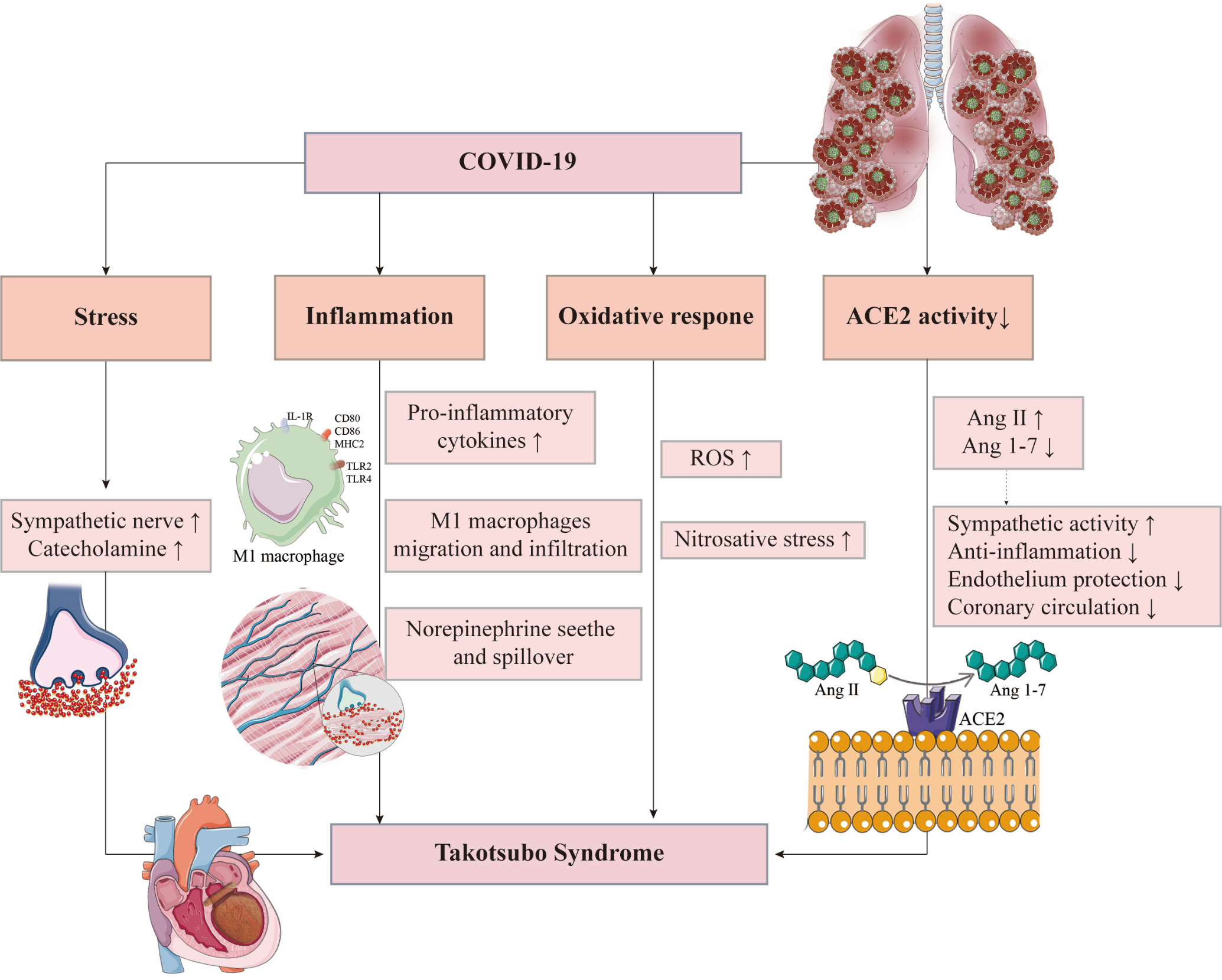 Fig. 2.
Fig. 2.Possible mechanisms of Takotsubo syndrome in COVID-19 patients. ACE2, angiotensin-converting enzyme 2; Ang, angiotensin; IL-1R, interleukin-1 receptor; MHC2, major histocompatibility complex 2; ROS, reactive oxygen species; TLR, Toll-like receptor.
Acute psychological and physiological stressors caused by COVID-19 are one of the important triggers of TTS. The COVID-19 epidemic can cause great anxiety and depression [41]. Chadha et al. [33] reported a case of TTS in an elderly woman without SARS-CoV-2 infection who developed anxiety because of the COVID-19 epidemic that triggered her illness. Compared with the general population, patients with COVID-19 in a closed unit not only face fatal physical complications but also social and psychological stress, which may cause greater anxiety, depression, and other psychological disorders [42]. These predisposing factors are more likely to induce TTS.
Physical stressors in COVID-19, another important trigger of TTS, should not be ignored. Pneumonia and hypoxemia induced by acute respiratory distress syndrome (ARDS) can result in enhanced sympathetic stimulation via the carotid and aortic body receptors [43]. In addition, strong acute systemic inflammatory response leads to decreased vascular resistance, followed by the sympathetic activation to ensure a sufficient blood supply for vital organs, which would be an important trigger for TTS [44].
SARS-CoV-2 causes ARDS by infecting the alveolar epithelial cells, resulting in
the release of cytokines such as IL-1, IL-6, and TNF-
TTS is pathologically characterized by inflammatory infiltration of myocardial
M1 macrophage (Mon 1) [47]. During the acute inflammatory phase, Mon 1 is
recruited and accumulates in the injured myocardial areas, which aggravates
inflammation by protein degradation [46]. Notably, myocardial macrophage
infiltration is more likely to result from the migration of circulating monocytes
into the heart, rather than the proliferation of intrinsic myocardial macrophages
[48]. Plasma levels of IL-6, IL-8, and chemokine ligand 1 (CXCL1) were
significantly increased in TTS patients. IL-8 and CXCL1 were related to the
adhesion and infiltration of macrophages. There is evidence that IL-6 is
correlated with the incidence of mortality and adverse effects of TTS [49]. The
risk of adverse effects was estimated to be as high as 67% with increased levels
of IL-6 and IL-10 on admission because of TTS [49]. Remarkably, cytokines such as
IL-1 and TNF-
SARS-CoV-2 causes pulmonary inflammatory
infiltration and edema via the invasion of alveolar epithelial cells.
Subsequently, alveolar gas exchange is interfered by the formation of hyaline
membrane and interstitial thickening. Ventilation-perfusion abnormality results
in hypoxemia, which could induce the imbalance between oxidation and
antioxidation and induce oxidative stress [51]. Notably, oxidative stress plays
an important role in the cardiac dysfunction in TTS. Interestingly, myocardial
oxidative stress level was markedly increased in patients with TTS, which is even
higher than that of acute myocardial infarction, and it was increased
proportionally with wall motion score and norepinephrine level [52]. The
imbalance between oxidation and antioxidation leads to reactive oxygen species
accumulation, resulting in the oxidation of nitric oxide to peroxynitrite anion
(ONOO
ACE2 is a SARS-CoV-2 receptor required for cell entry. With the help of TMPRSS2, ACE2 on the cell membrane could be cleaved, which plays a key role in the intracellular entry of SARS-CoV-2 [56]. TMPRSS2 enzymolysis causes ACE2 dysfunction, suggesting that SARS-CoV-2 infection would decrease ACE2 activity [57]. Meanwhile, ACE2 is a negative regulator of the renin-angiotensin system and plays an essential role in cardioprotective functions. ACE2 converts angiotensin II (Ang II) into Ang 1–7. The activation of Ang 1–7/Mas pathway and reduced Ang II would have protective effects for TTS, such as anti-inflammation, protection of vascular endothelial function, improvement of coronary microcirculation, and reduction of oxidative stress and sympathetic activity [58, 59]. Therefore, ACE2 dysfunction caused by SARS-CoV-2 infection is likely to be the predisposing factor that makes patients more susceptible to TTS.
TTS has been initially considered as a benign disorder. However, the morbidity and mortality of TTS were underestimated. Tornvall et al. [60] reported that the mortality curves in TTS patients were similar to that in patients with ACS. Any complications, such as heart failure and cardiogenic shock, might be life-threatening. The optimal treatments for acute and chronic TTS are needed for a favorable outcome.
Very recently, an international expert consensus on TTS summarized the
management for patients with TTS [35]. Pulmonary edema and cardiogenic shock are
the most severe complications in patients with TTS. The presence of left ventricular outflow tract obstruction (LVOTO) in
shock patients should be immediately evaluated using angiography. Fluid therapy,
COVID-19-related TTS is widely reported with the increasing number of confirmed COVID-19 cases. This syndrome automatically recovers when pneumonia subsides (Supplementary Material 1). Monoclonal antibody, such as tocilizumab, may help reduce the excessive release of catecholamines caused by the cytokine storm, which may be beneficial to reduce physical stress [64]. However, the adverse effects of drug interactions between treatments of COVID-19 and TTS are worth mentioning. A joint statement issued by the American College of Cardiology, the American Heart Association, and the American Heart Rhythm Society urgently pointed out that anti-COVID-19 drugs, such as hydroxychloroquine and azithromycin, may prolong QT intervals or lead to the occurrence of torsades de pointes-type ventricular tachycardia [65].
For patients with relatively stable TTS, ACEI,
SARS-CoV-2 invasion leads to the downregulation of surface ACE2 expression [67], while ACEI, angiotensin receptor blockers, and mineralocorticoid receptor antagonist will increase ACE2 expression [67]. However, given the lack of data on the link between the downregulation of ACE2 and patients’ susceptibility to COVID-19, these drugs should remain for patients with cardiovascular diseases [68]. Meanwhile, considering the uncertainty, calcium channel blockers, if necessary, could be taken into consideration.
TTS is prevalent in menopausal women. The role of estrogen has also been emphasized. Experimental studies reported that a weaker estrogen signaling leads to increased morbidity and mortality in both male and female mice infected with respiratory virus [69]. However, currently, there are few clinical trials to support this therapy.
A total of 119 patients of COVID-19-related TTS have been reported. This acute and reversible cardiac syndrome may be directly caused by SARS-CoV-2, the massive release of catecholamine triggered by hypoxemia, cytokine storm, or extreme anxiety caused by the COVID-19 pandemic. The increase of myocardial biomarkers is a sensitive warning of COVID-19-related cardiac injury. Patients with acute chest pain, ECG abnormality, and myocardial biomarkers should be suspected of cardiac complications. Echocardiography or LV angiography could help confirm the diagnosis. The portable echocardiography should be promptly used for patients with urgent medical conditions to identify LVOTO. Emergent treatment for cardiogenic shock should be implemented promptly, including mechanical ventilation or circulatory support devices. Medications, particularly QT-prolonging drugs, must be taken cautiously for TTS patients with COVID-19.
ACE2, angiotensin-converting enzyme 2; ACS, acute coronary syndrome; AF, atrial fibrillation; Ang II, angiotensin II; ARDS, acute respiratory distress syndrome;
CY, LY, and CL designed the review. LZ, ZC, RJ, YH, and BZ performed the research. All authors provided advice and analyzed the data. LZ and ZC wrote the manuscript’s first draft. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This research was funded by the National Natural Science Foundation of China, grant number no. 81703482 and 81974171 to CY, and grant number no. 82070405 to LY. This research was also funded by the Innovative and Entrepreneurial Team of Jiangsu Province, grant number no. JSSCTD202144 to CY, the Science and Technology Support (Social Development) Project of Bureau of Science and Technology of Changzhou, grant number no. CE20195044 to LY, and the Natural Science Foundation of Jiuangsu Province, grant number no. BK20211382 to CL.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.rcm2309298.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.