1 The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, NY 10029-6574, US
2 Cardiovascular Department, Humanitas Gavazzeni, 24125 Bergamo, Italy
Academic Editor: Federico Ronco
Abstract
Historically, prevention from ischemic events with dual antiplatelet therapy
(DAPT) post percutaneous coronary intervention (PCI) took precedence over
protection from bleeding. However, increasing data suggest that major bleeding
complications are as detrimental as ischemic events. Awareness about the
prognostic impact of bleeding prompted the search for new strategies aimed at
maximizing both ischemic and bleeding protection. This is noteworthy because
patients at high bleeding risk (HBR) have generally been underrepresented in
clinical trials on DAPT and they often are at increased risk of ischemic events
as well. The present review discusses the evidence base for new
pharmacotherapeutic strategies to decrease bleeding risk without compromising
ischemic protection among HBR patients undergoing PCI, including shortening DAPT
duration, early aspirin withdrawal, and P2Y
Keywords
- high bleeding risk
- HBR
- percutaneous coronary intervention
- antiplatelet therapy
- antithrombotic therapy
- DAPT
Antiplatelet agents constitute the foundation therapy for secondary prevention
of thromboembolic events after percutaneous coronary intervention (PCI) with
drug-eluting stent (DES) [1]. Guidelines currently recommend, the use of dual
antiplatelet therapy (DAPT), a combination of aspirin and a P2Y
Advancements in PCI technologies have allowed extending this treatment option to high-risk patients who were traditionally managed conservatively. These patients typically have extensive CAD and multiple comorbidities that, not only increase their risk for thromboembolic events, but also for bleeding complications. Indeed, a study of an all-comer population undergoing DES implantation has shown that as high as 1 in every 15 patients experienced post-discharge bleeding at a median of 300 days after the procedure [5]. Interestingly, the impact of bleeding on two-year mortality was significantly larger compared with post-discharge MI [5]. This study, together with other observational studies, shed light on the prognostic relevance of post-discharge bleeding after PCI [10].
Finding patients at HBR is of highest importance for the management of
antithrombotic therapy after PCI. Nonetheless, a lack of standardization in
defining this population limits the generalizability of trial results as well as
clinical decision-making. Based on review of the literature, the Academic
Research Consortium (ARC) recently published an agreement definition of patients
at HBR based on fulfillment of specific criteria (Fig. 1, Ref. [11]) [12].
Several registry-based studies have validated the ARC-HBR definition by showing
an incidence of Bleeding Academic Research Consortium (BARC) 3 or 5 bleeding risk
of
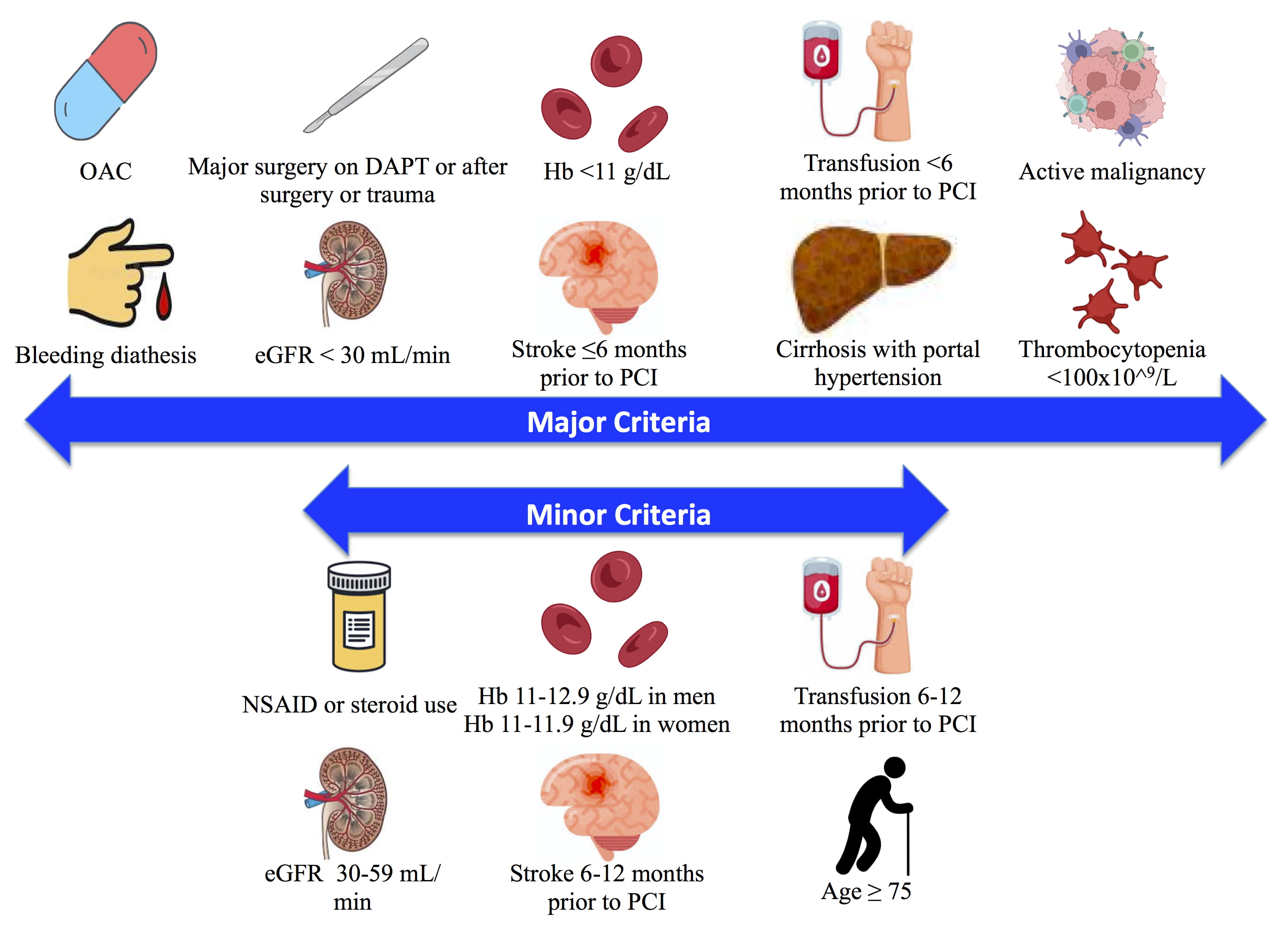 Fig. 1.
Fig. 1.ARC-HBR definition of HBR. Major and minor risk factors used in
the definition for HBR [11]. 1 major or
Over the past years, numerous risk scores have been designed to inform and guide decision making on DAPT duration and intensity after PCI. The European Society of Cardiology guidelines recommend using the DAPT and PRECISE-DAPT risk scores [16, 17]. The DAPT scoring system was developed using predictors of both ischemic and bleeding events to identify patients who derive the greatest benefit over harm from prolonging DAPT beyond 12 months of PCI [18]. Conversely, the PRECISE-DAPT was developed to assess the risk of out of hospital bleeding up to 2 years post-PCI [19]. The PARIS score encompasses two separate prediction models to evaluate ischemic and bleeding risks after PCI [20]. Although these scores share similar components, each has its own features (Table 1). The DAPT score included somewhat lower risk patients who were event-free at 12 months post-PCI, while both PRECISE-DAPT and PARIS included patients immediately after discharge of index PCI. While HBR patients composed approximately 25% of all subjects considered, each scoring system identified different rates of bleeding ranging from 1% to 10% [11]. Therefore, it has been difficult to confidently use such scoring systems in HBR patients undergoing PCI. Recently, Urban et al. [12] developed the ARC-HBR trade off model, which predicts the risk of non-periprocedural major bleeding and thrombotic events at one year among HBR patients who have undergone PCI. Although this was the first risk score especially dedicated to HBR patients, it should be noted that this tool was derived from studies using different DAPT durations (i.e., driven by the protocol of the study or guideline-based) and recommendations should not be solely made based on its risk predictions.
| REACH-39 | DUTCH ASA Score37 | DAPT41 | PARIS38 | PRECISE-DAPT32 | BleeMACS36 | |
| Year | 2010 | 2014 | 2016 | 2016 | 2017 | 2018 |
| Database | REACH | Dutch ASA registry | DAPT randomized trial | PARIS | 8 randomized trials | BleeMACS |
| Number | 56616 | 235531 | 11648 | 4190 | 14963 | 15401 |
| Population | Risk of atherosclerosis | New low-dose ASA | Post-PCI patients event free 12 mo after index | All PCI | Patients undergoing PCI | ACS undergoing PCI |
| Definition | Non-fatal hemmorhage or bleeding leading to both hospitalization and transfusion at 2 years | UGIB at median follow up 530 d | GUSTO moderate or severe bleeding | BARC 3 or 5 after 2 y | TIMI major or minor with median follow up 552 d | Intracranial bleed or any bleed requiring hospitalization or transfusion at 1 year |
| Bleeding risk score factors | Age, PAD, CHF, DM, HLD, HTN, Smoking, Anti-platelet, OAC | Age, Anemia, DM, Other anti-platelet, OAC | Age, PAD, HTN, Renal insuffiency | Age, BMI, Anemia, Triple therapy, Smoking, Renal insuffiency | Age, Previous bleed, WBC, Hb, Cr clearance | Age, HTN, PAD, Prior bleed, Malignancy, Cr clearance, Hb |
| Validation discrimination | AUC 0.68 | AUC 0.64 | AUC 0.68 | AUC 0.72 | AUC 0.73 | AUC 0.71 |
| External validation | CHARISMA | Dutch Health Insurance Database | PROTECT | ADAPT-DES | PLATO and BernPCI Registry | SWEDEHEART |
| External validation discrimination | AUC 0.64 | AUC 0.63 | AUC 0.64 | AUC 0.64 | AUC 0.70 and 0.66 | AUC 0.65 |
| ASA, aspirin; AUC, area under curve; BARC, Bleeding Academic Research Consortium; BMI, body mass index; CHF, congestive heart failure; Cr, creatinine; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; GUSTO, global utilization of streptokinase and TPA for occluded arteries; Hb, hemoglobin; HTN, hypertension; HLD, hyperlipidemia; OAC, oral anticoagulant; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction; UGIB, upper gastrointestinal bleed; WBC, white blood cell. | ||||||
The paradigm in interventional cardiology research has shifted over the past few
years into testing strategies that unite modern DES platforms with short DAPT
durations (Table 2, Ref. [21, 22, 23, 24, 25]). LEADERS-FREE was a randomized double blind
trial comparing outcomes of HBR patients receiving the polymer-free
biolimus-eluting BioFreedom stent vs. a similar bare metal stent (BMS); patients in
both arms were maintained on DAPT for one month after PCI [21]. The BioFreedom
stent was found to be superior to BMS with regards to the composite of cardiac
death, MI, or stent thrombosis, largely driven by decreased rates of MI.
Conversely, the ONYX ONE trial examined the same BioFreedom stent in comparison
to the durable-polymer zotralimus-eluting Resolute Onyx stent in a similar HBR
population [22]. The Resolute Onyx stent was found to be non-inferior to the
BioFreedom stent with respect to the same primary outcome as above. However,
since both the LEADERS-FREE and ONYX ONE trials preceded the ARC-HBR consensus,
definitions of HBR differed, making comparisons among studies difficult to
interpret. Most notably, LEADERS-FREE and ONYX ONE considered age
| Trial | N | Population | DAPT | Intervention | Control | Primary outcome | Result |
| LEADERS-FREE [21] | 2466 | CAD requiring PCI | 1 month | BioFreedom DCS | BMS | 1: Cardiac death, MI, or stent thrombosis | 1: 9.4% vs. 12.9%, HR 0.71, 95% CI 0.56–0.91, p |
| 2: TLR | 2: 5.1% vs. 9.8%, HR 0.5, 95% CI 0.37–0.69, p | ||||||
| SENIOR [23] | 1200 | 1 month stable CAD, 6 months ACS | Synergy DES | BMS | MACCE at 1 year | 12% vs. 16%, RR 0.71, 95% CI 0.52–0.94, p = 0.02 | |
| ONYX-ONE [22] | 1996 | HBR patients undergoing PCI | 1 month | Resolute Onyx DES | BioFreedom DCS | Non-inferiority for cardiac death, TVMI, TLR at 1 year | 17.1% vs. 16.9%, p = 0.01 |
| EVOLVE Short DAPT [25] | 2009 | HBR patients with stable or unstable angina | 3 months | SYNERGY DES | DES | 1: Death or MI | 1: 5.6% vs. 5.7%, p = 0.0016 non-inferiority |
| 2: stent thrombosis | 2: 0.2%, p = 0.0005 for comparison to 1% performance goal | ||||||
| XIENCE 28 [24] | 1392 | HBR patients undergoing PCI | 1 month | XIENCE DES | DES | 1: Death or MI between 1 and 6 months | 1: 3.5% vs. 4.2%, p |
| 2: BARC 2,3,5 bleeding between 1 and 6 months | 2: 4.9% vs. 5.9%, p = 0.19 | ||||||
| XIENCE 90 [24] | 1693 | HBR patients undergoing PCI | 3 months | XIENCE DES | DES | 1: Death or MI between 3 and 12 months | 1: 5.4% vs. 5.4%, p |
| 2: BARC 2,3,5 bleeding between 3 and 12 months | 2: 5.1% vs. 7.0%, p = 0.0687 | ||||||
| 3. Stent thrombosis between 3 and 12 months | 3: 0.2%, p | ||||||
| ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; BMS, bare metal stent; CAD, coronary artery disease; CI, confidence interval; DAPT, dual anti-platelet therapy; DCS, drug coated stent; DES, drug-eluting stent; HBR, high bleeding risk; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; N, Number of patients; PCI, percutaneous coronary intervention; RR, relative risk; TLR, target lesion revascularization; TVMI, target vessel myocardial infarction. | |||||||
More recently, the EVOLVE Short DAPT registry enrolled n = 1437 HBR patients
treated with the Synergy stent followed by 3-month DAPT [25]. Such short DAPT
duration was found to be non-inferior to a historical cohort of patients treated
with 12-month DAPT with respect to death or MI but failed to show and advantage
in terms of bleeding. Notably, the study was non-randomized and the control group
was not uniform as it included multiple different stent types, potentially
limiting the generalizability of the results. The XIENCE Short DAPT program
included 3 registries (XIENCE 28 Global and 28 USA, and XIENCE 90) for a total of
n = 3652 HBR patients undergoing PCI with the fluoropolymer-based cobalt-chromium
Everolimus-eluting Xience stent who discontinued DAPT at 1 or 3 months post-PCI
if event-free and treatment-adherent [27]. Both short DAPT regimens (1 and 3
months) were non-inferior to standard DAPT (6 to 12 months) with respect to death
or MI and superior with respect to major bleeding, after propensity
score-stratification vs. an historical group of patients receiving the same stent
[24]. In a subsequent exploratory analysis from the XIENCE data, 1 month of DAPT
was shown to have comparable ischemic outcomes and lower bleeding risk compared
with 3-month DAPT [28]. MASTER DAPT was the first RCT testing different DAPT
durations in HBR patients treated with a new-generation DES. The trial included
4434 HBR patients who underwent placement of the biodegrable-polymer
sirolimus-eluting Ultimaster stent [29]. Subjects who were event free after 1
month of index PCI were either randomized to DAPT discontinuation followed by
either aspirin or a P2Y
Aspirin has been the mainstay therapy for long-term secondary prevention of
ischemic events for decades. Recently, its undisputed benefits have been
challenged for several reasons: (1) increased risk of intracranial and
extracranial bleeding, especially in HBR patients, (2) widespread use of optimal
medical therapy including disease-modifying drugs (i.e., angiotensin converting
enzyme-inhibitors, angiotensin receptor blockers, statins, etc.), and (3) the
introduction of more potent antiplatelet agents. However, the introduction of new
antiplatelet agents in PCI practice has always requested these new agents to
prove their benefits on a background of aspirin therapy such that their
individual effects have never been truly assessed. The PLATO trial showed that
the more potent P2Y
The TWILIGHT study examined ticagrelor monotherapy following 3-month DAPT in
high-risk patients undergoing PCI. Patients were considered at high risk for
ischemic and bleeding events if they fulfilled at least one clinical and one
angiographic high-risk feature. This double-blinded placebo-controlled study
randomized patients to receive either ticagrelor monotherapy or ticagrelor plus
aspirin for 12 months after being event free for 3 months post PCI. Ticagrelor
monotherapy was shown to reduce the incidence of the primary endpoint of BARC 2,
3, or 5 bleeding without an increase in ischemic events [41]. A sub-analysis of
the TWILIGHT trial looking at patients who qualify as HBR based on ARC-HBR
criteria showed consistent results, with larger absolute risk reduction in major
bleeding observed with ticagrelor monotherapy in HBR versus non-HBR patients
[42]. Several meta-analyses of the above studies showed decreased risks of
bleeding, while no concomitant increase in events [43, 44, 45]. Although many of
these studies do not specifically examine HBR patients, a short DAPT duration
followed by P2Y
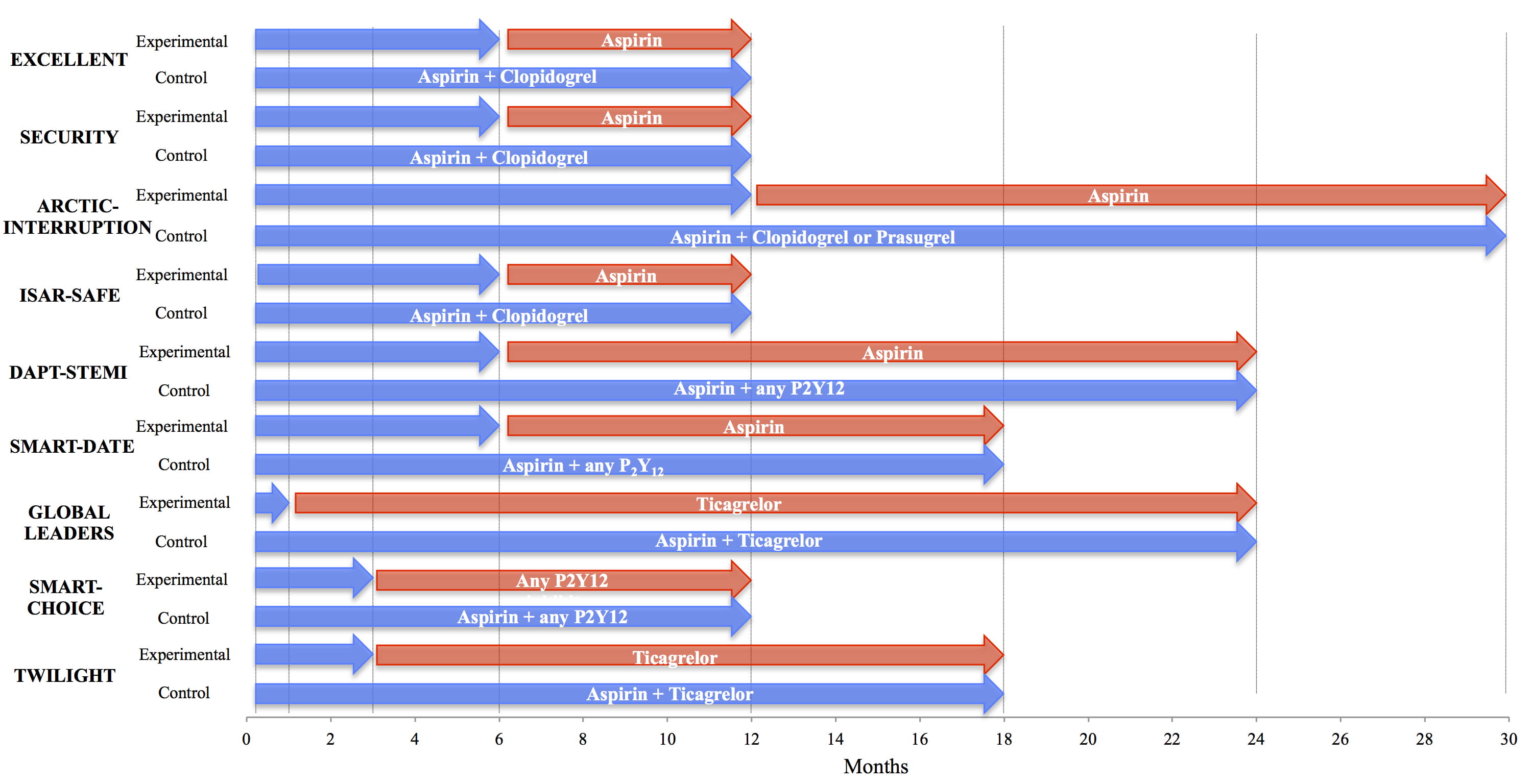 Fig. 2.
Fig. 2.Trial designs of shortening DAPT after PCI regardless of stent design. Comparison of studies of short DAPT duration regardless of specific stent designs [34, 37, 41, 46, 47, 48, 49, 50, 51]. Blue arrows indicate duration of DAPT, red arrows indicate duration of monotherapy. DAPT, dual antiplatelet therapy.
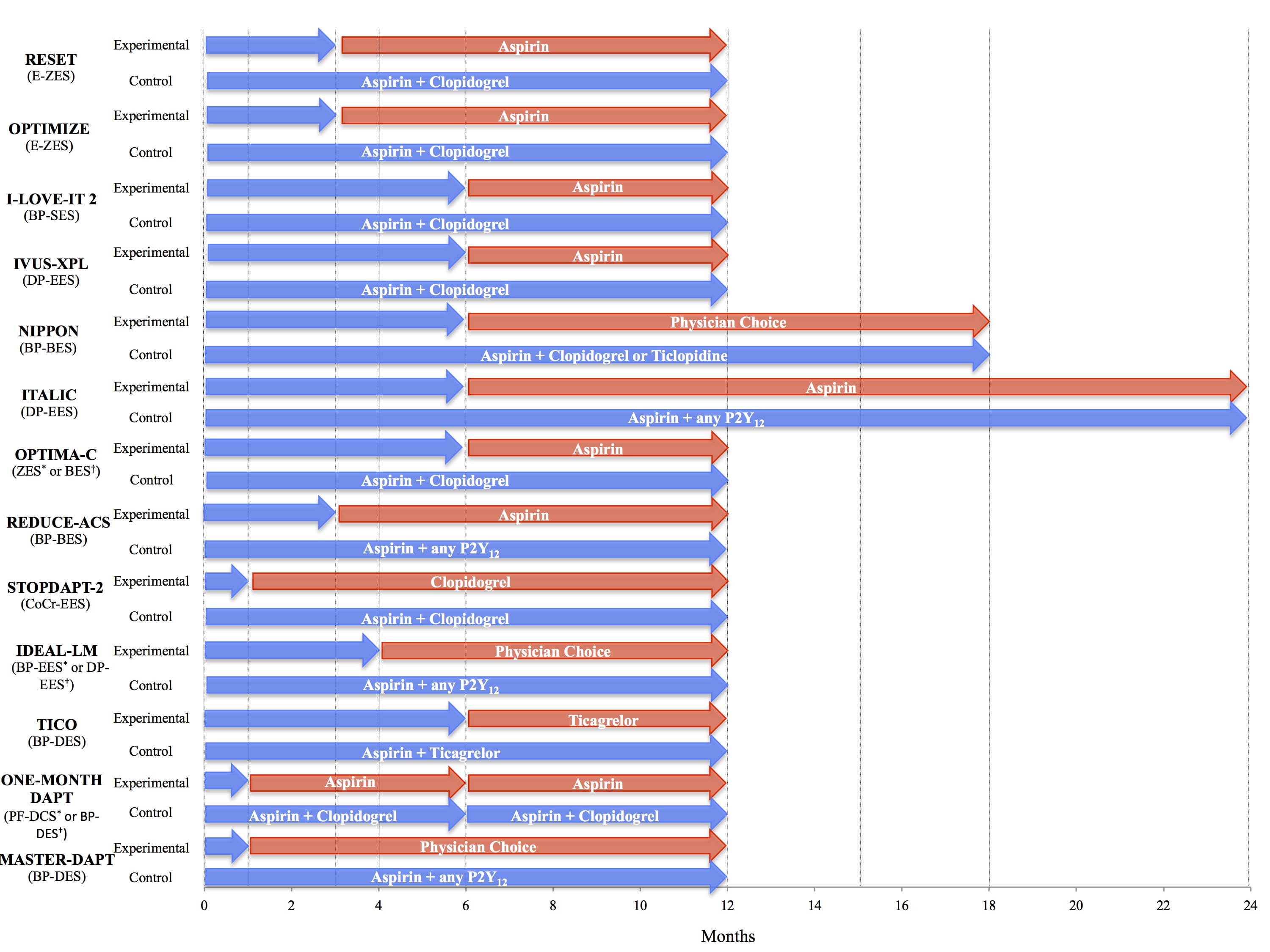 Fig. 3.
Fig. 3.Short DAPT trial with specific stent designs. Comparison of studies with short DAPT designs with specific stent designs [29, 38, 40, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61]. Blue arrows indicate duration of DAPT, red arrows indicate duration of monotherapy. BES, biolimus eluting stent; BP-BES, bioresorbable polymer biolimus-eluting stent; BP-DES, bioresorbable polymer drug-eluting stent; BP-EES, bioresorbable polymer everolimus-eluting stent; BP-SES, bioresorbable-polymer sirolimus-eluting stent; CoCr-EES, cobalt chromium everolimus-eluting stent; DAPT, dual antiplatelet therapy; DP-EES, durable polymer everolimus-eluting stent; E-ZES, endeavor zotarolimus-eluting stent; PF-DCS, polymer free drug coated stent; ZES, zotarolimus-eluting stent. * indicates experimental group. † indicates control group.
| Trial | N | Population | Major inclusion and exclusion criteria | Intervention | Control | Primary outcome | Result |
| GLOBAL LEADERS [34] | 15968 | Stable CAD or ACS with biolimus A9-eluting stent | Inclusion: 50% of more stenosis in |
ASA + ticagrelor for 1 month followed by 23 months ticagrelor monotherapy | DAPT for 12 months followed by ASA monotherapy | Composite of all-cause mortality or non-fatal new Q-wave MI at 2 years | 3.81% vs. 4.37%, RR 0.87, 95% CI 0.75–1.01, p = 0.073 |
| Exclusion: Chronic oral anti-coagulation | |||||||
| STOPDAPT-2 [38] | 3045 | PCI | Inclusion: PCI with CoCr-EES stent without complications post PCI | 1 month DAPT followed by clopidogrel monotherapy | DAPT | Composite of CV death, MI, stroke, stent thrombosis, major or minor bleeding at 1 year | 2.36% vs. 3.70%, HR 0.64, 95% CI 0.42–0.98, p |
| Exclusion: Need for oral anticoagulation, history of intracranial bleeding | |||||||
| SMART-CHOICE [37] | 2993 | PCI with DES placement | Inclusion: 50% or more stenosis in |
DAPT for 3 months followed by monotherapy | DAPT | Composite of death, MI, stroke at 1 year | 2.9% vs. 2.5%, one-sided 95% CI -∞-1.3%, p = 0.007 non-inferiority |
| Exclusion: Cardiogenic shock, active bleeding | |||||||
| TWILIGHT [41] | 7119 | High risk for bleeding or ischemia undergoing PCI | Inclusion: 1 clinical feature and one angiographic feature with high risk ischemia or bleeding events | 3 months DAPT followed by monotherapy | DAPT | 1: BARC 2,3 or 5 bleeding at 1 year | 1: 4.0% vs. 7.1%, HR 0.56, 95% CI 0.45–0.68, p |
| Exclusion: STEMI, cardiogenic shock, oral anticoagulation | 2: Composite of death, MI, stroke | 2: 3.9% vs. 3.9%, HR 0.99, 95% CI 0.78–1.25, p | |||||
| TICO [40] | 3056 | ACS requiring PCI | Inclusion: PCI with Orsiro stent for ACS | Ticagrelor monotherapy after 3 months DAPT | DAPT | Composite of major bleeding, death, MI, stent thrombosis, stroke, or TVR at 1 year | 3.9% vs. 5.9%, HR 0.66, 95% CI 0.48–0.92, p = 0.01 |
| Exclusion: prior hemorrhagic stroke, internal bleeding in last 6 weeks, hemoglobin |
|||||||
| MASTER-DAPT [29] | 4434 | HBR receiving TANSEI DES | Inclusion: |
1 month DAPT followed by ASA or P2Y |
DAPT | 1: NACE | 1: 7.5% vs. 7.7%, AD –0.23%, 95% CI –1.8–1.33, p |
| Exclusion: Treatment for ISR, BARC |
2: MACCE | 2: 6.1% vs. 5.9%, AD 0.11%, 95% CI –1.29–1.51, p = 0.001 non-inferiority | |||||
| 3: MCB at 12 months | 3: 6.4% vs. 9.2%, AD –2.78%, 95% CI –4.37 to –1.20, p | ||||||
| ACS, acute coronary syndrome; AD, absolute difference; ASA, aspirin; BARC, Bleeding Academic Research Consortium; BMS, bare metal stent; CAD, coronary artery disease; CoCr-EES; cobalt chromium everolimus-eluting stent; CI, confidence interval; CV, cardiovascular; DAPT, dual anti-platelet therapy; DES, drug-eluting stent; HBR, high bleeding risk; HR, hazard ratio; ISR, in-stent restenosis; MACCE, major adverse cardiac and cerebrovascular events; MCB, major or clinically relevant bleeding; MI, myocardial infarction; N, Number of patients; NACE, net adverse clinical events; PCI, percutaneous coronary intervention; RR, relative risk; TVR, target vessel revascularization. | |||||||
In patients presenting with ACS, guidelines recommend DAPT with a potent
P2Y
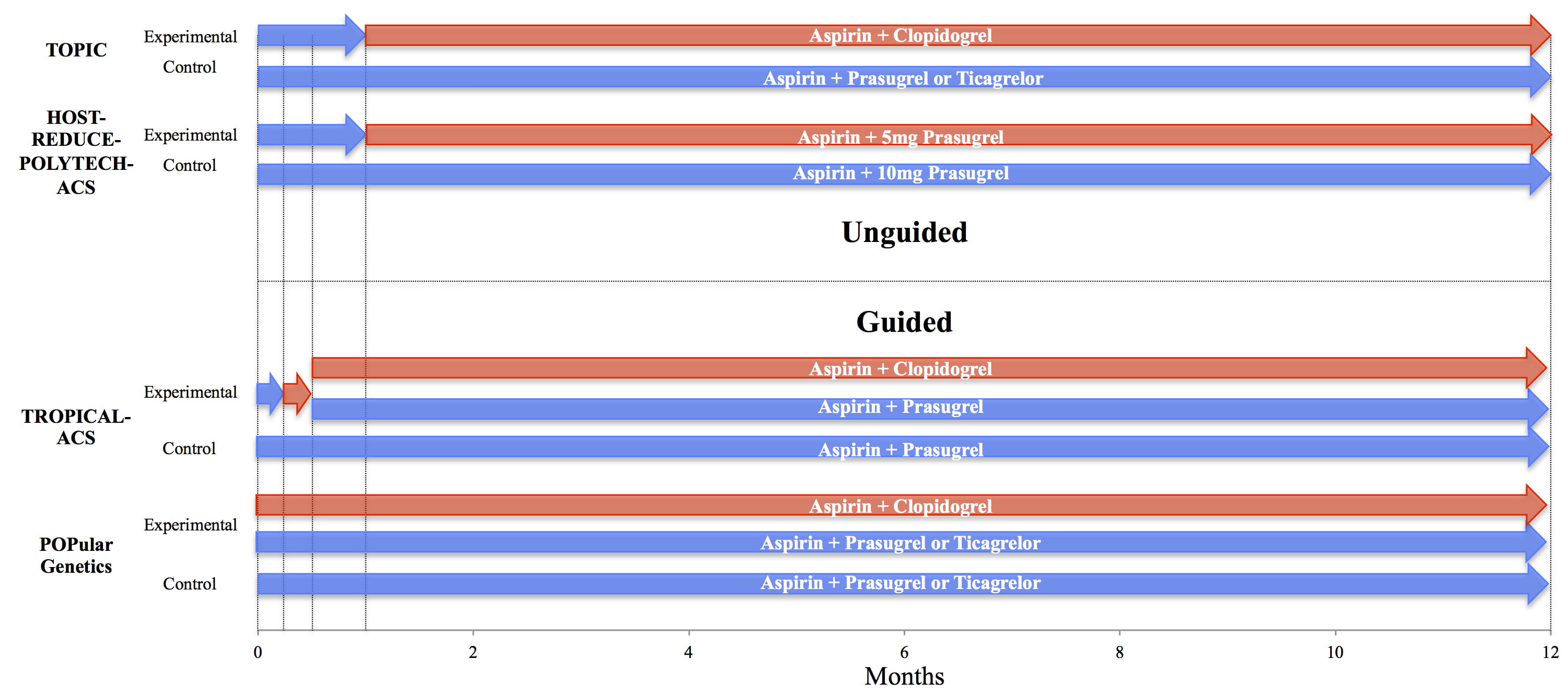 Fig. 4.
Fig. 4.Guided and Unguided de-escalation trials. Comparison of trial designs of guided and unguided de-escalation strategies. Blue arrows indicate duration of potent therapies, red arrows indicate duration of less potent therapies (i.e., lower doses of prasugrel or ticagrelor vs. changing from prasugrel or ticagrelor to clopidogrel) [64, 65, 66, 67].
De-escalation of antiplatelet therapy can be guided by platelet function testing
(PFT) or genetic testing. Although multiple modalities have been developed to
determine platelet function, they all serve the purpose to determine how well
platelets work to stop bleeding. They determine the residual ability of platelets
to aggregate after doses of antiplatelet drug therapy Patients on P2Y
The variable platelets reactivity to clopidogrel may lead to suboptimal
antithrombotic protection [72]. Loss of function alleles, specifically the
CYP2C19*2 and CYP2C19*3 alleles, have been identified as
genetic causes for the decreased response to clopidogrel and its decreased
efficacy [73, 74]. In those without this loss of function allele, clopidogrel was
found to be equally effective as more potent P2Y
| Trial | N | Population | Major inclusion and exclusion criteria | Intervention | Control | Primary outcome | Result |
| TOPIC [64] | 646 | ACS requiring PCI | Inclusion: ACS, no adverse events at 1 month after ACS | Switch to ASA + clopidogrel if 1 month event free after index PCI | Previous DAPT | Composite of cardiovascular death, urgent revascularization, stroke, and BARC |
13.4% vs. 26.3%, HR 0.48, 95% CI 0.34–0.68, p |
| Exclusion: History of intracranial bleeding, thrombocytopenia, long term anticoagulation | |||||||
| TROPICAL-ACS [66] | 2610 | ACS requiring PCI and 12 months DAPT | Inclusion: ACS, planned treatment of prasugrel 12 months after PCI | Stepdown with 1 week prasugrel followed by 1 week clopidogrel and PFT guided maintenance therapy | DAPT with ASA + Prasugrel | Non-inferiority. Composite of cardiovascular death, myocardial infarction, stroke, or BARC |
7% vs. 9%, HR 0.81, 95% CI 0.62–1.06, p = 0.004 non-inferiority |
| Exclusion: Cardiogenic shock in last 2 weeks, oral anticoagulation, indication for surgery | |||||||
| POPular-Genetics [67] | 2488 | Primary PCI with stent | Inclusion: Treated MI within 12 hours of symptoms | Genetic testing determining P2Y12 inhibitor therapy | DAPT | 1: NACE | 1: 5.1% vs. 5.9%, AD: –0.7 percentage points, 95% CI –2.0–0.7, p |
| Exclusion: Malignancy with increase in bleeding, dialysis, severe HTN, cardiogenic shock | 2: major or minor PLATO bleeding | style="border-bottom:1px solid #000;"2: 9.8% vs. 12.5%, HR 0.78, 95% CI 0.61–0.98, p = 0.04 | |||||
| TAILOR-PCI [80] | 1849 | Carriers of CYP2C19 undergoing PCI for ACS or stable CAD | Inclusion: ACS or stable CAD | Ticagrelor DAPT in carriers | Clopidogrel DAPT in non-carriers | Composite of CV death, MI, stroke, stent thrombosis, severe recurrent ischemia at 1 year | 4.0% vs. 5.9%, HR 0.66, 95% CI 0.43–1.02, p = 0.06 |
| Exclusion: Known CYP2C19, Cr |
|||||||
| HOST-REDUCE-POLYTECH ACS [65] | 2338 | ACS requiring PCI | Inclusion: Clinical ACS of |
low dose prasugrel + ASA after 1 month DAPT | DAPT | Composite of death, MI, stent thrombosis, repeat revascularization, stroke, BARC |
7.2% vs. 10.1%, HR 0.70, 95% CI 0.52–0.92, p |
| Exclusion: major or active bleeding | |||||||
| ACS, acute coronary syndrome; AD, absolute difference; ASA, aspirin; BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; CI, confidence interval; Cr; creatinine; CV, cardiovascular; DAPT, dual anti-platelet therapy; HR, hazard ratio; HTN, hypertension; MI, myocardial infarction; N, Number of patients; NACE, net adverse clinical events; PCI, percutaneous coronary intervention; PLATO, PLATelet inhibition and patient Outcomes. | |||||||
Coronary artery bypass graft (CABG) surgery is usually the mainstay of therapy
among patients with left main (LM) disease. However, with recent advances in
intravascular imaging and coronary stent technologies, PCI has become a safe and
viable alternative to surgical management [82, 83, 84]. Stenting of LM lesions are
connected with an increased incidence of ischemic events and thus a prolonged
DAPT duration is usually required [85, 86]. Nonetheless, a significant proportion
of patients undergoing PCI for LM disease are at HBR [87]. Data on optimal DAPT
in HBR patients undergoing PCI for LM disease remains sparse. A complex PCI
sub-study of TWILIGHT included patients with left main disease, as well as
bifurcation lesions treated with two stents, 3 vessels treated, 3 or more lesions treated,
total stent length of
Additionally, DAPT in PCI for CTO remains limited, especially in the HBR
population. However, a study looking at over 1000 patients undergoing PCI for CTO
showed increased rates of death and MI among short (
With increasing emphasis on the prognostic relevance of bleeding events after PCI, the need for studies designed to examine various antiplatelet strategies in HBR patients is paramount. Over the past few years, more studies have investigated antithrombotic therapy in HBR patients undergoing PCI. Although a lot of knowledge has been generated, there remain many unanswered questions. Indeed, the optimal duration and intensity of DAPT after PCI in HBR patients is yet to be defined. Shorter or longer DAPT durations might be beneficial in specific HBR subgroups, depending on the risk-benefit tradeoff of individual patients. In order to allow for direct comparison among RCTs, studies should adopt similar criteria to define HBR, used standardized endpoints for bleeding (BARC type 3 or 5) and ischemic events, and evaluate these two outcomes separately, whenever possible [8].
Significant work has been done with regards to the efficacy and safety of
different P2Y
However, it is not understood which P2Y
Ongoing studies are investigating short DAPT regimens with new stent technologies. TARGET SAFE will assess 1 month versus 6 months of DAPT among HBR patients receiving the Firehawk sirolimus eluting stent (NCT03287167) and BIOFLOW-DAPT will assess shorter DAPT among patients receiving either the new Orsiro stent compared to the Resolute Onyx stent (NCT04137510).
Remarkable advances in PCI technologies and techniques over the last decade have enabled more high-risk patients than ever before to undergo PCI. In particular, HBR patients constitute a third of those undergoing PCI and their management remains challenging periprocedurally and in the long-term. Due to an overlap between ischemic and bleeding risk factors in HBR patients, meticulous choice of antithrombotic therapy intensity and duration is imperative. Several bleeding avoidance strategies have recently been developed and tested in clinical trials, though only few enrolled HBR patients. Further large and well-powered studies dedicated to HBR patients are needed to establish the optimal management strategy in these vulnerable patients.
Conceptualization—DJ, JN, and FB; Methodology—DJ, JN, and FB; Writing—original draft preparation—DJ, JN and FB; Writing—review and editing—DJ, JN, FB, MS, DF, DC, AS, and RM; Visualization—DJ, JN and FB; Supervision—RM. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.




