†These authors contributed equally.
Academic Editor: Konstantinos P. Letsas
Cardiac simulation has moved from early life-saving pacemakers meant only to prevent asystole to current devices capable of physiologic stimulation for the treatment of heart rhythm and heart failure, that are also intended for remote patient and disease-progression monitoring. The actual vision of contemporary pacing aims to correct the electrophysiologic roots of mechanical inefficiency regardless of underlying structural heart diseases. The awareness of the residual cardiac dyssynchrony related to customary cardiac pacing has changed the concept of what truly represents “physiologic pacing”. On a different perspective, leadless stimulation to abolish CIED surgery and prevent lead-related complications is becoming a priority both for young device recipients and for frail, elderly patients. Careful clinical evaluation attempts to bridge decision-making to patient-tailored therapy.
The aim to restore a normal cardiac physiology in patients with conduction system disease is still ongoing after 60 years of cardiac pacing, in a quest of truly physiologic stimulation that stems from suboptimal outcome, namely occurrence of symptomatic LV dysfunction/heart failure, in 12–20% patients despite maintenance of atrio-ventricular (AV) synchrony [1].
An ideal model of stimulation is based on restoration of the physiologic cardiac activation: interatrial, atrioventricular, interventricular and intraventricular. The goal is to achieve the optimal ventricular preload by enabling normal interatrial and AV coupling, that portends a normal stroke volume once physiologic ventricular activation ensues. Cardiac stimulation should aim to correct the electrical delays leading to uncoordinated contraction of cardiac chambers and to untimely opening/closure of AV valves [2].
Meanwhile, awareness of complications related to repeated surgery and to long-term lead survival has promoted the development of leadless pacing systems to minimize acute and long-term complications, beyond providing a solution for patients without superior vein access to the heart.
Achievement of the most physiological stimulation and of complications/hardware minimization may represent divergent priorities at the individual-patient level, given the technology actually available. This review focuses on their difference, to highlight their specific value in clinical practice.
Right ventricular pacing (RVP) has been the conventional ventricular pacing
modality in patients with AV block (AVB) for several decades; however, it is
recognized that it may cause left ventricular systolic dysfunction and heart
failure symptoms in up to 15% of patients over the long term [1, 2]. This
peculiar clinical situation is termed right ventricular pacing-induced
cardiomyopathy (RVPIC), is inversely related to pre-implantation left ventricular
(LV) function, to RV pacing
His Bundle Pacing (HBP) was firstly brought into clinical practice, by pacing the His bundle either at the atrial or at the ventricular aspect of the tricuspid valve. His bundle excitation may occur directly without depolarization of myocardial fibers (selective HBP), or entwined with capture of local myocardial fibers (non-selective HBP) when the His bundle has a deep septal location, thus being surrounded by ventricular myocardium. From an electrocardiographic viewpoint, selective HBP is observed when a pacing artifact is followed by an isoelectric interval (corresponding to the HV interval) before the QRS onset, which is similar to the intrinsic conduction (Fig. 1), correction of a bundle branch block (BBB) being also observed (Figs. 2,3) [10]. Non-selective HBP stimulation occurs when a “pseudo-delta” wave due to capture of local myocardium is observed (Fig. 3C). The pseudo-delta wave may differ in amplitude and duration, depending on the mass of myocardial fibers in proximity of the lead and on the H-V conduction time. No significant mechanical difference has been reported between selective and non-selective HBP [11], though a possible relationship with the extent of pre-excitation by the “pseudo-delta” wave was not investigated. The selective or non-selective type of HBP stimulation can make the ECG interpretation particularly challenging, especially in the event of a coexistent fascicular/bundle branch block and of complete/incomplete correction of bundle branch block by HBP. This task can be accomplished by 12-ECG recording during pacing output manipulation, as thoroughly described in the excellent review by Burri et al. [10].
 Fig. 1.
Fig. 1.Selective His Bundle Pacing (HBP) in a patient with 2nd degree atrio-ventricular block. Please note that the paced QRS is identical to the intrinsically conducted upon temporary pacing inhibition, and that the pacing artifact is followed by an isoelectric line before QRS onset, equaling a normal H-V interval (50 ms in V3).
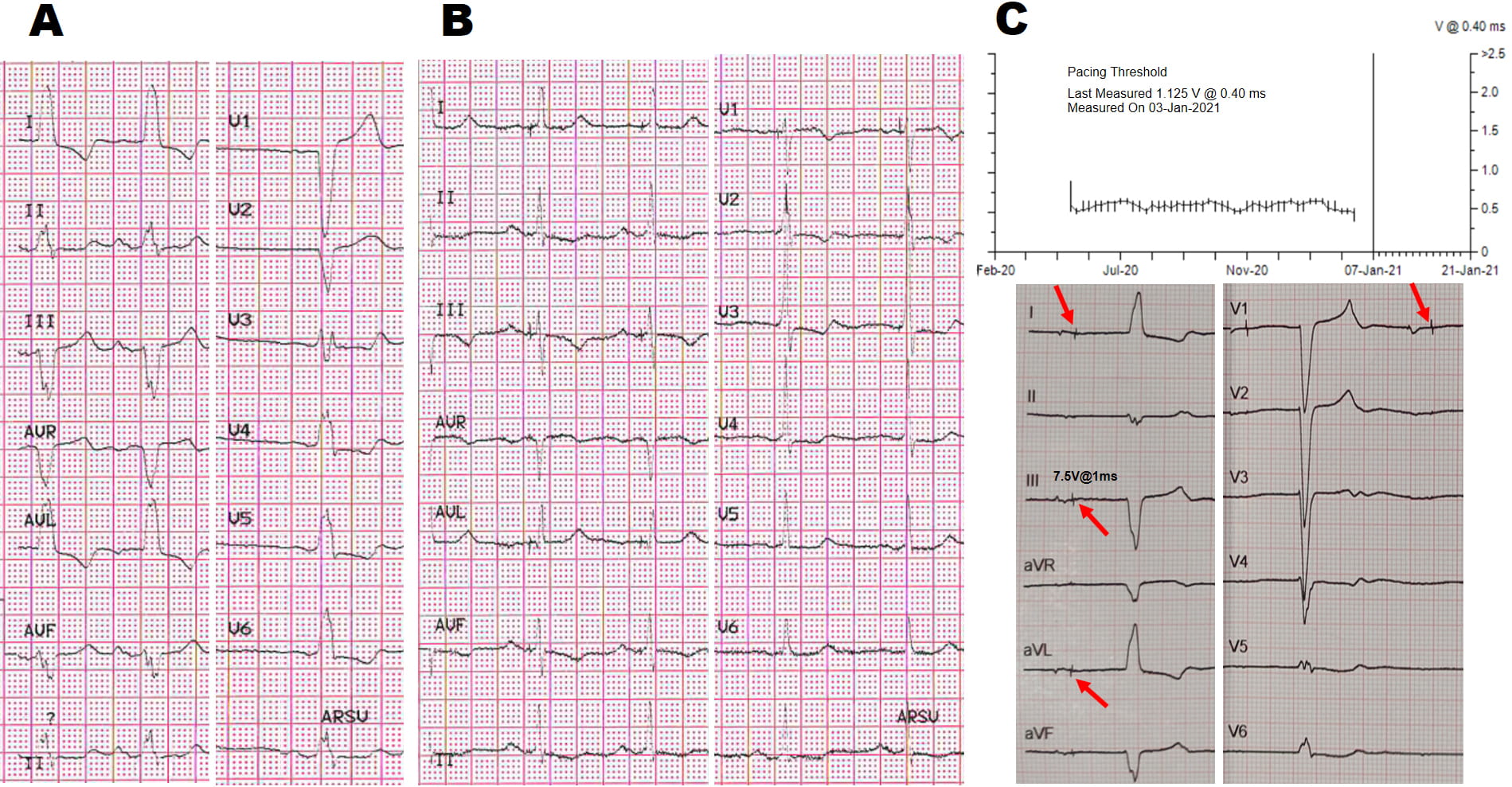 Fig. 2.
Fig. 2.Selective His Bundle Pacing (HBP) with LBBB correction. (A,B) 83 years old lady with recurrent syncopal sinoatrial block and systolic dysfunction. (C) At 12 months follow-up complete infra-His block developed, despite a normal His threshold behavior until January 2021: all of a sudden, threshold measurements failed (upper panel C) and the patient developed shortness of breath. Note the new-onset complete AVB distal to the His pacing site (panel C), with an escape rhythm different from the baseline LBBB pattern observed at baseline when she had normal 1:1 atrio-ventricular conduction (panel A): drop of the R wave in Lead II, aVF, V3–V5 (lower panel C). This case highlights the risk of truly selective HBP in the event of conduction disease progression distally to the pacing site. Red arrows point to pacing artifacts not followed by His-to-ventricular (H-V) conduction despite programming the maximum output.
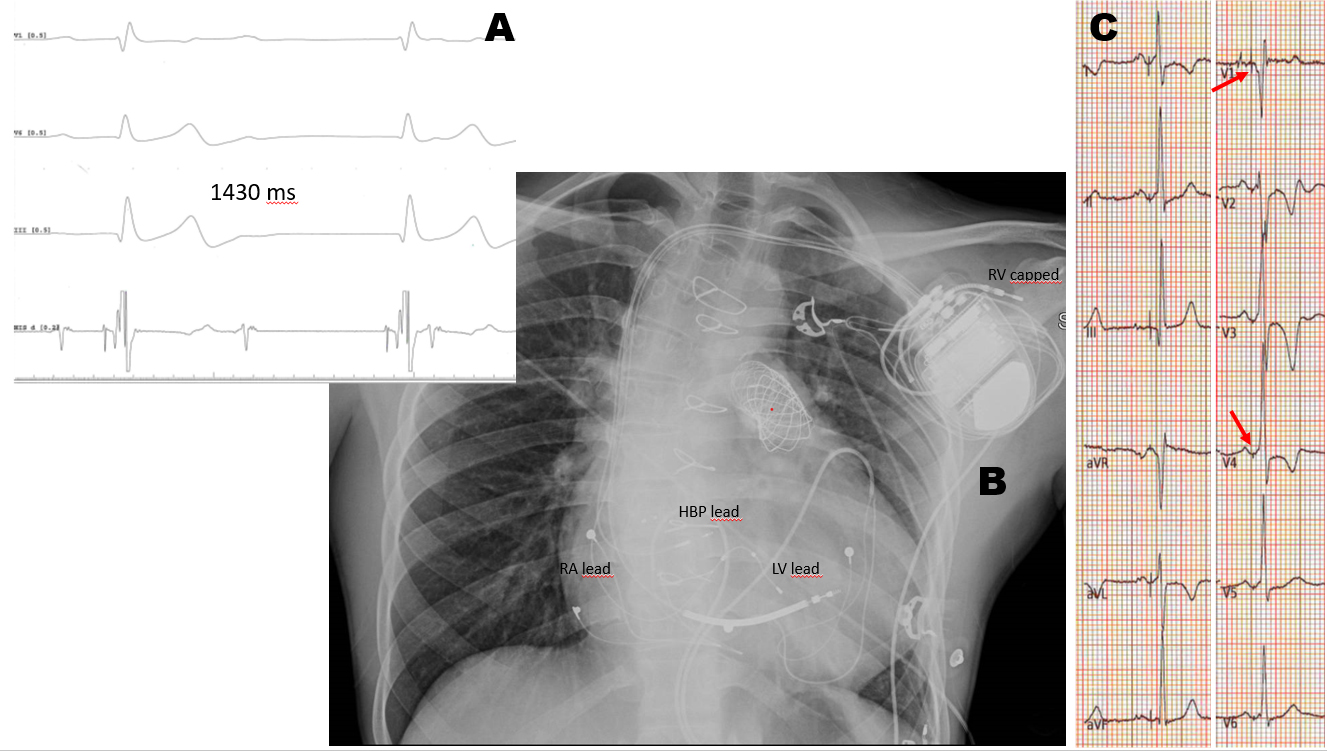 Fig. 3.
Fig. 3.Non-Selective His Bundle Pacing (HBP) with RBBB correction. (A) Young NYHA 3 heart failure male previously operated on, with severe left ventricular dysfunction, complete atrio-ventricular block and RBBB. (B) Sensing occurs via an active fixation LV lead plugged into the RV port, while pacing occurs via the HBP lead plugged in the LV port. The existing epicardial lead was tunneled to the pocket for atrial sensing, while the dipole of the ICD lead was capped (panel B). (C) Red arrows point to the pseudo-delta wave indicating non-selective HBP with RBBB correction.
A recent meta-analysis of single-center studies has shown that HBP is feasible
with acceptable and stable pacing thresholds (average threshold 1.7 V [95% CI
1.42–2.01 V], most often reported at a pulse width of 0.5 ms–1 ms) at
follow-up, and a low rate of complications in clinical practice [12]. The
clinical outcome of HBP has never been addressed in a randomized controlled
trial, however its effect seems favorable in many clinical settings: reversal of
RVPIC after 6 years of RV pacing by HBP was observed in a multi-center experience, improved ventricular function being associated with shortened QRS
duration [13]. In a direct comparison with RVP, HBP showed better clinical
outcomes: the combined primary endpoint (death, HF hospitalizations and upgrade
to CRT) significantly improved (25% during HBP vs 32% during RVA, p =
0.02, 25% vs 36% in patients with VP
A significant improvement of LV function by HBP occurred in several studies, especially in HF patients (Table 1, Ref [13, 14, 16, 17, 18, 19, 20, 21]), thus being the preferred pacing strategy in patients with systolic dysfunction and a narrow QRS. Though a significantly higher rate of lead revisions than with RV pacing burdens HBP (7% vs 3% at mid-term), the improvement of tools and device technology is expected to lessen the complication rate in the future. Beyond bradycardia pacing, HBP has gained acceptance in lieu of BIV when CRT is indicated [14, 15, 16]. Indeed, correction of LBBB by HBP has proved feasible and effective at long term, with similar clinical effects in the single parallel multicenter comparison of HBP and BIV pacing for CRT [16]. Owing to its relevant cross-over rate, HBP should be considered in case of failure to achieve CRT by LV on BIV pacing for any reason. HBP may offer a specific advantage in right BBB patients with systolic dysfunction and HF symptoms, where BVP has less robust evidence than in LBBB patients (Fig. 3): preliminary results in this subset of HF patients [22] are very promising, and hint at a possibility of clinical improvement in a population who would otherwise remain undertreated.
| Main clinical trials with CSP (n° of patients) | Comparison | Mortality | HF events | AF | Main findings |
| Abdelrahman et al. [14] 2018 (765) | HBP vs conventional Ventricular Pacing (mixed population) | = | NA | HBP feasible and safe. | |
| Vijayaraman et al. [13] 2018 (192) | HBP vs conventional Ventricular Pacing (mixed population) | = | = | HBP feasible and safe. | |
| Upadhyay et al. [16] 2019 (41) | His-CRT vs BIV CRT | = | = | NA | Crossover rate greater with His-CRT |
| Trend to greater Echocardiographic response with His-CRT | |||||
| Su et al. [17] 2020 (94) | HBP vs conventional Ventricular pacing (Ablate and Pace in chronic AF with HF, narrow QRS) | = | NA | EF and NYHA improvement | |
| 4% HBP lead revision | |||||
| Wu et al. [18] 2020 (137) | HBP or LBBAP vs BVP (EF |
= | = | = | Superior EF improvement with CSP |
| Su et al. [19] 2021 (632) | LBBAP observational (mixed population, 15% CRT indication) | NA | NA | NA | 1% loss of CSP, 8% permanent RBB injury |
| Wang et al. [20] 2020 (131) | LBBAP vs RV pacing (mixed bradycardia indications) | NA | NA | NA | Narrower paced QRS |
| Vijayaraman et al. [21] 2021 (325) | LBBAP retrospective observational (CRT candidates) | NA | NA | NA | ECHO responders 72%, super-responders 31%; No HF events in 73% |
| Better outcome in NICM and in LBBB patients | |||||
| Legend: AF, atrial fibrillation; BVP, biventricular pacing; CRT, cardiac resynchronization therapy; CSP, conduction system pacing; ECHO, echocardiography; HBP, His Bundle Pacing; HF, heart failure; LBBAP, left bundle branch area pacing; LBBB, left bundle branch block; NICM, non-ischemic cardiomyopathy; MVP, minimization of ventricular pacing; RBB, right bundle branch. | |||||
HBP is currently acknowledged indication for the ablate-and-pace strategy for AF patients (IIa in the recent ESC guidelines [23]), based on 7 observational studies gathering about 310 patients which showed a favorable clinical outcome (Table 1), though the risk of pacing threshold increase and lead complications seems somewhat higher in this setting [19]. The use of a ventricular back up lead may be particularly warranted in this clinical scenario, and while it is technically simple in permanent AF/AT patients by the use of a dual chamber pacemaker, a biventricular device is needed when an atrial lead is to be implanted. From the technical viewpoint, the delivery of CRT may be particularly challenging when His-optimized CRT is achieved by HBP+LV stimulation, since sensing the RV signal for rhythm detection and classification may be inadequate by the HBP lead. In CRTP, this can be obviated by devices that can use the LV channel for ventricular sensing instead of the RV channel, while the only practical solution in a CRTD setting is enabled by IS-1 connectors, that ensure superior flexibility (Fig. 3B) [24]. A recent retrospective analysis of 24 patients with moderate systolic LV dysfunction (EF in the range 35–50%) undergoing ablate-and-pace reported a similar effect of BIV or HBP on NYHA class, while a superior improvement of EF and a trend towards decreased LV end-diastolic index was observed for HBP [25]. Despite its inner methodologic limitations, this study sets the premises for a randomized comparison, though some technical aspects need to be a priori defined (selective vs non-selective HBP; back up lead implantation, RV or LV lead as back-up?).
HBP in lieu of conventional RV pacing has been acknowledged a class IIb
indication in patients with EF
As an alternative to BVP, His bundle pacing (HBP) can preserve and restore optimal physiological ventricular synchronization, however, relative high stimulation thresholds, low R-wave amplitude, and the long-term persistence of conduction distally to the pacing site and in the Purkinje network are of concern (Fig. 2).
Huang et al. [26] firstly demonstrated the direct capture of left bundle branch (LBB) by placing the lead deep inside the septum, resulting in synchronized activation of the ventricles. Since then, left bundle branch area pacing (LBBAP) has emerged as an alternative modality to deliver physiological pacing, as it overcomes several limitations of HBP.
LBBAP is based on the following anatomical considerations: unlike the thin His bundle running at variable depth for variable length within the central fibrous body, the LBB fibers lie on the LV septal plane giving a ribbon-like appearance beneath the endocardium of the subaortic septal region. This broader LBB structure may be easier to target and capture then the His bundle, that is on the contrary a smaller discrete target. LBB area stimulation may thus have a lower and more stable pacing threshold, and a normal R-wave sensing amplitude is the usual finding.
LBB area implant technique can be summarized in 3 different steps: (1) locating the target area, (2) septum penetration, and (3) confirmation of LBBAP criteria.
Commonly, the 4.1-F diameter 3830 SelectSecure pacing lead (Medtronic Inc., Minneapolis, MN, USA) is used, but stylet-driven leads like Solia (Biotronik, Berlin, Germany) are also used.
Locating the target area. The target area for LBBAP lead implantation is
located on the upper mid interventricular septum. This region can be identified
using the His bundle as a reference and once localized, advancing the sheath with
the lead 1.5–2 cm towards the RV apex [27]. Once the sheath has been advanced to
the upper mid-septum, perpendicular orientation of the sheath against the septum
is confirmed in LAO 45
Septum penetration. Once this paced QRS pattern is identified, and initial fixation is achieved by 3–5 lead turns, QRS morphology and pacing impedance can then be evaluated. Additional turns may be necessary to penetrate the lead into the septum, maintaining a perpendicular orientation in LAO view [23]. Monitoring the pacing lead impedance is a recommended security measure as an abrupt drop of pacing impedance usually indicates perforation to the LV. Besides, the sudden loss of current of injury (COI) can also be used as a marker of lead protrusion in the LV chamber.
Jastrzębski et al. [28] as well as Ponnusamy et al. [29] have described a new and rather sensitive technique to identify LBB capture and to prevent septal perforation based on the presence of “fixation beats”, defined as ventricular premature complexes typically eliciting while the lead is being screwed in the septum. When these “fixation beats” show the typical LBBAP paced QRS morphology, no additional turns should be applied to the lead, and electrical parameters should be tested.
Lead perforation into the LV cavity can be also recognized by a significant drop
in impedance of
Confirmation of LBBAP criteria. Once the paced QRS morphology changes to a RBB-like appearance in V1, LBBAP criteria must be demonstrated to confirm LBB capture [18]. LBBAP is defined as pacing the area where the proximal left bundle is located, resulting in capture of the LBB together with stimulation of the LV septal myocardium at a variable extent. LBBAP capture can be determined by the presence of an RBBB pattern paced morphology and one or more of the following criteria [30]:
• Visualization of LBB potential and evidence of LBB capture [31];
• Transition from non-selective to selective LBB capture or non-selective to LV septal myocardial capture during threshold testing [18, 30, 32, 33];
• Abrupt shortening of R-wave peak time (RWPT)
• Demonstration of short stim-retrograde His (
• Demonstration of distal conduction system potentials (stim to LBB
potential
• Time from LBB potential to R-wave peak in V6 equals the time from pacing stimulus to R-wave peak in V6 [31];
• In patients with LBBB correctable with HBP, difference (Delta) in
RWPT in V6 during HBP and LBBAP
An incomplete right bundle branch block (RBBB) pattern rather than complete block may appear, and the paced QRS morphology may vary, depending on the capture of the distal His bundle or of the proximal LB, on the coexistence of distal conduction system disease and Purkinje connections. LV septal pacing without capturing the LBB can also produce an RBB-like pattern but with prolonged LV free wall activation [33]. Indeed, a recent study has compared ventricular depolarization during NS-LBBAP, LVSP, and NS-HBP using ultra-high-frequency ECG (UHF-ECG) in 68 patients with either broad or narrow QRS complex. This study showed that NS-HBP causes a more physiological ventricular depolarization than either type of LV stimulation [33]. These two latter have a different effect, in that NS-LBBAP increases interventricular dyssynchrony owing to a longer septal LV-to-RV activation in comparison with LVSP, but provides a faster left ventricular lateral wall activation thanks to depolarization of the posterior fascicle. LVSP seem to provide better interventricular synchrony, and may deserve clinical interest respect to RV pacing [33]. Alternately, in patients with normal His conduction, pacing near the proximal LBB may rapidly conduct to the right bundle and result in normal QRS morphology (Fig. 4) [18].
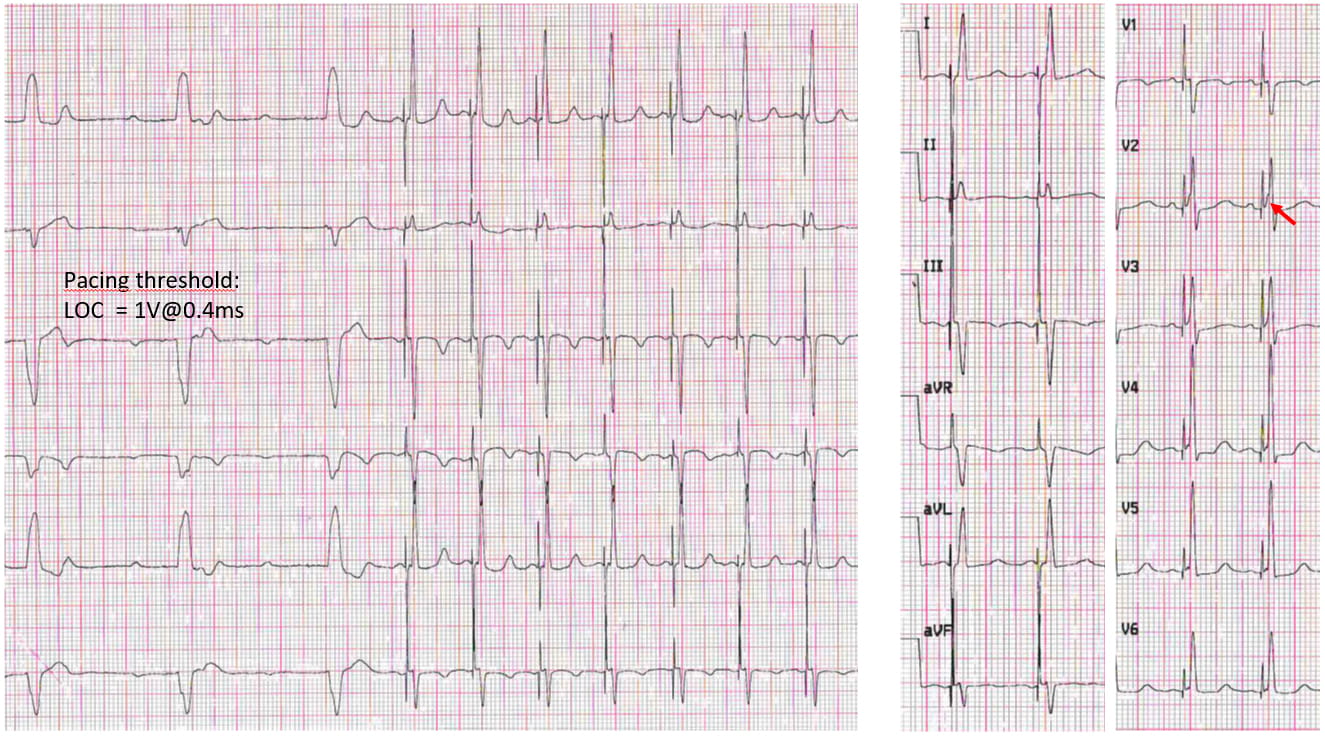 Fig. 4.
Fig. 4.Selective left bundle area pacing (LBBAP) in a patient with complete atrio-ventricular block and LBBB. Please note the nearly normal QRS duration and morphology of the paced QRS, that has only minimal septal capture (pseudo-delta wave, see red arrow), and the pacing threshold around 1.2 V @ 0.4 ms. LOC, loss of capture.
Left ventricular septal pacing (LVSP) occurs when the pacing lead is placed deep
in the left septal sub endocardium but no LBB capture can be demonstrated. A
RBBB-like paced QRS morphology is also usually observed. Typically, RWPT in V6
will be
LVSP resulted in optimal haemodynamic effects when compared with epicardial LV
free wall sites and RV apex/septal pacing, as resulted from previous animals’
studies [34, 35]. Indeed, physiological electrical activation of the working
myocardium begins at the LV septal endocardium and propagates to the epicardium,
during sinus rhythm. Salden et al. [36] compared the acute
electrophysiological and haemodynamic effects of LVSP with BVP and HBP in 27
patients with an indication for CRT. They found that single site LVSP provides
short-term haemodynamic improvement and electrical resynchronization at least as
good as that occurring either during BVP or HBP. Interestingly, the basal, mid,
and apical LVs pacing placements resulted in similar results, indicating that
within the LV septum the position of the pacing electrode is not critical.
However, the mid- and long-term clinical outcome of single-lead LVSP need further
evaluation in future studies. Currently, no definitive conclusions can be drawn
about the clinical implications of LBBAP in comparison with LVSP. Hou et
al. [37] indicate that LBBAP capture may provide superior benefit compared with
myocardial capture, with shorter Stim-LVAT (73.1
Like HBP, LBBAP can also be selective (S-LBBAP) and nonselective (NS-LBBAP). Transition from nonselective to selective LBB capture or nonselective LBB capture to LV septal myocardial only capture can be usually demonstrated during threshold testing or with other pacing methodologies [18, 21, 31, 38].
Selective capture of LBB shows a distinct isoelectric interval preceding the local EGM at low pacing output, its duration corresponding to the interval between LBB potential to surface QRS onset. This can also be demonstrated by change in paced QRS morphology from qR to rSR in lead V1 with fixed peak Left Ventricular Activation Time (pLVAT, measured from the pacing artifact to the R peak in lead V6) during unipolar threshold measurement.
In non-NS selective LBB capture no distinct isoelectric segment and QRS onset are detected, with a pseudo delta wave following the pacing artifact.
In NS-LBBAP to LV septal transition, paced QRS morphology remains the
same in V1 (qR) but the QRS duration broadens with prolongation of pLVAT. On the
contrary, in LVSP to non-selective LBBAP transition, the most obvious
change is abrupt shortening of Stim-LVAT
The paced QRS duration (QRSd) may not be of much diagnostic value as it is affected by myocardial capture, pacing site along the LBB and presence of distal conduction system disease. A paced RBBB pattern is a necessary but not sufficient criterion to prove LBB capture because many patients with LVSP without LBB capture also demonstrate an RBBB pattern. Paced QRSd is also not sufficient to help distinguish LVSP from LBB capture.
Differently to HBP, where engagement of the conduction system can be easily
proven by changing the pacing output, LBB and septal myocardium thresholds are
usually similar, thus limiting this maneuver. Using deep septal stimulation,
Jastrzębski et al. [39] have recently demonstrated that
programmed ventricular stimulation can be used to differentiate LBBAP from LVSP.
They classified the response to programmed stimulation based on the presence of a
sudden change of the QRS morphology during the extrastimulus in “myocardial
response”, “selective LBB response” or nondiagnostic. These responses are
based on the presence of a different effective refractory period of the
myocardium and the LBB. They found that the average septal myocardial refractory
period was shorter than the LBB refractory period: 263.0
The largest series of LBBAP published so far [19] reported a 97.8% implant
success rate using strict criteria for LBBAP among 632 patients with either
bradycardia or CRT indication because of heart failure (Table 1). Although mean
follow up was 18.6
Wang et al. [20] randomized 131 patients with single or dual
chamber pacemaker indication (sinus node disease 32.1%, AV block 54.2%, chronic
atrial fibrillation with bradycardia 13.7%) to receive either LBBAP (n = 65) or
RV septal pacing (n = 66). Successful LBBAP was achieved in 92% of the cases and
was associated with a narrower QRS when compared with RV septal pacing (121.49
LBBAP has also been tested in other scenarios such as bradycardia pacing
indications after transcatheter aortic valve replacement and proved to be
successful in 26/28 patients (93%) in a multicenter observational study. In this
series, LBBAP showed higher success rate (93% vs 63%, p
Another scenario in which the advantages of LBBP appear obvious is the AF/AT ablation-and-pace strategy where stable pacing thresholds, lead stability and patient safety without a back-up lead are of clinical concern [40].
Although HBP theoretically preserves both RV and LV synchrony while LBBAP would only preserve LV synchrony, some studies have shown that LBBAP can achieve comparable LV activation times and synchronicity as HBP, especially when AV interval has been tailored [37, 41]. Moreover, myocardial septal capture and possible retrograde conduction from the LBB to the RBB appear to minimize the theoretical loss of RV synchrony during LBBAP.
Chan et al. [42] found that both LBBAP and HBP show similar and significant improvement in LV activation sequence using the non-invasive global epicardial imaging in a patient with LBBB. This was further verified in Hou’s study, which shows better LV mechanical synchrony than BVP utilizing phase analysis of gated SPECT myocardial perfusion imaging [37].
Wu et al. [43] studied 137 consecutive patients referred for CRT
who received either BVP (n = 54), HBP n = 49), or LBBAP (n = 32). The paced QRS
width significantly improved from baseline in the 3 groups although QRS narrowing
was grater for HBP and LBBAP. LBBAP was associated with better pacing threshold
and sensed R wave when compared with HBP, but no LV-only pacing with fusion with
intrinsic conduction was used in this study. At 1-year follow up, HBP and LBBAP
showed significantly greater improvement of LVEF when compared with BVP and
significantly higher rates of LVEF normalization (74.4%, 70.0%, and 44.9% in
HBP, LBBAP, and BVP groups p
Recently, the International LBBAP Collaborative Study Group result have been
published [47]. This was a retrospective, observational study carried out at 8
institutions from 5 different countries including 325 patients with a CRT
indication in which LBBAP was attempted. LBBAP was successful in 277 patients
(85%). Importantly, the electrical parameters obtained at implant were optimal
and remained stable during a mean follow-up of 6
Owing to the capability of correcting conduction system disease at a more distant site than HBP, and to achieve reliably adequate sensing and pacing parameters, LBBAP seems to enable a possibly easier approach to CSP in all clinical pacing settings. Moreover, it would theoretically minimize lead redundancy by sparing back-up leads, which has a positive effect on thoracic veins patency. Clinically relevant questions are yet answered, namely the clinical effect of selective vs non-selective HBP or LBBAP, in the light of the different electrical activation observed with HBP compared to LBBAP [33]. Though, concerns are also different: a relevant portion of the lead is buried inside the septum with LBBAP and the long-term effect of myocardial contraction on lead welding and insulation is unknown; the risk of injury to coronary artery branches injury and late lead migration in the LV chamber with potential thromboembolic complications are actually unknown, though they seem a rare possibility. Further improvements in delivery sheaths and lead manufacturing are needed to ease the procedure and enable 100% success rate to consistently capture the conduction system.
Leadless stimulation concept dates to the middle seventies, aiming to overcome the risks of device surgery. This aspect is pivotal in frail patients and in young patients who face multiple surgeries in lifetime. Freedom from long-term lead-related issues and cosmetic needs are additional advantages.
The first VVIR leadless systems entered clinical practice in a view to avoid short-term pacemaker complications. Haunting of the guilty led to blaming the leads as the main responsible for major complications, where adequate training and operator experience are an important bias in understanding pacemaker-related complications [48]. The most appropriate indications to leadless pacing are:
• superior access to the heart occluded, or already colonized by indwelling catheters for dialysis/chemotherapy, or to be preserved for future indwelling catheters;
• patients with mechanical heart valves on anticoagulation and prone to bleeding, or frail patients on dual antiplatelet/anticoagulation + antiplatelet;
• previous CIED extraction due to pocket/lead infection;
• patients on hemodialysis or immunocompromised;
• less than 20% Vp needed in patients with borderline ventricular function.
In the recent ESC guidelines [23], Leadless Pacing has gained a class IIa indication for the clinical settings described above at the first 4 points, who represent the subgroups mostly vulnerable to device pocket infection, whereas a class IIb has been recommended as an alternative to single-lead ventricular pacing, based on patients’ life expectancy and clinical status. This is based on the absence of a reliable comparison in an actual population of single-chamber pacemaker recipients according to the best practice to minimize surgical and long-term lead-related complications as per current recommendations [49]. The benefits of leadless pacing, aside from specific clinic settings, are at present unclear.
The first leadless pacing experience enrolled 33 patients with a single chamber RV pacing indication, who received a Nanostim pacer [50]: complication-free rate at 90 days was 94%, (cardiac perforation and tamponade in a single patient, Table 2). A successful implant rate of 96% and a 6.7% rate of major adverse effects occurred at six months in the LEADLESS II trial [51] (1.3% perforation; 1.3% elevated pacing threshold requiring reintervention, 1.2% vascular complications and 1.7% device dislodgement), while stable and acceptable parameters were measured in 90% of the total cohort (Table 2). Regretfully, the Nanostim experience was terminated by significant serious events and a device battery recall, with several indications to pacemaker retrieval. Thirty-four 34 battery failures due to increased battery resistance [52] occurred over 1423 Nanostim units worldwide; device failure was higher than 40% after 3 years [53]. in a series of 14 consecutive patients. Nanostim implantation was stopped in 2016 with an indication to replacement in pacemaker-dependent patients. This challenging situation prompted Nanostim retrieval, which proved successful in nearly 90% of patients by snaring the device docking button [54], which pitifully showed either inaccessibility or detachment of the docking button, or migration to the pulmonary artery [52].
| Leadless pacemaker trials | Leadless | Leadless II | Micra trial (IDE) | Micra post approval registry (PAR) | Leadless II–phase 2 |
| Enrollment period | December 2012–April 2013 | February 2014–June 2015 | December 2013–May 2015 | July 2015–ongoing | November 2020–June 2021 |
| System | Nanostim | Nanostim | Micra | Micra | Aveir |
| Population (n) | 33 | 526 | 725 | 1817 | 200 |
| Follow-up (days) | 90 | 180 | 180 | 210 | 118 |
| Success rate (%) | 97% | 95.8% | 99.2% | 99.1% | 98% |
| Acute major complication rate (%) | 6% | 6.7% | 4% | 2.26% | 3% |
| Main aspects of leadless pacing | Leadless pacing systems | Transvenous pacing systems | |||
| Total short terms complications | 3.4–4.8% | 6.4–12% | |||
| Pericardial effusion | 0.6–1.5% | 0.3–1.2% | |||
| Cardiac perforation | 1.3–1.5% | 0.1–0.8% | |||
| Access site | 0.7–1.2% | 1.2–2.2% | |||
| High pacing threshold | 0.3–1.3% | 0.8–1.6% | |||
Owing to these issues, the newly developed leadless VVIR pacer AveirTM Abbott has substantial technical innovations: a standard transvenous pacemaker battery chemistry (Lithium Carbon-Monofluoride) with 12% longer battery life (1.1 years longer to 10.4 years), altered form factor (10% shorter, 1.5 Fr wider to 19.5 Fr), modified docking button (enabling retrievability), modified delivery system with an ergonomic design, and a new ASIC chip designed to provide an expandable platform (to later support a dual-chamber pacing system once approved). The safety and efficacy have been recently reported on 200 patients (Table 2), highlighting that training to avoid complications remains a key aspect: 3 cardiac tamponades (2 requiring sternotomy), and 3 premature device deployments [55].
The Micra Transcatheter Pacing International Trial [56] demonstrated the efficacy and safety of the Medtronic Micra system with a 3.4% rate of complications at six months in 725 patients (1.5% perforation; 0.7% vascular complications), and successful implantation in 99.2% of patients and adequate six-month pacing threshold (Table 2). A real-life ongoing analysis, the Micra PAR registry [57], reporting 2.2% of major complications seems to confirm the previous data (Table 2). A small study of age and sex-matched transvenous vs leadless VVIR recipients reported no difference in terms of overall complications, but disappointed the major expectations of leadless implantation: procedure time and fluoroscopy time were greater in leadless recipients, endocarditis occurred in transvenous pacemaker recipients only, while pericardial effusions occurred only in leadless recipients [58] (Table 2). Indeed, the PAR registry reported pericardial effusion in 14 patients (0.78%), 8 requiring pericardiocentesis. Subanalysis of the PAR registry provides evidence that Micra implantation under uninterrupted anticoagulation is safe, and that Micra implantation in 105 patients extracted because of pre-existing CIED infection is not burdened by further device infection [59, 60]. In 197 patients on hemodialysis there were no pacemaker infection, although 3 procedure-related deaths were observed (2 perforations). A real comparison of transvenous and leadless VVIR pacemakers in adequately trained centers is currently unavailable though of key importance, being the age of first-time VVIR recipients close to 80 years, which carries several associated co-morbidities.
According to two contemporary registries [61, 62], VVIR pacemakers are indicated in around 10% of pacemaker recipients. As reported by a recent EHRA survey [63] involving 52 high-volume European centers, anticipated difficult vascular access, history of complicated conventional PM implantation, permanent atrial fibrillation and an anticipated higher risk of infections were the features that dictated the choice of a leadless pacemaker. Limited availability and economic issues, such as lack of reimbursement or high cost of the device, hinder the use of leadless pacemakers.
More data are needed to assess the longevity of leadless batteries. According to current studies, expected service-of-life is strongly dependent on the effective pacing settings, being estimated as 10 years for Micra [64]. Moreover, the computational work is decreased and remote monitoring is not available to minimize current drain.
In 2020 both FDA and CE approved Micra AV, a leadless pacing system which aims to synchronize ventricular stimulation with atrial activity, building a VDD software in the same VVIR Micra. This single-chamber VDD detects the mechanical atrial activity by the 3-axis accelerometer, that senses the 4 blood flow accelerations corresponding to the heart sounds, as a surrogate of the electrical p-wave. The fourth signal (A4) of each cycle represents the atrial contraction (the A wave of Doppler trans-mitral flow), thus triggering ventricular stimulation. Diastolic ventricular filling (or E wave) is recorded as the A3 signal.
Feasibility and efficacy of atrioventricular synchrony (AVS) delivered by Micra
AV in patients with high degree AV block have been demonstrated in acute studies
with a downloaded software. In MARVEL2 study 38/40 patients with AVB and intact
conduction system achieved
MARVEL and MARVEL2 studies showed that stroke volume, assessed by
echocardiography, was improved by AVS compared to VVI pacing, thus reinforcing
the superiority of AVS pacing. In MARVEL2, patients had lower sinus rates in VDD
mode than in VVI mode, meaning a decreased sympathetic activity when a more
physiological pacing is achieved. However, studies of long-term benefits of Micra
AV are lacking [66]. Sensing atrial activity via its mechanical counterpart may
pose some issues. Firstly, atrial undersensing is a major concern in several
scenarios: with ineffective atrial contraction, no A4 is detected and AVS is not
achieved (an E/A
 Fig. 5.
Fig. 5.Micra AV functioning in two patients. Patient 1 has undersensing of atrial mechanical activity (AM) for 42.8% of time, and was sometimes symptomatic because of retrograde ventriculo-atrial conduction (red arrows); incomplete racking of AM was obtained after careful reprogramming. Patient 2 was on dialysis via a trans-jugular catheter (red arrow): loss of AM tracking occurs for 14.3% of time only, whereas 27.7% of time recovery of intrinsic conduction is detected.
Oversensing of A3 signal and subsequent pacemaker-induced tachycardia may be prevented by increasing A3 threshold and by the tracking check feature, though this may escape at lower heart rates (Fig. 5, Patient 1).
It is known that right ventricular pacing with or without AV synchrony may promote RVPIC. Micra AV aims to transfer AV coupling in the leadless world, but still provides only non-physiological right ventricular stimulation with unreliable and often non-physiologic AV intervals (Fig. 5, Patient 1). Indeed, the electromechanical delay between electrical P wave and A4 signal may cause long AV intervals or even AV uncoupling, that warn about the incidence of new-onset AF, HF, and mortality. This pitfall is partially mitigated by the intact AV conduction mode switch algorithm, which periodically checks for intrinsic conduction and reduces unnecessary pacing (Fig. 5, Patient 2).
There is currently paucity of data on Micra AV performance in real clinical
practice. In our experience, based on observation of 16 consecutive recipients
because of 2nd–3rd degree AV block (Table 3) the performance in term of AVS
(defined as presence of a P wave in the 300 ms preceding the paced QRS) was
disappointing, with only 62% of patients maintaining atrio-ventricular coupling
for
| Micra AV performance in consecutive recipients | Clinical profile of the population (n = 16) |
| Male sex | 12 (75%) |
| Age (years) | 71.5 |
| AV block persistent | 11 (69%) |
| LV EF | 52 |
| Hypertesion | 13 (81%) |
| Diabetes | 9 (56%) |
| Previous MI | 6 (37%) |
| Previous COI | 6 (37%) |
| Previous valvular surgery | 6 (37%) |
| Current dialysis | 6 (37%) |
| Recent heart transplantation | 2 (12%) |
| Previous CIED extraction | 2 (12%) |
| All-type complications | 0 |
| Follow-up (months) | 7 |
| % VP | 84 |
| Patients with loss of AVS |
10 (62%) |
| AV, atrio-ventricular; AVS, atrio-ventricular synchrony; CIED, cardiac implantable electronic device; COI, coronary artery interventions; EF, ejection fraction; LV, left ventricular; %VP, percentage of ventricular stimulation. | |
Micra AV has offered the opportunity for leadless CRT by coupling with WISE leadless LV stimulation either in sinus rhythm or in AF patients, thus overcoming the barriers to transvenous CRT implementation [69]. Notably, recent experience with transeptal WISE implantation enable also leadless CSP by targeting the left-sided prospect of the LBB.
However, the current technology unveils its inner limitation, making true AVS a
priority for the future of leadless stimulation. The recently presented animal
data on the AveirTM leadless atrial pacemaker make dual chamber leadless
pacing a real possibility in a 2-year horizon. In an ovine model, atrial
AveirTM performance at medium term was optimal, while no perforation,
epicardial/pericardial adhesion, or device dislodgement were observed [70]. In a
leadless dual chamber ovine model, bidirectional communication of atrial and
ventricular units was demonstrated: the i2iTM communication modality uses
energy-efficient subthreshold electrical pulses conducted through blood and
myocardial tissue. Over an average of 8715
The WISE system was developed to enable CRT at the LV endocardial level in the perspective of targeted CRT unrestricted by factors like: difficult access to the heart; inaccessibility of the coronary sinus or of coronary veins; unavailability of coronary veins at the target spot; high LV pacing threshold or delayed LV activation due to scarred epicardial/midventricular layers; phrenic stimulation impossible to manage. The system consists of a transmitter unit placed underneath the superficial intercostal muscle at an ultrasound-unrestricted view in all body posture in the anterior chest wall (Fig. 6A), and of a receiver unit (9.1 mm in length * 2.6 mm in width) deployed at the LV endocardium via the arterial or transeptal route (Fig. 6B–D). The system is currently working synergistically to a co-implanted device: upon detection of a pacing pulse of given programmable width, the transmitter locates the receiver in the ventricle, then delivers an ultrasound train that is converted in an excitatory pulse by the receiver within 4 ms by the co-implanted device delivered pulse (Fig. 6A, Fig. 7). The WISE has proven effective in CRT candidates who had previously failed the conventional procedure, who had a high risk of mechanical or infective complications, or who were non-responders to conventional CRT, similarly to endo-ventricular LV stimulation by conventional leads [73, 74, 75]. The implantation consists of two stages (intercostal transmitter placement + endoventricular unit insertion) that require different expertise, but can be accomplished in a single session as in our practice. The main limitation of WISE is the high energy drain dictated by the receiver localization process and by delivery of pacing with ultrasounds, that make a co-implant mandatory for sensing atrial and ventricular activity and a large battery size to ensure a 4-year service-of-life. The system is actually undergoing fast transformation: the battery is going to become a rechargeable one, thus overcoming the issue of power supply in a high-drain setting: this will enable the WISE system to work out sensing of the cardiac activity, and paves the way to a stand-alone device. Moreover, device downsizing around 25% of its actual size will be possible, so co-implant of a subcutaneous/extravascular defibrillator in a view of a total extravascular CRTD will also be enabled. Another very important benefit of a rechargeable device is the potential decrease of pocket infections, that burden patients’ outcome when multiple pocket entries occur [76].
 Fig. 6.
Fig. 6.WISE LV endoventricular pacing. (A) Schematic representation of the WISE system components. (B) Endocardial receiver implantation in the animal model, showing multiple device placement both at the LV septum and free wall either in basal, mid and apical sites. (C) Implantation of the receiver (red arrow) at the posterolateral LV wall under visualization by dye injection. (D) Release (red arrow) of the receiver after insertion in the ventricular wall.
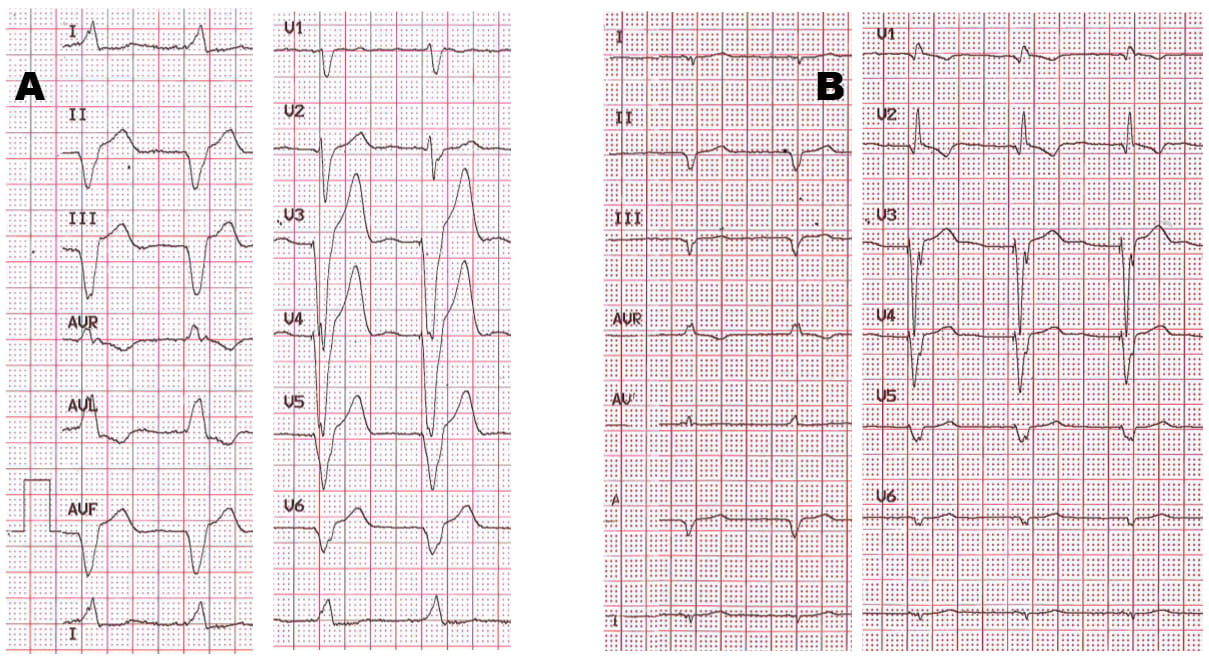 Fig. 7.
Fig. 7.CRT enabled by the WISE system in a heart failure patient with permanent AF, AV block and unsuitable coronary veins for conventional CRT. Note the impressive change in QRS duration and morphology, fostered by the fast LV activation via endocardial pacing.
Baseline ventricular function, HF status, and expected amount of ventricular pacing should drive the decision-making of cardiac stimulation modality [2, 3, 4]. The coexistence of a high-risk clinical profile for HF development and advanced AV block should prompt the seek of a more physiologic, though more challenging, pacing modality. On the contrary, a high-risk profile for infective or bleeding or lead complications should pave the way to a leadless system to minimize serious complications. Conventional RV ventricular stimulation would probably stand in the remaining population in need of cardiac pacing, unless improvement in LBBAP technology and leadless pacing will make it as fast and as reliable at long term. Though attractive from a pathophysiological viewpoint, the new pacing modalities need to prove their clinical efficacy on sound clinical endpoints, compared respectively to conventional RV pacing and CRT, that represent the actual gold-standard. Durability of the pacing systems and sustainability for the health system need also to be evaluated as end-points in the long term.
Conceptualization—MB, ID, GPP, ST, AS; Methodology—CC, GS, CM, MZ; Software—AA, GM; Validation—AM, CC; Formal analysis—GS, SS, AM; Investigation—LB, SS; Data curation—MB, MZ, CM, ID, SS, GS, LB; Writing—original draft preparation—GPP, AS, ST, MB; Writing—review and editing—ID, CC, GPP; Visualization—MZ, AA, CM, LB; Supervision—MB, AS; All authors have read and agreed to the published version of the manuscript.
All patients treated by transvenous or leadless CIEDs gave their written consent for participation in clinical trials or registries, based on the approval by our Hospital ethic committee (10/2016/U/Oss).
Not applicable.
This research received no external funding.
Mauro Biffi: educational activity and Speaker’s bureau for Biotronik, Boston Scientific, Medtronic, and Zoll. The other authors declare no conflicts of interest.
