Academic Editor: Daphne Merkus
Background: Neuregulin-1 (NRG-1) is a stress-mediated transmembrane
growth factor. Reduced myocardial damage and higher NRG-1 levels upon treatment
with remote ischemic conditioning (RIC) has been described in rats. However, the
role of NRG-1 in patients with acute myocardial infarction (MI) is unknown. Thus,
we conducted a post hoc analysis of a randomized controlled trial that tested RIC
in patients with MI scheduled for primary percutaneous coronary intervention
(PCI). Methods: Blood was drawn from 30 patients before RIC/PCI, within
1 hour, 4 days and 1 month later. Median left ventricular ejection fraction
(LVEF) in the overall study population following MI was 48.5%. Results:
NRG-1 plasma levels decreased significantly following PCI/RIC and remained
decreased up to 1 month following MI (p
Neuregulin-1 (NRG-1) is a stress-mediated paracrine transmembrane growth factor
deriving, among others, from endothelial cells [1, 2]. Emerging evidence reveals
the cardiovascular functions and benefit of NRG-1
In line with that, we previously demonstrated that remote ischemic conditioning (RIC), a tissue protective intervention based on short, intermitted ischemia/reperfusion episodes, stimulated NRG-1 expression in the infarcted tissue area and subsequently improved cardiac function in rats with reperfused myocardial infarction (MI) [8]. This study suggests that reduction of NRG-1 levels post MI may contribute to the progression of adverse remodeling and HF. Controversially, a recent study [9] demonstrated that the recombinant form of NRG-1 application worsened left ventricular (LV) ejection fraction (LVEF) in rats with chronic MI.
Although the administration of human-recombinant NRG-1 to patients with HF resulted in a significant improvement in cardiac output and LVEF [10], its circulating expression and functional importance as a biomarker in progression of adverse post MI remodeling in patients with ST-elevation myocardial infarction (STEMI) is yet unclear.
Thus, we aimed to study plasma NRG-1 concentrations in patients with acute STEMI and, additionally, its association with patient characteristics, cardiac function following STEMI and RIC.
We conducted a post-hoc analysis of a randomized-controlled trial investigating
RIC in STEMI patients, that was previously reported [11]. In brief, patients with
STEMI and planned primary percutaneous coronary intervention (PCI) at
presentation to the emergency department were enrolled. Among others, those with
symptoms
We fitted mixed-models specifying the patient as random factor to account for the repeated measurements design and all other included variables as fixed factors. All models included the time of measurement as a fixed effect. We report the derived estimates of the fixed effects together with 95% confidence intervals (CI). Additionally, comparisons within one group at two time points were performed using paired Student’s t-test for parametric variables, which were tested using Shapiro-Wilk normality test.
In total, we included 30 patients in the present analysis with available data
for NRG-1. The mean age was 60.7 (
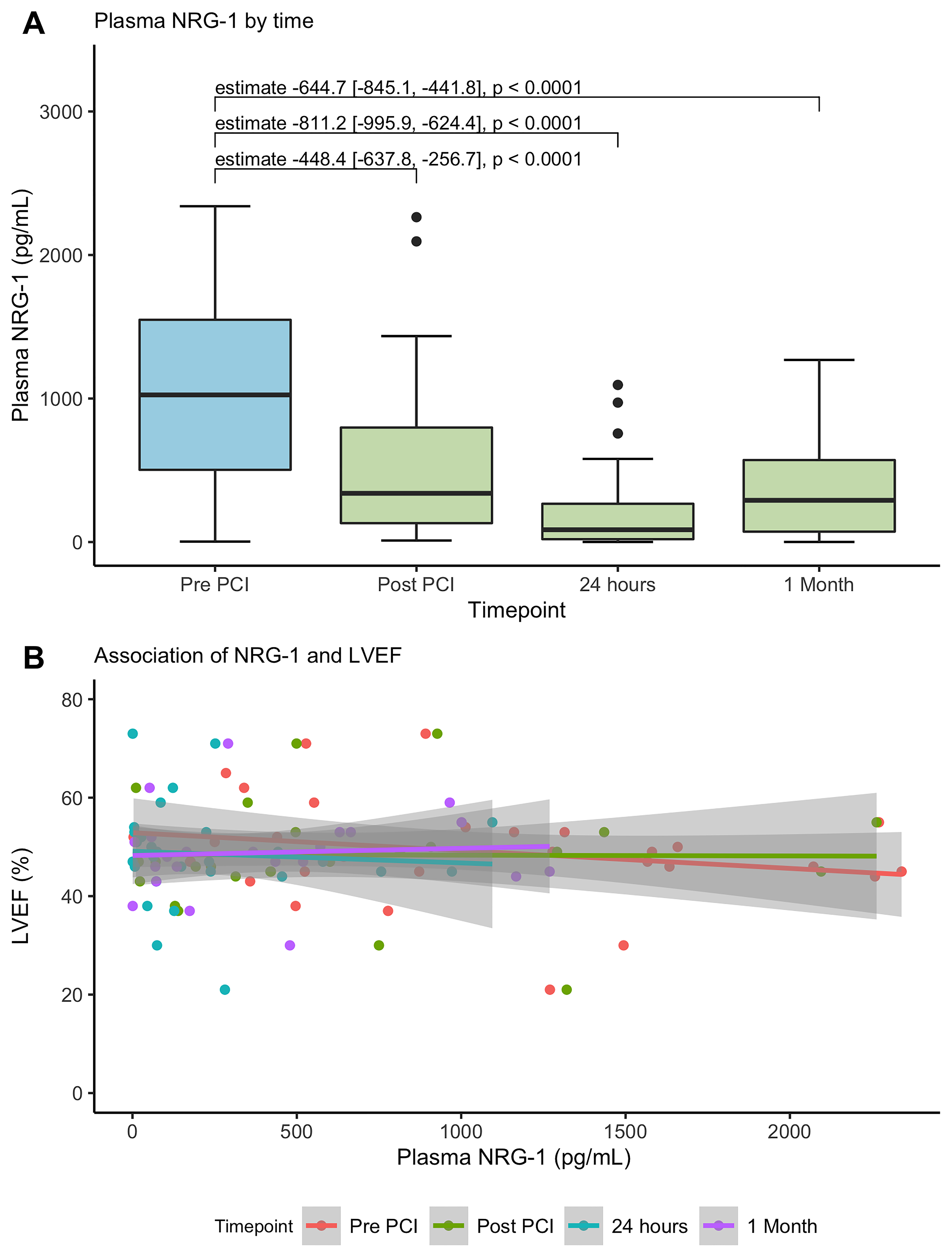 Fig. 1.
Fig. 1.Plasma Neuregulin-1 (NRG-1) levels over time (A) and in relation to left ventricular ejection fraction (LVEF; B).
In summary, we investigated plasma NRG-1 concentrations for the first time in patients with acute STEMI. Although being investigated broadly in heart failure patients, NRG-1 has not been widely studied in the context of acute MI. Thus, for the first time we demonstrate that NRG-1 plasma levels are critically affected in patients with STEMI during its acute phase. Our study also highlights the importance and difference of changes in circulating NRG-1 in STEMI and HF patients. Accordingly, we found that circulating NRG-1 levels were declined after PCI in STEMI patients. On the other hand, patients with advanced stage of HF were characterized with a significant increase of circulating NRG-1 levels [12] but mRNA expression of ErbB2 and ErB4 were decreased [13].
In our previous study, we reported on the effect of RIC on NRG-1 plasma and
tissue concentrations, respectively, in experimental myocardial infarction in
rats [8]. Although effective in rats, we did not observe any effect of RIC on
plasma NRG-1 levels. Lately, the effect or RIC in humans has been questioned
[14]. Although some studies did reveal some positive effect on myocardial salvage
in the context of STEMI, the overall effect seems to be modest [15]. Also, a
large randomized controlled trial did not show any effect on survival or
hospitalization for heart failure following in the first year after STEMI [16].
Despite the previously described association of NRG-1 and cardiac function, we
did not observe any association with LVEF. Although this will at some point be
owed to the small number of patients included in this analysis, the early time of
LVEF determination following STEMI of only four days will most likely not fully
reflect the effect of STEMI on the developing heart failure. In line, the overall
reduction in LVEF observed in our trial was small, with only three patients
having a LVEF
In summary, our findings highly suggest a further role of NRG-1 in humans with acute MI, despite its known role in heart failure. Considering the beneficial effects of the administration of recombinant NRG-1 to patients with heart failure [10], patients with STEMI could be a further target population of interest. Future studies will need to address the function of NRG-1 during acute MI and its relation to LVEF and other cardiac parameters, in mid- and long-term follow-up.
Conceptualization and methodology: PMH, AK, BKP, JW and KH. Formal analysis: PMH and AK. Investigation: PMH, IFG, EA, BJ, PMP, JW and KH. Writing—Original draft preparation: PMH and AK. Writing—Review and editing: all authors. Supervision: KH and BKP. Project administration: PMH, AK and KH. Funding acquisition: PMH, JW, AK, BKP and KH.
All patients provided written informed consent, the study was approved by the competent ethics committee (EK 16-009-0216) and was performed according to Good Clinical Practice and the Declaration of Helsinki.
We would like to thank the staff of Wojta’s lab for continuous advice throughout the study and the peer reviewers for their opinions and suggestions.
This work was supported by the Ludwig Boltzmann Institute for Cardiovascular Research, by a grant of the Austria-Hungary Action Foundation (No. 92öu8) and the Association for Research on Arteriosclerosis, Thrombosis and Vascular Biology (ATVB).
The authors declare no conflict of interest. Attila Kiss is serving as one of the Guest editors of this journal. We declare that Attila Kiss had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Daphne Merkus.
The datasets used and/or analyzed during the current study are available from the first author on reasonable request.
See Table 1.
| Study population | ||
| Age | 60.7 (3.8) | |
| Females | 7 (23.3%) | |
| Hypertension | 16 (53.3%) | |
| Hyperlipoproteinaemia | 11 (36.6%) | |
| Diabetes | 1 (3.3%) | |
| Smoking history | ||
| Previously | 11 (36.7%) | |
| Continued | 14 (46.7%) | |
| History of Coronary Artery Disease | 2 (6.7%) | |
| Total ischemic time, minutes | 234.7 (127.4) | |
| TIMI flow pre PCI | 0 (0, 3) | |
| TIMI flow post PCI | 3 (3, 3) | |
| Maximum Creatine Kinase | 1865 (122, 20023) | |
| Maximum Troponin | 72.5 (0.5, 975.0) | |
| Maximum NT-proBNP | 1460.0 (654.5, 3515.2) | |
| eGFR at presentation | 64.9 (8.4) | |
| BMI | 27.2 (25.6, 30.8) | |
| Left ventricular ejection fraction following STEMI | 48.5 (45.0, 53.0) | |
| Left ventricular ejection fraction | ||
| Severe ( |
2 (6.7%) | |
| Moderate (31 |
7 (23.3%) | |
| Slight (46 |
9 (30.0%) | |
| Normal ( |
12 (40.0%) | |
| Categorial variables are presented as n (%) and scale variables as mean
( | ||
