† These authors contributed equally.
Academic Editors: Brian Tomlinson and Takatoshi Kasai
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy caused by defective desmosomal proteins. The typical histopathological finding of ARVC is characterized by progressive fibrofatty infiltration of the right ventricle due to the dysfunction of cellular adhesion molecules, thus, developing arrhythmogenic substrates responsible for the clinical manifestation of ventricular tachycardia/fibrillation (VT/VF). Current guidelines recommend implantable cardiac defibrillator (ICD) implantation to prevent sudden cardiac death (SCD) in ARVC, especially for those experiencing VT/VF or aborted SCD, while antiarrhythmic drugs, despite their modest effectiveness and several undesirable adverse effects, are frequently used for those experiencing episodes of ICD interventions. Given the advances in mapping and ablation technologies, catheter ablation has been implemented to eliminate drug-refractory VT in ARVC. A better understanding of the pathogenesis, underlying arrhythmogenic substrates, and putative VT isthmus in ARVC contributes to a significant improvement in ablation outcomes through comprehensive endocardial and epicardial approaches. Regardless of ablation strategies, there is a diversity of arrhythmogenic substrates in ARVC, which could partly explain the nonuniform ablation outcome and long-term recurrences and reflect the role of potential factors in the modification of disease progression and triggering of arrhythmic events.
Arrhythmogenic right ventricular cardiomyopathy (ARVC), characterized by progressive fibro-fatty replacement of the right ventricle (RV), is an inherited disease [1]. Mutation in genes associated with desmosomal proteins is considered the cornerstone of ARVC, leading to pathological disruption of cell-to-cell adhesion. The clinical manifestations of ARVC include asymptomatic carriers, syncope, heart failure, and ventricular tachycardia/fibrillation (VT/VF). Through the classification of structural, histological, electrocardiographic, arrhythmic, and genetic characteristics as major and minor criteria, a quantitative and modified Task Force has been proposed to facilitate the diagnosis of ARVC. Furthermore, implantable cardioverter defibrillator (ICD) implantation should be considered in patients with ARVC who experience sudden cardiac death (SCD) due to VT/VF, sustained VT, or who present with high-risk characteristics [2].
Notably, antiarrhythmic drugs (AADs) remain the fundamental management to prevent the recurrence of ventricular tachyarrhythmias in ARVC. In the North American ARVC Registry, amiodarone showed superior efficacy in preventing ventricular arrhythmia [3], although the safety and efficacy of flecainide in combination with other AADs have also been reported [4, 5]. However, AADs may not be effective and are frequently limited by well-known acute and long-term toxicities, especially in young patients. Therefore, catheter ablation has been considered an alternative management method for drug-refractory VT in ARVC. The broad application of epicardial mapping provides a better delineation of arrhythmogenic substrates in ARVC, particularly those with failed endocardial ablation or recurrence. Recent studies have also aimed to correlate the nonuniform distribution of arrhythmogenic substrates within the RV epicardium with clinical characteristics. Several ablation strategies, not individualized for ARVC, have been proposed to yield better outcomes. Irrespective of the ablation strategies, the diverse substrate characteristics due to different extents of disease progression in ARVC could contribute to the heterogeneous ablation outcome and VT recurrence. In addition to heart transplantation, there are still clinical hurdles to explore novel strategies, such as sympathetic denervation or radioablation, for patients with drug-refractory VT who have received failed endocardial/epicardial ablation. In this study, we review the characteristics of arrhythmogenic substrates and the associated ablation outcomes in patients with ARVC.
According to the current guideline, the EP study is not routinely recommended for the diagnosis or risk stratification of patients with ARVC [2, 6]. Previous studies have demonstrated inconsistent positive inducibility values to predict the future occurrence of VT/VF in patients with ARVC [7, 8]. Therefore, the decision on preventive ICD implantation should not be solely based on the inducibility test of EP studies, especially for patients with high-risk characteristics. Furthermore, based on current guidelines [9], DNA variants might have different impacts on the survival and prognosis of arrhythmogenic cardiomyopathy. Therefore, by identifying DNA variants individually, genetic analysis may help to better stratify the risk of VT in patients with ARVC.
However, an EP study might help differentiate VT in ARVC from idiopathic VT. Denis et al. [10] showed the utility of high dose isoproterenol to facilitate the diagnosis of early-stage ARVC. Moreover, regardless of the ablation strategies, an attempt to induce clinically documented VT through a comprehensive EP study is frequently performed before catheter ablation. Induced VTs could provide clues regarding the location of the putative VT isthmus, guide and tailor ablation strategies individually, and help determine ablation endpoints and predict long-term outcomes [11, 12, 13, 14].
To date, no randomized controlled trials have demonstrated the superiority of catheter ablation compared to antiarrhythmic drugs. Moreover, studies exploring the precise timing of VT ablation in patients with ARVC who experience limited episodes of VT are scarce. Since ARVC is a progressive inherited disease, catheter ablation for patients with ARVC is frequently reserved for those with recurrent or drug-refractory VT/VF to reduce frequent ICD therapies and improve quality of life [6]. However, given the advances in mapping and ablation techniques, catheter ablation might be promising and has been implemented in patients without background ICD therapy due to patient refusal or financial hardship, with 81% of ARVC patients free from recurrent VT during 46 months follow-up [11].
A discrepancy of abnormal substrates between the epicardium and the endocardium often exists, being a larger epicardial scar [11]. In general, mapping of the endocardial substrate serves as the first step; however, the necessity of mapping and ablation of abnormal epicardial substrates should be considered to achieve successful ablation of VT [11, 15, 16]. Despite the possibility that the epicardial substrates might be predicted by endocardial mapping and pre-procedural images [17], and certain VT circuits could be ablated from the endocardium only [18], growing evidence supports the pivotal role of simultaneous endocardial and epicardial mapping/ablation in ARVC [19], particularly for high-volume and experienced EP laboratories.
From the viewpoint of pathogenesis, abnormal substrates typically involved the
tricuspid annulus (TA) and RV outflow tract (RVOT) and could extend to the RV
free wall and RV apex [20]. Abnormal substrates located in the basal perivalvular
area of the left ventricle have been reported [21]. Detailed substrate mapping
during sinus or paced rhythm is often required to thoroughly reveal critical
zones responsible for the origins of VT [22]. Traditionally, low-amplitude,
fractionated electrograms, late potentials, and local abnormal ventricular
activities are considered abnormal substrates critical for VT circuits [23, 24, 25]
and the abolition of these signals have been shown to reduce VT recurrences
[25, 26]. In our laboratory, RV endocardial abnormal substrates are defined as the
bipolar voltage
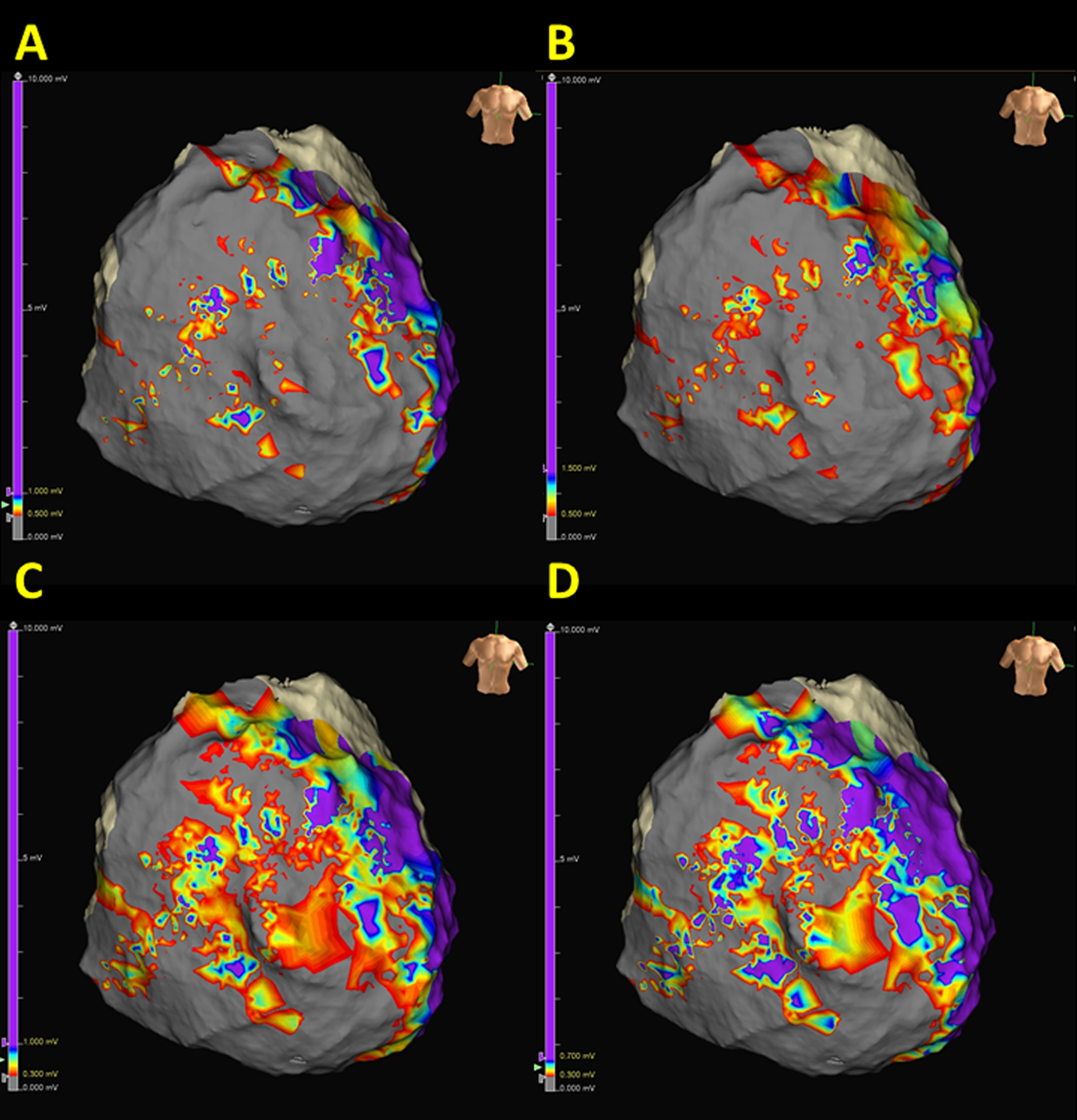 Fig. 1.
Fig. 1.Identification of potential epicardial channels by adjusting the bipolar voltage threshold. Possible channels could be observed within the superior tricuspid annulus, lower tricuspid annulus, and right ventricle (RV) free wall. Voltage thresholds were 0.5–1.0 mV, 0.5–1.5 mV, 0.3–1.0 mV, and 0.3–0.7 mV in (A–D), respectively.
In addition to the above, advances in high-density mapping using closely spaced multielectrode mapping catheters provide better delineation and higher resolution of ventricular scars [35, 36, 37, 38, 39]. Since the critical VT isthmuses responsible for clinically documented VT are usually complicated and frequently localized within dense scars, high-density mapping also yields a better illustration of channels within the scar [40]. However, the appropriate cut-off value of scarring, particularly in patients with ARVC, remains unknown and requires further investigation.
Since substrate mapping focused on bipolar voltage, electrogram duration, and fractionated or isolated late potentials, our group proposed a novel Simultaneous Amplitude Frequency Electrogram Transformation (SAFE-T) to recognize critical substrates correlated to the VT isthmus, and ablation targeting these abnormal substrates could yield better outcomes [25, 41, 42, 43]. Recently, functional substrate mapping has been proposed by annotating the offset of the local bipolar electrogram deflection to create isochronal late activation mapping (ILAM). The isochronal crowding regions, i.e., the deceleration zone identified by ILAM, are frequently correlated with the critical zone of VT circuits. Substrate modification based on the ILAM approach has been reported to be an effective and promising strategy to achieve noninducibility of VT [44, 45]. A representative case using ILAM to localize the critical zone of VT circuits is illustrated in Fig. 2.
 Fig. 2.
Fig. 2.A representative case demonstrates the correlation between
epicardial substrate and isochronal late activation map (ILAM) in arrhythmogenic
right ventricular cardiomyopathy (ARVC). (A) An extensive scar is observed
within the inferior wall of the basal to middle right ventricle (RV). However, no
remarkable channels could be identified. (B) The creation of an ILAM demonstrated
the functional property of abnormal substrates during sinus rhythm. The latest
deflection of the bipolar electrograms was annotated. The activation sequence
initiated from white, red, orange, yellow, green, blue, indigo, to violet region
and the propagation wavefront slowed down in the isochronal crowding area during
sinus rhythm (Supplementary Movie 1). The isochronal crowding region
(deceleration zone) identified within the scar was defined by regions with
isochronal crowding with
Among noninvasive modalities, 12-lead electrocardiography (ECG) remains the most ubiquitous tool. Several studies have demonstrated a correlation between depolarization and repolarization features on 12-lead ECG and the localization of abnormal substrates in ARVC. Tanawuttiwat et al. [46] reported that the timing of epsilon waves on the 12-lead ECG was associated with electrical activation of the sub-tricuspid region. Similarly, Tschabrunn et al. [47] found that the extent and distribution of abnormal RV substrates were correlated with region-specific ECG depolarization changes. Additionally, Kubala et al. [48] showed that areas of abnormal substrates were comparable to the degree of T wave inversion, while more advanced transmural lesions were found if a downsloping elevated ST-segment pattern in V1 and V2 was observed. Moreover, our recent study also demonstrated that the presence of J waves, which has been dominantly observed in the inferior leads, was associated with the discordance of endocardial and epicardial activation patterns, emphasizing the pivotal role of transmural depolarization discrepancy in the electrocardiographic manifestation [49]. The ECG image, which noninvasively maps cardiac electrical activity on the heart epicardial surface, has been reported to assess arrhythmogenic substrates in ARVC [50].
Electrocardiographic features could also help predict the requirement of the epicardial approach. Bazan et al. [51] reported that a Q wave or QS in regional leads could represent epicardial local activation. Our previous study studied the association between the epicardial scar and the extent of abnormal signal-averaged ECG, which could also predict the epicardial circuit and the need for epicardial ablation [52]. Moreover, the inter-lead QRS dispersion of precordial leads could be identified in patients who benefit from epicardial ablation [53].
Noninvasive imaging modalities, including cardiac computed tomography (CT) and cardiovascular magnetic resonance imaging (CMR), play an important role in detecting scars and facilitating clinical decisions on ablation strategies. Venlet et al. [54] reported a strong correlation between tissue heterogeneity in CT and low-voltage sites harboring late potentials. To date, the use of high-resolution late gadolinium-enhancement [55] and the use of regional myocardial strain in cine CMR might provide a high diagnostic value for localizing VT substrates [56].
Although scars in ARVC traditionally involve arrhythmogenic triangles, VTs arise predominantly from the free wall of TA and RV, followed by RVOT, and rarely originate at the RV apex [20], implying the role of heterogeneous substrates in ventricular arrhythmogenesis. Haqqani et al. [16] first described the layered activation pattern within the epicardial and endocardial scars in ARVC. Our previous study also demonstrated the scar transmularity in the clinical manifestations of ARVC. By categorizing scar patterns according to scar transmularity [15], we found that horizontal distribution rather than transmural extension of the scar was associated with the presence of VF and led to a worse ablation outcome, reflecting the consequence of heterogeneous substrates on clinical manifestations [15].
Irrespective of the diverse clinical manifestations, several factors are also associated with substrate heterogeneity in ARVC. First, we previously demonstrated that heterogeneous substrates were observed between different sexes in ARVC, and male patients generally had worse substrates in terms of larger epicardial low voltage zone, areas with late potential, and longer local abnormal ventricular activities [28]. Findings mentioned above might be caused by sex hormones [57] and could partly explain the nonuniform ablation outcome [28]. Given the progressive entity of ARVC, structural deterioration and scar progression might be observed as the disease evolves [58, 59]. Riley et al. [59] reported the nonuniform progression of endocardial scars in 11 patients undergoing repeat procedures for recurrent VT. In their study, only two patients developed a significant increase in scar areas, while 10 of 11 patients had a significant increase in RV volume. On the contrary, our previous study showed that scar progression accounted for 72.9% of recurrent VT during repeat procedures in patients with initially successful ablation [60]. Heterogeneous substrate characteristics and diverse study populations could explain the different findings of the above studies. Notably, scar progression was consistent with RV remodeling, which was predicted by the history of an athlete [60]. Endurance exercise has been shown to worsen structural remodeling [61], and the aforementioned findings again highlighted that factors promoting disease progression, such as tricuspid regurgitation resulting from RV dilatation, could also contribute to heterogeneous substrates and deteriorate the prognosis [62].
The predominant VTs in ARVC are usually macro-reentry and characterized by a left bundle branch block pattern with either superior or inferior axis [63]. Delineation of VT isthmuses not only provides mechanistic insights correlated to the underlying substrates but also facilitates complete VT elimination without extensive substrate modification. However, VT mapping is frequently confined by unstable hemodynamics, changing VT morphology during mapping, wobbling cycle length, inappropriate mapping modalities, inadequate mapping resolution, and non-inducibility during EP studies.
To map the VTs, the most important step is to induce clinically documented VT, although eliminating nonclinical VT is important from the viewpoint of preventing VT recurrence. In our laboratory, rapid ventricular pacing and programmed stimulation of up to three additional stimuli were conducted at the RV apex and/or RVOT to obtain VT morphology. Once VTs are induced, QRS morphology, either from 12-lead ECG or ICD, and cycle lengths were compared with those of clinically documented VTs.
Traditionally, activation mapping and entrainment mapping are still the mainstays to illustrate VT circuits, although entrainment might overestimate VT circuits [64]. For entrainment mapping, pacing of the mapping catheter with a cycle length shorter than the tachycardia cycle length by 20–30 ms is needed. VT isthmuses could be identified based on the following criteria: (1) concealed fusion of all 12-lead ECG is achieved during entrainment; (2) the postpacing interval is less than 30 ms of the VT cycle length; (3) the stimulus-to-electrogram interval is less than 20 ms of the electrogram-QRS interval after entrainment, and (4) the local electrogram to QRS interval should be between 30% and 70% of the VT cycle length [65]. Pace mapping, especially for hemodynamically unstable VT, might be used to search for the exit of VT [15, 60, 66]. Different EP mapping strategies have been reported to determine VT isthmuses, such as decrement evoked potential mapping [67] and hidden slow conduction electrograms [68].
Complementary with the process of disease progression, the peri-tricuspid regions frequently harbor the diastolic path of VT isthmuses [69, 70, 71]. Back in 1996, Stark et al. [72] delineated the slow conduction isthmus of VT in ARVC surrounding an aneurysm and TA with concealed entrainment. Miljoen et al. [70] reported that the critical isthmuses of peri-tricuspid VT were surrounded by TA with a low voltage area, while the isthmuses of RVOT VT were outlined by TA with a low voltage area located at RVOT posterior wall in 11 patients with ARVC shows a mappable VT circuit with localized reentry.
Growing evidence supports three-dimensional architecture of VT circuits in structural heart diseases [19] Given the frequent endocardial and epicardial involvement in ARVC [73], putative VT isthmuses could also display spatial and transmural properties. Jiang et al. [22] first reported 33 patients with ARVC who underwent simultaneous epicardial and endocardial mapping of VT circuits through the collaboration of four centers. In particular, complete tachycardia circuits could be mapped in the epicardial with endocardial activation gaps in 54% of VT circuits, and 21% of VT circuits were confined to the epicardial only with a focal endocardial breakout. Activation gaps were found on endocardial or epicardial maps, demonstrating intramural involvement in 21% of VT circuits in 35% of patients. Localized reentry, defined by more than half of the diastolic paths recorded within 1.5 cm, could be observed in 27% of patients. They also found that VT rarely originated from the RV apex and RVOT, while the RV inferior wall and free wall harbored VT circuits evenly [22]. Findings mentioned above reemphasize the importance of epicardial substrates on the contribution of VT circuits and the importance of epicardial mapping/ablation strategies, and the three-dimensional architecture of VT isthmuses in patients with ARVC [22].
Although the left bundle branch block pattern is more common [63], right bundle branch block pattern has also been documented in ARVC VTs, suggesting LV involvement [63, 74]; therefore, mapping and ablation of the LV might be necessary for these patients. However, Marchlinski et al. [75] recently reported that among 110 patients with ARVC presenting with VT for ablation, the right bundle branch block VT accounted for 17% of cases, most of which originated from the RV rather than the LV. Furthermore, the early precordial QRS transition (V2 or V3), with a frontal plane axis directed in the superior direction and typically leftward, was indicative of RV origin [75].
Given the observations mentioned above, RV mapping, including endocardial and epicardial mapping, serves as the first step for the VT isthmus localization.
Although VF/ventricular flutter is not uncommon, macroreentrant VTs remain the most common manifestation of ventricular tachyarrhythmias in ARVC [76]. Ideally, delineation of VT circuits and targeting the critical isthmuses of VTs to terminate VTs, especially mappable VTs, would be the perfect strategy for VT ablation in ARVC. However, due to the three-dimensional VT circuits in ARVC, an incomplete epicardial circuit with an activation gap with endocardial focal centrifugal activation pattern might be encountered (Figs. 3,4). Entrainment from the earliest activation sites and the adjacent scar might provide information on the potential exit or surrogates of the reentrant circuits (Fig. 3) [77].
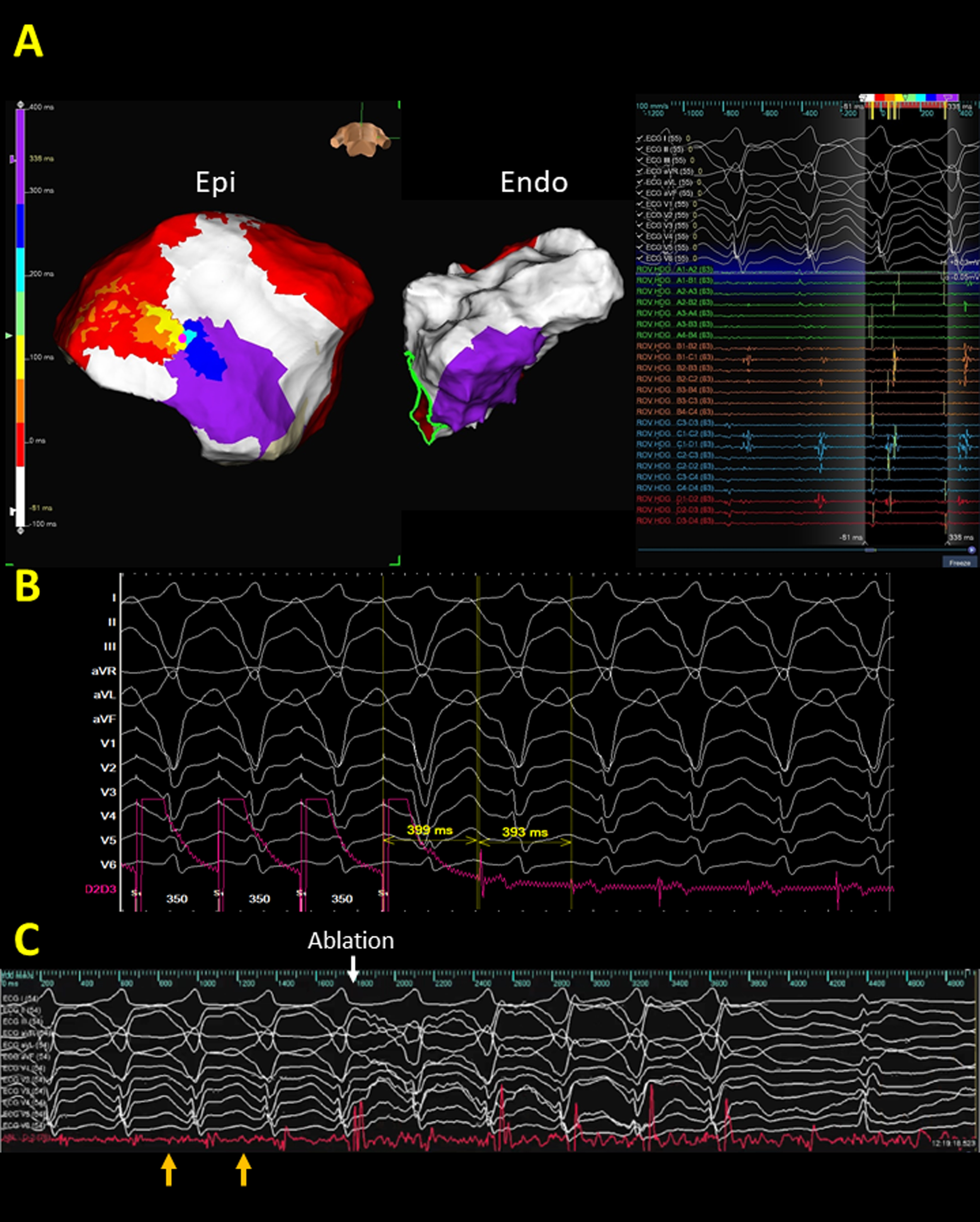 Fig. 3.
Fig. 3.A representative case of ventricular tachycardia (VT) isthmus localization by activation map and entrainment. (A) and (B) The VT circuit in arrhythmogenic right ventricular cardiomyopathy. Figs. 2,3 are the same case. The reentrant circuits could be recognized from the color-coded map, initiating from white, red, orange, yellow, green, blue, indigo, and violet region and the entrance to the exit of VT isthmus could be identified from the colored isochronal map from orange, yellow, green, to the blue region (Supplementary Movie 2). Notable, based on the mapping window between two onsets of QRS, the diastolic corridor is frequently located at the area with yellow, green, and blue colored region. The location of the VT isthmus was compatible with the location of the isochronal crowding region of isochronal late activation map (ILAM) (Fig. 2). Notably, an incomplete epicardial circuit with an activation gap with an endocardial focal centrifugal activation pattern is identified. (B) Concealed entrainment is achieved at the exit site of the VT isthmus. (C) Ablation at the pink dot area terminated the VT within 2 s. Diastolic potentials (orange arrows) were recorded by the distal electrode of the ablation catheter.
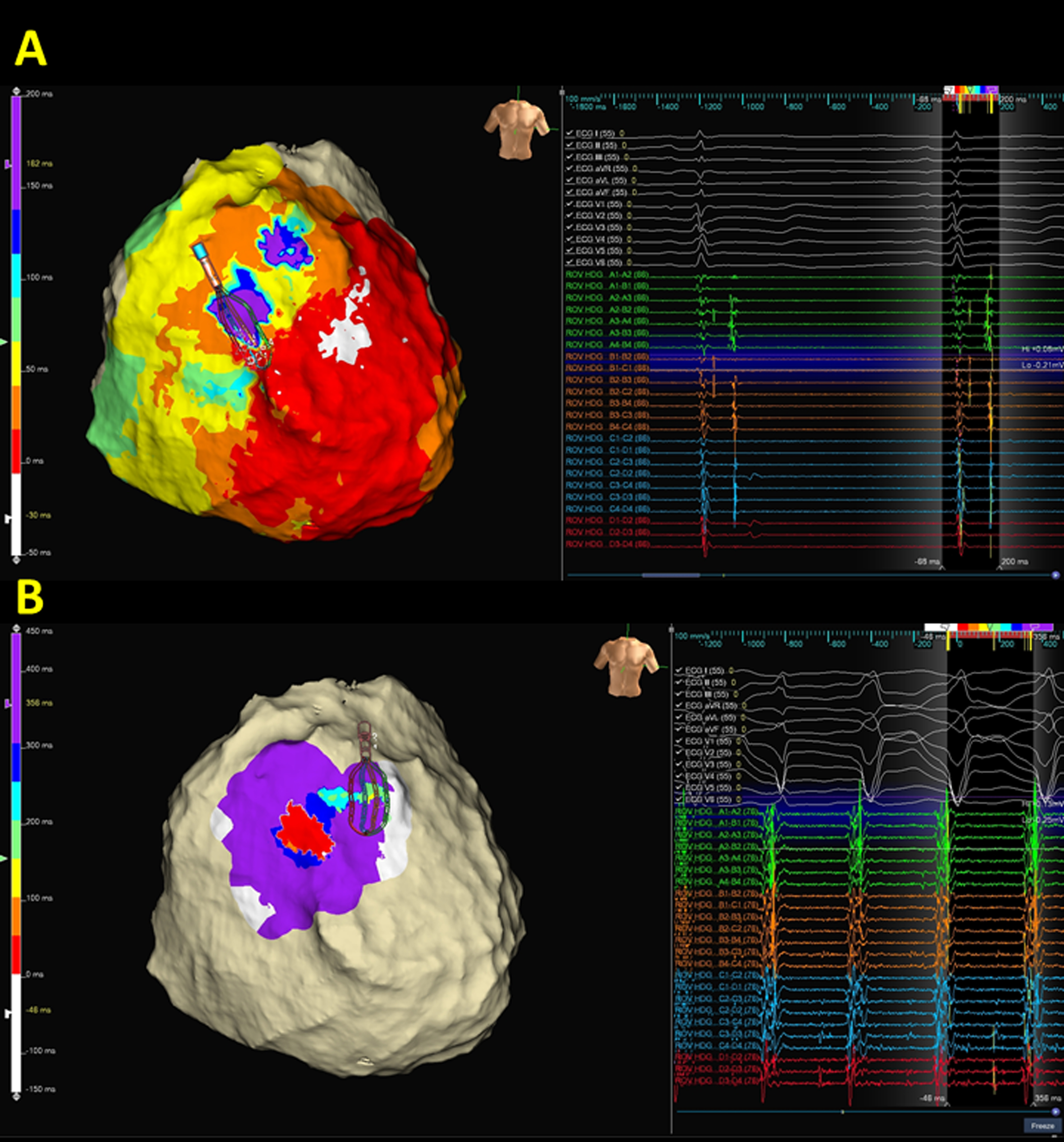 Fig. 4.
Fig. 4.A representative case demonstrates the correlation between deceleration zone and ventricular tachycardia (VT) isthmus. (A) isochronal late activation map (ILAM) of epicardial substrates. Compared to the bipolar voltage map (Fig. 2), ILAM provided informative and functional properties of the two deceleration zones within the RV outflow epicardium. (B) VT activation demonstrated that the VT circuit is compatible with the superior isochronal crowding region. Notably, an incomplete epicardial circuit with an activation gap is recorded, and both far-field and near-field potentials are recorded by high-density mapping.
Regardless of the mechanistic insights provided by the activation map of VT, substrate modifications, either through elimination of local abnormal ventricular activity [25] isolated delayed component ablation [42] core isolation [78], or scar dechanneling [79], have been used to facilitate non-inducibility of VT with promising results. Although extensive substrate modification might provide a better outcome [13], it could be time-consuming and may carry a higher risk of complications. In addition, it has been proven that not all abnormal substrates are actively involved in the VT isthmus [37]. Therefore, various ablation strategies have been proposed to target the abnormal substrates responsible for the VT isthmus without sacrificing clinical efficacy, such as decrement evoked potential mapping [67], hidden slow conduction electrograms [68], omnipolar mappings [80], and strategic multielectrode positioning for VT mapping (Fig. 4) [44].
Complications of VT ablation in ARVC include major vascular complications and SCD, which have also been reported in other VT ablation procedures [81, 82, 83]. Of note, since epicardial access is frequently needed in patients with ARVC, operators should also be aware of the complications related to the epicardial approach, such as liver laceration, RV perforation/rupture, coronary artery injury, pneumothorax, and phrenic paralysis [84]. Table 1 (Ref. [11, 12, 13, 15, 20, 49, 58, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94]) shows the reported complications during or after ablation in previous studies.
| Clinical studies | Mapping and/or ablation sites | Number of patients | Age | Acute success | Major complications | Follow-up | Short-term VA recurrences ( |
Long-term VA recurrences |
| Verma et al. (2005) [85] | Endocardial alone | 22 | 41 |
82% | 1 patient with cardiac tamponade | Median of 37 months | 23% | 47% |
| Satomi et al. (2006) [86] | Endocardial alone | 17 | 47 |
88% | No complications | 26 |
NA | 23.5% |
| Dalal et al. (2007) [87] | Endocardial alone | 24 | 36 |
Total procedural success: 46%; Partial procedural success: 31% | 1 patient with procedure-related death | 32 |
50% | 75% |
| Garcia et al. (2009) [58] | Endocardial & Epicardial | 13 | 43 |
92% | No complications | 18 |
NA | 23% |
| Bai et al. (2011) | Group 1: Endocardial alone | 49 | Group 1: 34 |
All patients achieved the | No major complications | Group 1: 1224 |
NA | Group 1: 47.8%; |
| [90] | Group 2: Endocardial & Epicardial | Group 1, n = 23; Group 2, n = 26 | Group 2: 37 |
procedural end point at the end of ablation. | days; Group 2: 1175 |
Group 2: 15.4% | ||
| Philips et al. (2012) [88] | Endocardial |
87 | 38 |
Complete success 47%; Partial success 38% | 1 patient with procedure-related death; 1 patient with delayed myocardial infarction | 88.3 |
53% | 85% |
| Philips et al. (2015) [91] | Endocardial |
30 | 33.1 |
97% | No major or minor complications | 19.7 |
24% | 30% |
| Santangeli et al. (2015) [13] | Endocardial |
62 | 39 |
VT noninducibility was achieved in 77% patients | 2 patients with DVT and pulmonary embolism; 1 patient with pericardial effusion; 1 patient with RV puncture; 1 patient with constrictive pericarditis | 56 |
NA | 29% |
| Müssigbrodt et al. (2017) [89] | Endocardial |
70 | 53.2 |
VT noninducibility was achieved in 84.4% patients | 1 transient ischemic attack, 2 acute pericardial effusions; 2 pulmonary thromboembolisms (one lethal) later during the hospital stay | 31.1 |
NA | 42.2% |
| Wei et al. (2017) [92] | Endocardial |
48 | 39.9 |
81.3% | No major complications | 71.4 |
NA | 43.7% |
| Kirubakaran et | Endocardial |
29 | Group 1: 38 |
VT noninducibility was | No major complications | 22 |
NA | 27% |
| al. (2017) [20] | Group 1: electrical cardiomyopathy (n = 14); Group 2: structural cardiomyopathy (n = 15) | Group 2: 47 |
achieved in 93% in Group 1 and 87% in Group 2. | |||||
| Lin et al. (2018) [15] | Endocardial |
80 | 47 |
100% | 2 patients with pulmonary edema; 1 patient with pseudo-anuerysm | 38 |
5% | 48.8% |
| Souissi et al. (2018) [93] | Endocardial |
49 | 47 |
71% | 1 patient with cardiac tamponade, hemothorax and DVT; 1 patient with femoral arterio-venous fistula; 1 patient with intestinal perforation | 64 |
63% | 86% |
| Mathew et al. (2019) [94] | Endocardial |
47 | 44 |
Complete success 80%; Partial success 16% | 1 patient with cardiac tamponade | Median follow-up of 50.8 months | 37% | 55% |
| Santangeli et al. (2019) [11] | Endocardial |
32 | 45 |
VT noninducibility was achieved in all patients | 1 patient with RV laceration | Median follow-up of 46 months | NA | 19% |
| Lin et al. (2021) | Endocardial |
45 | Group 1: 51.8 |
Successful ablation was | No major complications | 33.9 |
NA | 15.6% |
| [49] | Group 1: with J wave (n = 13); Group 2: without J wave (n = 32) | years; Group 2: 44.2 |
achieved in all patients | |||||
| Daimee et al. (2021) [12] | Endocardial |
116 | Median of 34.3 years | Total procedural success: 95.8%; Partial procedural success: 4.2% | 1 patient with delayed pericardial effusion | 5.2 |
Single procedure: 31.4%; Multiple procedure: 18.2% | Single procedure: 50.2%; Multiple procedure: 30.4% |
| ARVC, arrhythmogenic right ventricular cardiomyopathy; DVT, deep vein thrombosis; NA, not applicable; VT, ventricular tachycardia. | ||||||||
Ablation outcomes of VT in ARVC have been reported in previous studies (Table 1). Outcomes in studies before 2009 were limited, mainly due to the relatively small number of patients and endocardial-based ablation [85, 86, 87, 88]. However, the initial report of endocardial ablation demonstrated that only 25–53% of cases were free from VT recurrence [85, 86, 87, 88]. In a recent cohort study of the Johns Hopkins ARVC Program, which consisted of 116 patients with 166 ablation procedures, catheter ablation could yield VT-free survival with 68.6% and 49.8% at 1 and 5 years, respectively, after a single procedure [12]. In addition, multiple procedures could further provide VT-free survival with 81.8% and 69.6% at 1 and 5 years, respectively [12]. Additionally, the reduction of VT burden after catheter ablation can result in discontinuation of AAD, which could be toxic or intolerant for patients with ARVC [12]. Despite the abovementioned findings, Müssigbrodt et al. [89] reported a similar outcome of VT recurrences between endocardial vs. endocardial/epicardial ablation based on the inducibility-guided ablation strategy.
Although VT catheter ablation in ARVC is effective and the need for epicardial ablation is often needed to achieve better long-term VT freedom, it is should be noted that survival or acute benefits are not satisfactory at the expense of more major complications [14].
Similarly, recurrences have been heterogeneous in previous studies. These various results are likely to be linked to different mapping and ablation protocols (endocardial-only or combined endocardial epicardial ablation approach), different ablation endpoints, nonuniform follow-up assessment, and operator experience. Apart from the aforementioned issues, the most important factor associated with recurrence is the progression of the disease [95]. Notably, ARVC is a progressive cardiomyopathy, and progressive RV dilatation could result in increased severity of tricuspid regurgitation and a vicious cycle of structural remodeling, which may contribute to VT recurrences [62]. Briceño et al. [96] reported the characteristics of electroanatomic substrates in 19 patients with ARVC receiving repeat procedures separated by at least 9 months. The results demonstrated that the majority of recurrent VTs were confined to the previous abnormal substrate observed in the first procedure. On the contrary, our group found that 72.9% of recurrent VAs originated from newly developed circuits due to scar progression, which was in line with structural remodeling [60]. The abovementioned discrepancy between the two studies might be explained by the nonuniform severity of the study population, heterogeneous genetic mutations, duration of follow-up, and other precipitating factors for disease progression, such as exercise. Furthermore, we also reported several factors, such as male sex, absence of electrical regression identified by signal-averaged ECG, current/previous endurance, athlete activity, and lack of transmural scars that were associated with VT recurrence, reflecting certain factors that might modulate the substrate progression and ventricular arrhythmogenesis in ARVC.
First, repeat ablation could still be considered in patients with ARVC with recurrent sustained VT episodes or frequent appropriate ICD interventions in whom AAD is ineffective or not desired. Of note, the epicardial approach is recommended for patients who receive one or more attempts of endocardial ablation [6]. Since the epicardial approach is often needed for ARVC VT ablation, one of the most common difficulties encountered in the repeat procedure is the possibility of pericardial adhesions [97]. Tschabrunn et al. [98] reported 30 patients who underwent a repeat epicardial procedure for recurrent VT ablation, and significant epicardial adhesions interfering with catheter mapping were found in seven patients. Furthermore, Li et al. [99] reported that pericardial adhesions could still be encountered unanticipatedly in patients without a history of previous cardiac surgery or pericarditis. Pericardial adhesions might be managed by carefully manipulating a steerable flexed catheter tip coupled with a steerable sheath to allow adequate support [97]. However, in patients with severe adhesions, a surgical approach with sternotomy might be required to achieve complete dissection of the pericardium [97].
Recently, stereotactic radiotherapy has gained popularity in the treatment of cardiac arrhythmias [100]. By administering high-dose external-beam radiation noninvasively to achieve complete scar homogenization, case reports demonstrated that stereotactic radiotherapy could potentially be an alternative strategy for patients with recurrent drug-refractory VT in ARVC [100, 101].
In addition to the above, bilateral cardiac sympathetic denervation emerged as a
potential tool for refractory VT/VF treatment, especially for those experiencing
an electrical storm [102]. Assis et al. [103] reported the efficacy of
bilateral cardiac sympathetic denervation in eight patients with ARVC, and five
patients were free of VT recurrences during a mean follow-up of 1.9
Moreover, experience from the Nordic ARVC Registry showed that 10% of 31 patients who received heart transplantation for ventricular arrhythmia between 1988 and 2014 [104]. Heart transplantation should be considered for some selected patients with ARVC who suffer from frequent VTs that are refractory to the aforementioned strategies.
Last but not the least, worsening of RV structure in ARVC during long-term follow-up is inevitable, which may also account for VT recurrences. A double-blind parallel multicenter prospective randomized phase II study has been conducted to investigate the effect of spironolactone on the attenuation of right and/or left ventricular deterioration in patients with ARVC [105]. It is also expected that spironolactone may decrease arrhythmia, improve quality of life, and reduce hospitalizations.
In conclusion, ARVC is mainly an inherited progressive disease with fibrofatty infiltration and subsequent ventricular arrhythmogenesis. Through advances in understanding substrate characteristics and exploring electroanatomical mapping, both ablation strategies and outcomes of VT ablation in ARVC have improved and should be considered in high-volume and well-experienced EP laboratories. Pre-procedural 12-lead ECG and imaging modalities are vital and informative for localizing the scar and critical VT isthmuses. It should be noted that critical VT isthmuses and diseased substrates in ARVC are generally distributed in a three-dimensional fashion. Therefore, a thorough and meticulous examination of both the endocardium and epicardium should be reemphasized. The unsolved issue of catheter ablation in ARVC remains unsatisfactory for long-term survival. With the improvement of technology and understanding of the disease, further studies are warranted to better understand the mechanism and ablation targets and to prevent disease progression.
FPC, YJL, LWL, SLC, YFH, TCT, TFC, JNL, CYL, TYC, and KL conceived the presented idea and the study. WHC and FPC drafted the manuscript. YJL, LWL, SLC, YFH, TCT, TFC, JNL, CYL, TYC, KL, CIW, CML, and SHL verified and critically revised the manuscript. SAC supervised the findings and approved the study. All authors discussed the results and contributed to the final manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. We thank all peer reviewers for their opinions and suggestions.
This work was supported by the Ministry of Science and Technology (MOST 109-2314-B-075-075-MY3, MOST 109-2314-B-010-058-MY2, MOST 109-2314-B-075-074-MY3, MOST 109-2314-B-075-076-MY3, grant nos. 107-2314-B-010-061-MY2, MOST 106-2314-B-075-006-MY3, MOST 106-2314-B-010-046-MY3, and MOST 106-2314-B-075-073-MY3), Research Foundation of Cardiovascular Medicine, Szu-Yuan Research Foundation of Internal Medicine, and Taipei Veterans General Hospital (grant nos. V106C-158, V106C-104, V107C-060, V107C-054, V108C-107, V109C-113, and V110C-116).
The authors declare no conflict of interest.
