Academic Editor: Matina Kouvari
Medicinal plants have been used as an alternative medicine for obesity prevention, and Asian countries, which are major habitats of various medicinal plant species, have traditionally used these medicines for centuries. Obesity is a global health problem caused by excessive fat accumulation linked to abnormal lipid metabolism, such as adipogenesis, lipogenesis, and lipolysis. Accordingly, the effects of medicinal plants on obesity-related mechanisms and biomarkers have been evaluated in various experimental studies. For example, adipogenesis and lipogenesis are regulated by several transcription factors, such as peroxisome proliferator-activated receptor gamma, CCAAT/enhancer binding protein alpha, and fatty acid synthase. Moreover, activation of the adenosine monophosphate-activated protein kinase pathway is accompanied by promotion of lipolysis. However, few reports have consolidated studies of the effects of various Asian medicinal plants on obesity and related mechanisms. Therefore, in this review, we examined the associations of medicinal plants originating from Asian countries with obesity and discussed the related mechanisms and biomarkers from in vitro and in vivo studies.
Obesity is a global health problem that is expected to continuously increase in incidence in upcoming years [1]. According to the World Health Organization (WHO), in 2016, more than 650 million adults (approximately 13% of the population) worldwide were considered obese [2]. Because obesity causes various health complications, including insulin resistance, hepatic steatosis, and dyslipidemia [3, 4, 5], obesity is considered a major risk factor for chronic diseases, such as type 2 diabetes, cardiovascular diseases, and cancer [6, 7]. Thus, obesity prevention and management are essential for individual health and the national healthcare system.
To prevent obesity, it is generally recommended to reduce energy intake and improve lifestyle. When obesity becomes severe, anti-obesity drugs have been used as a mechanism to promote metabolism and suppress appetite; however, the use of these drugs is often limited owing to various side effects, such as neuropathy and cardiovascular disease [8]. For example, the appetite suppressant sibutramine was widely used after approval, but was then withdrawn from the market in 2010 due to the risk of cardiovascular disease [8]. Obesity is currently treated with long-term use of anti-obesity drugs that target various mechanisms of action, including gastric/pancreatic lipases, neurotransmitters, glucagon-like peptide-1 (GLP-1) analogs, and catecholamine release [9]. However, these drugs have been reported to exhibit various side effects, including insomnia, vomiting, hyperpyrexia, and constipation [9].
Accordingly, medicinal plants have emerged as alternative preventive agents because of their weak side effects, ease of availability, low cost, and richness in bioactive compounds [10]. A recent meta-analysis of clinical trials demonstrated that intake of green tea, Phaseolus vulgaris, and Nigella sativa improved obesity-related parameters, such as weight, body mass index, waist circumference, and lipid levels [11]. In addition, several plants, including garcinia and Yerba mate, have been developed as dietary supplements for weight management [12, 13, 14], and weight loss products in the form of pills have widely used for obesity management [15, 16].
Medicinal plants are currently used worldwide; in particular, Asian countries, including China, Japan, Thailand, Indonesia, and Himalayan countries, have traditionally used medicinal plants for more than two centuries [17, 18, 19, 20]. Asian medicinal plants also account for 45% of global profits in trades of medicinal plants, and approximately 39,000 species of medicinal plants are found naturally in Asian countries [21, 22]. However, an overview of the effects of various Asian medicinal plants on obesity and related mechanisms has rarely been reported.
Therefore, in this study, we study aimed to examine the association of medicinal plants originating from Asian countries with obesity and related mechanisms and biomarkers in vitro and in vivo.
The development of obesity can be influenced by various factors, such as diet, physical activity, environment and genetic susceptibilities [23]. In general, however, obesity is simply defined as status of abnormal fat accumulation in adipose tissue, and caused by excessive fat accumulation resulting from an imbalance between high energy intake and low energy expenditure at the cellular level [23]. Adipose tissue consists of white adipose tissue and brown adipocytes. White adipose tissue stores energy in the form of lipids or breaks down stored lipids to use them when energy is needed, whereas brown adipocytes use them to generate heat and contain large numbers of mitochondria [24]. In particular, adipose tissue generates heat and consumes energy through regulation of various proteins, such as uncoupling protein 1 (UCP1) [25]. Fat accumulation includes adipogenesis, which involves accumulation of lipids in the form of triglycerides (TGs) in adipocytes and increased size of adipocytes [26, 27]. Lipolysis, which is the alteration of stored TG to free fatty acids and glycerol, is also a process of abnormal lipid metabolism [28]. In addition, obesity is followed by dyslipidemia, which is characterized by high TG levels and low high-density lipoprotein cholesterol or high low-density lipoprotein cholesterol (LDL-C) levels [29].
Various adipogenic markers are also involved in the development of obesity.
Expression of CCAAT/enhancer binding protein (C/EBP)
We searched articles from PubMed and Google Scholar using the keywords “plant”, “obesity”, “anti-obesity”, “fat”, “lipid”, “cell”, and “mice” from April 2019 to October 2020. We considered only in vitro and in vivo studies using medicinal plants that generally originated from Asian countries in the article and mainly targeted adipogenesis, lipid or fat accumulation, and obesity. Articles written in languages other than English were excluded. As a result, fourteen medicinal plants from 12 articles were reviewed. We extracted the following information from the articles: general characteristics of medicinal plants, including order, family, genus, scientific name, major habitat, extraction method, plant part, and major components; in vitro or in vivo models used in the article; administration and dose; anti-obesity activities, such as anti-adipogenesis; and related biomarkers. The characteristics of medicinal plants were based on information in the original article; however, several parameters, including order, family, and genus, which were not specified in the original article, were based on the information of the National Institute of Biological Resources of the Ministry of Environment in Korea (URL: https://species.nibr.go.kr/).
The general characteristics of the Asian medicinal plants are described in Table 1 (Ref. [35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46]). The major habitat countries are North East Asia, including Korea, China, and Japan, as well as India, Malaysia, and Russia. In total, 11 orders, 12 families, and 14 genera were identified. The orders and families of medicinal plants were Apiales-Araliaceae, Asterales-Asteraceae, Capparales-Brassicaceae, Moringaceae, Cornales-Cornaceae, Dipsacales-Valerianaceae, Myrtales-Melastomataceae, Nymphaeales-Nelumbonaceae, Rosales-Saxifragaceae, Sapindales-Aceraceae, Urticales-Moraceae, and Violales-Violaceae. The types of plant extraction were generally water and ethanol, although petroleum ether and methanol were also used. The major compounds in medicinal plants were flavonoids, such as quercetin, as well as catechin, rutin, and phenolic acids. The effects of medicinal plants were demonstrated using in vitro models in nine studies and in vivo models in eight studies (Table 1).
| Order/Family/Genus | Plant name/Scientific name | Major habitat | Reference | ||
| Apiales | Dendropanax morbifera | Korea (Jeju Island) | Song et al. [35] | ||
| Araliaceae | |||||
| Dendropanax | |||||
| Asterales | Cirsium setidens Naki | Korea (Gangwon province) | Cho et al. [36] | ||
| Asteraceae | |||||
| Cirsium | |||||
| Cosmos | Cosmos caudatus Kunth | Malaysia | Rahman et al. [37] | ||
| Solidago | Solidago virgaurea var. gigantea | North East Asia | Wang et al. [38] | ||
| Capparales | Raphanus Sativus | China, Mostly Asia | Sim et al. [39] | ||
| Brassicaceae | |||||
| Raphanus | |||||
| Moringaceae | Moringa oleifera Lam. | India, Africa | Xie et al. [40] | ||
| Moringa | |||||
| Cornales | Cornus kousa | China, Japan, Korea | Khan et al. [41] | ||
| Cornaceae | |||||
| Cornus | |||||
| Dipsacales | Valeriana dageletiana Nakai ex F. Maek. | Korea (Ulung Island) | Wang et al. [42] | ||
| Valerianaceae | |||||
| Valeriana | |||||
| Myrtales | Melastoma malabathricum var Alba Linn | Malaysia | Karupiah et al. [43] | ||
| Melastomataceae | |||||
| Melastoma | |||||
| Nymphaeales | Nelumbo Nucifera L. | China (Mostly Asia) | Sim et al. [39] | ||
| Nelumbonaceae | |||||
| Nelumbo | |||||
| Rosales | Astilbe chinensis Franch. et Savat. | Russia, China, Japan, Korea | Zhang et al. [44] | ||
| Saxifragaceae | |||||
| Astilbe | |||||
| Sapindales | Acer okamotoanum Nakai | Korea (Ulung Island) | Kim et al. [45] | ||
| Aceraceae | |||||
| Acer | |||||
| Urticales | Morus Alba L. | China, Mostly Asia | Sim et al. [39] | ||
| Moraceae | |||||
| Morus | |||||
| Violales | Viola mandshurica W. Becker | China, Japan, Korea | Sung et al. [46] | ||
| Violaceae | |||||
| Viola | |||||
All in vitro studies selected in this study used 3T3-L1 cells to examine the effects of Asian medicinal plants on adipogenesis, lipogenesis, lipolysis, and other obesity-related activities (Table 2, Ref. [35, 36, 38, 39, 40, 41, 44]). Adipogenesis, also called adipocyte differentiation, is a process through which preadipocytes develop into mature adipocytes [47]. Adipocytes are major components of white adipose tissue that mediate physiological and pathological processes, such as appetite, immunological and inflammatory responses, glucose metabolism, and blood pressure regulation [47, 48]. 3T3-L1 cells are pre-adipocytes that originate from mouse embryonic fibroblasts and have been commonly used to evaluate anti-obesity effects [49]. Notably, many medicinal plants, including Acer okamotoanum Nakai and Astilbe chinensis Franch. et Savat., Cirsium setidens Naki, Cornus kousa, Dendropanax morbifera, Moringa oleifera, and a mixture of Nelumbo nucifera L., Morus alba L., and Raphanus sativus, attenuate pre-adipocyte differentiation by suppressing lipid accumulation and reducing the size and number of lipid droplets in adipocytes. In particular, studies of Astilbe chinensis Franch. et Savat., Dendropanax morbifera, and Moringa oleifera showed that these plants decrease TG accumulation in adipocytes. In addition, extracts of Acer okamotoanum Nakai, Cirsium setidens Naki, Cornus kousa, Dendropanax morbifera, and Moringa oleifera inhibit lipogenesis or stimulate lipolysis. Extracts of Cirsium setidens Naki increase glycerol release from mature adipocytes and stimulate triglycerol lipolysis. In particular, effect of glycerol release of extracts of Cirsium setidens Naki was stronger than the effect of Garcinia cambogia, a well-known plant for anti-adipogenic and anti-lipogenesis activities [50, 51]. Extracts of Dendropanax morbifera inhibit lipid accumulation by reducing glucose uptake, but do not significantly decrease lipolysis.
| Activities | Plants | Plant part | Extraction | Major components | Objects | Dose | References |
| Reduced intercellular lipid accumulation and lipid droplet sizes and numbers during adipogenesis | Acer okamotoanum Nakai | Leaf | Methanol extraction | - | 3T3-L1 cells | P, A + None, A + 50 μg/mL, A + 100 μg/mL | Kim et al. [45] |
| Astilbe chinensis Franch. et Savat. | Whole plant | Ethanol extraction | Astilbic acid (Triterpenoids) | 3T3-L1 cells | P, A + None, A + 20 μg/mL, A + 40 μg/mL | Zhang et al. [44] | |
| Cirsium setidens Naki | Leaf | Ethanol extraction | Pectolinarin | 3T3-L1 cells | P, A + PC (Garcinia cambogia extract) 100 μg/mL, A + 100 μg/mL, A + 200 μg/mL | Cho et al. [36] | |
| Cornus kousa | Leaf | Ethanol extraction | Anthocyanins | 3T3-L1 cells | A + None, A + 5 μg/mL, A + 30 μg/mL, A + 60 μg/mL, A + 100 μg/mL | Khan et al. [41] | |
| Dendropanax morbifera | Leaf | Water extraction | Vitamin C | 3T3-L1 cells | P, A + None, A + 50 μg/mL, A + 100 μg/mL, A + 300 | Song et al. [35] | |
| Tannic acid | μg/mL, A + 500 μg/mL | ||||||
| Moringa oleifera | Leaf | Petroleum ether extract | Isoquercitrin, Chrysin-7-glucoside, Quercitrin | 3T3-L1 cells | A + None, A + 25 μg/mL, A + 50 μg/mL, A + 100 μg/mL, A + 200 μg/mL, A + 400 μg/mL | Xie et al. [40] | |
| Nelumbo Nucifera L., Morus Alba L., Raphanus Sativus | Leaf, leaf, root | Ethanol extraction | Quercetin-3-O-glucuronide | 3T3-L1 cells | P, A + PC (Garcinia cambogia) 100 μg/mL, A + EM11 |
Sim et al. [39] | |
| Solidago virgaurea var. gigantea | Whole plant | Ethanol extraction | Protocatechuic acid, Chlorogenic acid, Rutin | 3T3-L1 cells | P, A + None, A + water extract 10 μg/mL, A + 10% ethanol extract 10 μg/mL, A + 30% ethanol extract 10 μg/mL, A + 50% ethanol extract 10 μg/mL, A + 70% ethanol extract 10 μg/mL, A + 100% ethanol extract 10 μg/mL | Wang et al. [38] | |
| Decreased TG accumulation in adipocytes | Astilbe chinensis Franch. et Savat. | Whole plant | Ethanol extraction | Astilbic acid (Triterpenoids) | 3T3-L1 cells | P, A + None, A + 20 μg/mL, A + 40 μg/mL | Zhang et al. [44] |
| Dendropanax morbifera | Leaf | Water extraction | Vitamin C | 3T3-L1 cells | P, A + None, A + 50 μg/mL, A + 100 μg/mL, A + 300 | Song et al. [35] | |
| Tannic acid | μg/mL, A + 500 μg/mL | ||||||
| Moringa oleifera | Leaf | Petroleum ether extract | Isoquercitrin, Chrysin-7-glucoside, Quercitrin | 3T3-L1 cells | A + None, A + 25 μg/mL, A + 50 μg/mL, A + 100 μg/mL, A + 200 μg/mL, A + 400 μg/mL | Xie et al. [40] | |
| Inhibited lipogenesis or promoted lipolysis | Acer okamotoanum Nakai | Leaf | Methanol extraction | - | 3T3-L1 cells | P, A + None, A + 50 μg/mL, A + 100 μg/mL | Kim et al. [45] |
| Cirsium setidens Naki | Leaf | Ethanol extraction | Pectolinarin | 3T3-L1 cells | C, A + PC (Garcinia cambogia extract) 100 μg/mL, A + 100 μg/mL, A + 200 μg/mL | Cho et al. [36] | |
| Cornus kousa | Leaf | Ethanol extraction | Anthocyanins | 3T3-L1 cells | A + None, A + 5 μg/mL, A + 30 μg/mL, A + 60 μg/mL, A + 100 μg/mL | Khan et al. [41] | |
| Dendropanax morbifera | Leaf | Water extraction | Vitamin C | 3T3-L1 cells | P, A + None, A + 50 μg/mL, A + 100 μg/mL, A + 300 | Song et al. [35] | |
| Tannic acid | μg/mL, A + 500 μg/mL | ||||||
| Moringa oleifera | Leaf | Petroleum ether extract | Isoquercitrin, Chrysin-7-glucoside, Quercitrin | 3T3-L1 cells | A + None, A + 25 μg/mL, A + 50 μg/mL, A + 100 μg/mL, A + 200 μg/mL, A + 400 μg/mL | Xie et al. [40] | |
| P, preadipocyte; A, adipocyte (differentiated cell); PC, positive control.
| |||||||
As shown in Table 3 (Ref. [36, 40, 41, 44, 45]), the expression of adipogenesis-
and lipogenesis-related biomarkers is downregulated by treatment with
Acer okamotoanum Nakai, Astilbe chinensis Franch. et Savat.,
Cirsium setidens Naki, Cornus kousa, Dendropanax
morbifera, and Moringa oleifera in differentiated adipocytes.
Additionally, treatment with Cirsium setidens Naki extract reduces the
expression of PPAR
| Mechanisms | Plants | Related biomarkers | References |
| Adipogenesis, lipogenesis, and lipolysis | Acer okamotoanum Nakai | PPAR |
Kim et al. [45] |
| Astilbe chinensis Franch. et Savat. | PPAR |
Zhang et al. [44] | |
| Cirsium setidens Naki | PPAR |
Cho et al. [36] | |
| phospho-HSL | |||
| Cornus kousa | PPAR |
Khan et al. [41] | |
| Dendropanax morbifera | PPAR |
Song et al. [35] | |
| Moringa oleifera | PPAR |
Xie et al. [40] | |
| HSL | |||
| Activation of the AMPK pathway | Astilbe chinensis Franch. et Savat. | Phospho-AMPK |
Zhang et al. [44] |
| Cirsium setidens Naki | Phospho-AMPK |
Cho et al. [36] | |
| Cornus kousa | Phospho-AMPK |
Khan et al. [41] | |
| Moringa oleifera | Phospho-AMPK |
Xie et al. [40] | |
| Inactivation of PI3K/AKT signaling | Acer okamotoanum Nakai | PI3K 110 |
Kim et al. [45] |
| phospho-AKT (Ser 473) | |||
| phospho-mTOR (Ser 2481) | |||
| phospho-p70S6K (Ser371) | |||
| Activation of |
Acer okamotoanum Nakai | Kim et al. [45] | |
| phospho- | |||
| phospho- | |||
| phospho-GSK3 |
By contrast, promotion of lipolysis is also related to inhibition of lipid accumulation, and lipolysis-related biomarkers have been evaluated in adipocyte cell model studies. In this review, we found that treatment with Moringa oleifera and Cirsium setidens Naki upregulated hormone-sensitive lipase (HSL) protein levels and phosphorylation, respectively. HSL is a key regulator of TAG lipolysis [57].
The effects of Astilbe chinensis Franch. et Savat. and Cornus
kousa on adipogenesis inhibition or lipolysis stimulation are mediated by
activation of the adenosine monophosphate (AMP)-activated protein kinase (AMPK)
pathway. Extracts of Astilbe chinensis Franch. et Savat. upregulate
phospho-AMPK
Leaf extracts of Acer okamotoanum Nakai show anti-adipogenic activity
by regulating phosphatidylinositol-3 kinase (PI3K)/AKT and
Treatment with Acer okamotoanum Nakai extract stimulates
As described in Table 4 (Ref. [36, 37, 38, 39, 40, 42, 43, 44, 46]), major anti-obesity activities in vivo, such as reduced body weight, adipose tissue weight (e.g., epididymal or retroperitoneal fat), and adipocyte size, have been demonstrated by all Asian medicinal plants discussed in this review. A high-fat diet (HFD) supplemented with extracts of Astilbe chinensis Franch. et Savat., Cirsium setidens Naki, Cosmos caudatus Kunth, Melastoma malabathricum var Alba Linn, Moringa oleifera, a mixture of Nelumbo nucifera L., Morus alba L., and Raphanus sativus, Solidago virgaurea var. gigantea, Valeriana dageletiana Nakai ex F. Maek., and Viola mandshurica W. Becker was provided to C57BL/6 or Sprague-Dawley rats for 7 to 14 weeks. The findings showed that supplementation with the extracts of most plants resulted in a decrease in serum lipid levels. Extracts of Astilbe chinensis Franch. et Savat., Cosmos caudatus Kunth, Melastoma malabathricum var Alba Linn, and Viola mandshurica W. Becker reduce serum TG, total cholesterol (TC), and LDL-C levels. Moringa oleifera extracts decrease TC and LDL-C levels, and a mixture of Nelumbo nucifera L., Morus alba L., and Raphanus sativus extracts decreases TC levels. Extracts of Solidago virgaurea var. gigantea and Valeriana dageletiana Nakai ex F. Maek. decrease TG and TC levels, whereas extracts of Astilbe chinensis Franch. et Savat., Cosmos caudatus Kunth, and Viola mandshurica W. Becker also show potential for controlling diabetes-related parameters by regulating glucose or insulin plasma levels. Additionally, extracts of Nelumbo nucifera L., Morus alba L., and Raphanus sativus improve glucose levels in HFD-fed mice, as demonstrated in a glucose tolerance test. The effects of extracts of Cirsium setidens Naki, Moringa oleifera, a mixture of Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus, Solidago virgaurea var. gigantea, Valeriana dageletiana Nakai ex F. Maek, and Viola mandshurica W. Becker were also similar when compared to positive controls, Garcinia cambogia, Lovastatin, or Orlistat.
| Activities | Plants | Plant part | Extraction | Major components | Animal model | Administration/Dose | Period | References |
| Reduced body weight, adipose tissue weight, and adipocyte size | Astilbe chinensis Franch. et Savat. | Whole plant | Ethanol extraction | Astilbic acid (Triterpenoids) | C57BL/6N | Supplementation with HFD | 8 weeks | Zhang et al. [44] |
| ND, HFD, HFD + 100 mg/kg, HFD + 200 mg/kg | ||||||||
| Cirsium setidens Naki | Leaf | Ethanol extraction | Pectolinarin | C57BL/6J | Oral administration with HFD | 14 weeks | Cho et al. [36] | |
| ND, HFD, HFD + 25 mg/kg/day, HFD + 50 mg/kg/day, HFD + 100 mg/kg/day, HFD + 200 mg/kg/day, HFD + PC (Garcinia cambogia extract) 100 mg/kg/day | ||||||||
| Cosmos caudatus Kunth | Leaf | Ethanol extraction | Quercetin, Catechin, Rutin, Chlorogenic acid | C57BL/6N | Supplementation with HFD | 10 weeks | Rahman et al. [37] | |
| ND, ND + 175 mg/kgBW, ND + 350 mg/kgBW, HFD, HFD + 175 mg/kgBW, HFD + 350 mg/kgBW | ||||||||
| Melastoma malabathricum var Alba Linn | Whole plant | Methanol extraction | Epicatechin, Flavonoids | Sprague-Dawley rats | Supplementation with HFD | 8 weeks | Karupiah et al. [43] | |
| ND, HFD, HFD + 5% | ||||||||
| Moringa oleifera | Leaf | Petroleum ether extract | Isoquercitrin, Chrysin-7-glucoside, Quercitrin | C57BL/6N | Supplementation with HFD | 10 weeks | Xie et al. [40] | |
| ND, HFD + PC (Lovastatin) 10 mg/kg, HFD + 0.125 g/kg, HFD + 0.25 g/kg, HFD + 0.5 g/kg | ||||||||
| Nelumbo Nucifera L., Morus Alba L., Raphanus Sativus | Leaf, leaf, root | Ethanol extraction | Quercetin-3-O-glucuronide | C57BL/6J | Oral administration with HFD | 8 weeks | Sim et al. [39] | |
| ND, HFD, HFD + PC (Garcinia cambogia) 245 mg/kg, HFD + EM11 | ||||||||
| Solidago virgaurea var. gigantea | Whole plant | Ethanol extraction | Protocatechuic acid, Chlorogenic acid, Rutin | C57BL/6J | Oral administration with HFD | 7 weeks | Wang et al. [38] | |
| ND, HFD, HFD + 1% PC (Garcinia cambogia extract), HFD + 0.5% of 10% ethanol extract, HFD + 2% of 10% ethanol extract | ||||||||
| Valeriana dageletiana Nakai ex F. Maek | Stem and leaf | Ethanol extraction | Camphene, |
Sprague-Dawley rats | Supplementation with HFD | 8 weeks | Wang et al. [42] | |
| ND, HFD, HFD + 1% PC (Garcinia combogia extract), HFD + 1% | ||||||||
| Viola mandshurica W. Becker | Whole plant | Ethanol and water extraction | Esculetin, Schaftoside | Sprague-Dawley rats | Oral administration with HFD | 11 weeks | Sung et al. [46] | |
| ND, HFD, HFD + 200 mg/kg of ethanol extract, HFD + 100 mg/kg of ethanol extract, HFD + 50 mg/kg of ethanol extract, HFD + 200 mg/kg of water extract, HFD + 100 mg/kg of water extract, HFD + 50 mg/kg of water extract, HFD + PC1 (Orlistat) 50 mg/kg, HFD + PC2 (Garcinia combogia extract) 100 mg/kg | ||||||||
| Decreased lipid levels of serum | Astilbe chinensis Franch. et Savat. | Whole plant | Ethanol extraction | Astilbic acid (Triterpenoids) | C57BL/6N | Supplementation with HFD | 8 weeks | Zhang et al. [44] |
| ND, HFD, HFD+100 mg/kg, HFD+200 mg/kg | ||||||||
| Cosmos caudatus Kunth | Leaf | Ethanol extraction | Quercetin, Catechin, Rutin, Chlorogenic acid | C57BL/6N | Supplementation with HFD | 10 weeks | Rahman et al. [37] | |
| ND, ND + 175 mg/kgBW, ND + 350 mg/kgBW, HFD, HFD + 175 mg/kgBW, HFD + 350 mg/kgBW | ||||||||
| Melastoma malaba-thricum var Alba Linn | Whole plant | Methanol extraction | Epicatechin, Flavonoids | Sprague-Dawley rats | Supplementation with HFD | 8 weeks | Karupiah et al. [43] | |
| ND, HFD, HFD + 5% | ||||||||
| Moringa oleifera | Leaf | Petroleum ether extract | Isoquercitrin, Chrysin-7-glucoside, Quercitrin | C57BL/6N | Supplementation with HFD | 10 weeks | Xie et al. [40] | |
| ND, HFD + PC (Lovastatin) 10 mg/kg, HFD + 0.125 g/kg, HFD + 0.25 g/kg, HFD + 0.5 g/kg | ||||||||
| Nelumbo Nucifera L., Morus Alba L., Raphanus Sativus | Leaf, Leaf, Root | Ethanol extraction | Quercetin-3-O-glucuronide | C57BL/6J | Oral administration with HFD | 8 weeks | Sim et al. [39] | |
| ND, HFD, HFD + PC (Garcinia cambogia) 245 mg/kg, HFD + EM11 100 mg/kg, HFD + EM12 100 mg/kg, HFD + EM01 50 mg/kg, HFD + EM01 100 mg/kg, HFD + Q3OG 10 mg/kg | ||||||||
| Solidago virgaurea var. gigantea | Whole plant | Ethanol extraction | Protocatechuic acid, Chlorogenic acid, Rutin | C57BL/6J | Oral administration with HFD | 7 weeks | Wang et al. [38] | |
| ND, HFD, HFD + 1% PC (Garcinia cambogia extract), HFD + 0.5% of 10% ethanol extract, HFD + 2% of 10% ethanol extract | ||||||||
| Valeriana dageletiana Nakai ex F. Maek. | Stem and leaf | Ethanol extraction | Camphene, |
Sprague-Dawley rats | Supplementation with HFD | 8 weeks | Wang et al. [42] | |
| ND, HFD, HFD + 1% PC (Garcinia combogia extract), HFD + 1% | ||||||||
| Viola mandshurica W. Becker | Whole plant | Ethanol and water extraction | Esculetin, Schaftoside | Sprague-Dawley rats | Oral administration with HFD | 11 weeks | Sung et al. [46] | |
| ND, HFD, HFD + 200 mg/kg of ethanol extract, HFD + 100 mg/kg of ethanol extract, HFD + 50 mg/kg of ethanol extract, HFD + 200 mg/kg of water extract, HFD + 100 mg/kg of water extract, HFD + 50 mg/kg of water extract, HFD + PC1 (Orlistat) 50 mg/kg, HFD + PC2 (Garcinia combogia extract) 100 mg/kg | ||||||||
| Regulated plasma glucose and insulin levels | Astilbe chinensis Franch. et Savat. | Whole plant | Ethanol extraction | Astilbic acid (Triterpenoids) | C57BL/6N | Supplementation with HFD | 8 weeks | Zhang et al. [44] |
| ND, HFD, HFD + 100 mg/kg, HFD + 200 mg/kg | ||||||||
| Cosmos caudatus Kunth | Leaf | Ethanol extraction | Quercetin, Catechin, Rutin, Chlorogenic acid | C57BL/6N | Supplementation with HFD | 10 weeks | Rahman et al. [37] | |
| ND, ND + 175 mg/kgBW, ND + 350 mg/kgBW, HFD, HFD + 175 mg/kgBW, HFD + 350 mg/kgBW | ||||||||
| Nelumbo Nucifera L., Morus Alba L., Raphanus Sativus | Leaf, Leaf, Root | Ethanol extraction | Quercetin-3-O-glucuronide | C57BL/6J | Oral administration with HFD | 8 weeks | Sim et al. [39] | |
| ND, HFD, HFD + PC (Garcinia cambogia) 245 mg/kg, HFD + EM11 100 mg/kg, HFD + EM12 100 mg/kg, HFD + EM01 50 mg/kg, HFD + EM01 100 mg/kg, HFD + Q3OG 10 mg/kg | ||||||||
| Viola mandshurica W. Becker | Whole plant | Ethanol and water extraction | Esculetin, Schaftoside | Sprague-Dawley rats | Oral administration with HFD | 11 weeks | Sung et al. [46] | |
| ND, HFD, HFD + 200 mg/kg of ethanol extract, HFD + 100 mg/kg of ethanol extract, HFD + 50 mg/kg of ethanol extract, HFD + 200 mg/kg of water extract, HFD + 100 mg/kg of water extract, HFD + 50 mg/kg of water extract, HFD + PC1 (Orlistat) 50 mg/kg, HFD + PC2 (Garcinia combogia extract) 100 mg/kg | ||||||||
| Decreased liver weight and lipid accumulation in the liver | Cosmos caudatus Kunth | Leaf | Ethanol extraction | Quercetin, Catechin, Rutin, Chlorogenic acid | C57BL/6N | Supplementation with HFD | 10 weeks | Rahman et al. [37] |
| ND, ND + 175 mg/kgBW, ND + 350 mg/kgBW, HFD, HFD + 175 mg/kgBW, HFD + 350 mg/kgBW | ||||||||
| Melastoma malabathricum var Alba Linn | Whole plant | Methanol extraction | Epicatechin, Flavonoids | Sprague-Dawley rat | Supplementation with HFD | 8 weeks | Karupiah et al. [43] | |
| ND, HFD, HFD + 5% | ||||||||
| Moringa oleifera | Leaf | Petroleum ether extract | Isoquercitrin, Chrysin-7-glucoside, Quercitrin | C57BL/6N | Supplementation with HFD | 10 weeks | Xie et al. [40] | |
| ND, HFD + PC (Lovastatin) 10 mg/kg, HFD + 0.125 g/kg, HFD + 0.25 g/kg, HFD + 0.5 g/kg | ||||||||
| Nelumbo Nucifera L., Morus Alba L., Raphanus Sativus | Leaf, leaf, root | Ethanol extraction | Quercetin-3-O-glucuronide | C57BL/6J | Oral administration with HFD | 8 weeks | Sim et al. [39] | |
| ND, HFD, HFD + PC (Garcinia cambogia) 245 mg/kg, HFD + EM11 100 mg/kg, HFD + EM12 100 mg/kg, HFD + EM01 50 mg/kg, HFD + EM01 100 mg/kg, HFD + Q3OG 10 mg/kg | ||||||||
| Solidago virgaurea var. gigantea | Whole plant | Ethanol extraction | Protocatechuic acid, Chlorogenic acid, Rutin | C57BL/6J | Oral administration with HFD | 7 weeks | Wang et al. [38] | |
| ND, HFD, HFD + 1% PC (Garcinia cambogia extract), HFD + 0.5% of 10% ethanol extract, HFD + 2% of 10% ethanol extract | ||||||||
| Valeriana dageletiana Nakai ex F. Maek | Stem and leaf | Ethanol extraction | Camphene, |
Sprague-Dawley rats | Supplementation with HFD | 8 weeks | Wang et al. [42] | |
| ND, HFD, HFD + 1% PC (Garcinia combogia extract), HFD + 1% | ||||||||
| Viola mandshurica W. Becker | Whole plant | Ethanol and water extraction | Esculetin, Schaftoside | Sprague-Dawley rats | Oral administration with HFD | 11 weeks | Sung et al. [46] | |
| ND, HFD, HFD + 200 mg/kg of ethanol extract, HFD + 100 mg/kg of ethanol extract, HFD + 50 mg/kg of ethanol extract, HFD + 200 mg/kg of water extract, HFD + 100 mg/kg of water extract, HFD + 50 mg/kg of water extract, HFD + PC1 (Orlistat) 50 mg/kg, HFD + PC2 (Garcinia combogia extract) 100 mg/kg | ||||||||
| Reduced hepatic lipid metabolites | Solidago virgaurea var. gigantea | Whole plant | Ethanol extraction | Protocatechuic acid, Chlorogenic acid, Rutin | C57BL/6J | Oral administration with HFD | 7 weeks | Wang et al. [38] |
| ND, HFD, HFD + 1% PC (Garcinia cambogia extract), HFD + 0.5% of 10% ethanol extract, HFD + 2% of 10% ethanol extract | ||||||||
| Valeriana dageletiana Nakai ex F. Maek. | Stem and leaf | Ethanol extraction | Camphene, |
Sprague-Dawley rats | Supplementation with HFD | 8 weeks | Wang et al. [42] | |
| ND, HFD, HFD + 1% PC (Garcinia combogia extract), HFD + 1% | ||||||||
| ND, normal diet; HFD, high-fat diet; PC, positive control. | ||||||||
Anti-adipogenic effects on the liver have also been observed in medicinal plants. For example, extracts of Cosmos caudatus Kunth, Melastoma malabathricum var Alba Linn, Moringa oleifera, and a mixture of Nelumbo nucifera L., Morus alba L., and Raphanus sativus, Solidago virgaurea var. gigantea, Valeriana dageletiana Nakai ex F. Maek., and Viola mandshurica W. Becker decrease liver weight and lipid accumulation in the liver. In particular, Moringa oleifera reduces hepatic TG levels. Furthermore, several medicinal plants, including Solidago virgaurea var. gigantea and Valeriana dageletiana Nakai ex F. Maek, lower hepatic lipid metabolite levels, as measured using nuclear magnetic resonance. Both plants reduce the levels of fatty acids, cholesterol, phospholipids, and lipid moieties, which are induced by consumption of an HFD. Extracts of Cosmos caudatus Kunth or a mixture of Nelumbo nucifera L., Morus alba L., and Raphanus sativus, Solidago virgaurea var. gigantea, Viola mandshurica W. Becker, and Valeriana dageletiana Nakai ex F. Maek. were also used for evaluation of liver function and toxicity by analyzing aspartate transaminase, alanine transferase, and gamma-glutamyl transferase levels.
Similar to the results of in vitro studies, obesity-related mechanisms
and biomarkers have also been identified from in vivo studies (Table 5,
Ref. [38, 39, 40, 42, 44, 46]). Supplementation with extracts of Astilbe
chinensis Franch. et Savat. downregulates PPAR
| Mechanisms | Plants | Related organs | Related biomarkers | References |
| Adipogenesis and lipogenesis in adipose tissue and the liver | Astilbe chinensis Franch. et Savat. | Epididymal adipose tissue | PPAR |
Zhang et al. [44] |
| Moringa oleifera | Epididymal adipose tissue, liver | PPAR |
Xie et al. [40] | |
| Nelumbo Nucifera L., Morus Alba L., Raphanus Sativus | Liver, epididymal adipose tissue | PPAR |
Sim et al. [39] | |
| Adipose tissue, abdominal subcutaneous fat tissue | PPAR | |||
| Solidago virgaurea var. gigantea | Epididymal adipose tissue | PPAR |
Wang et al. [38] | |
| Liver | SREBP1c | |||
| Valeriana dageletiana Nakai ex F. Maek. | Epididymal white adipose tissue | PPAR |
Wang et al. [42] | |
| Liver | SREBP1c | |||
| Viola mandshurica W. Becker | Epididymal adipose tissue | C/EBP |
Sung et al. [46] | |
| Adipose tissue, abdominal subcutaneous fat tissue | UCP-2 | |||
| Lipolysis in adipose tissue and the liver | Astilbe chinensis Franch. et Savat. | Epididymal adipose tissue | ATGL |
Zhang et al. [44] |
| Moringa oleifera | Epididymal adipose tissue, liver | ATGL |
Xie et al. [40] | |
| Inhibitory effects of lipid accumulation by AMPK activation in adipose tissue and the liver | Astilbe chinensis Franch. et Savat. | Epididymal adipose tissue | Phospho-AMPK |
Zhang et al. [44] |
| Moringa oleifera | Epididymal adipose tissue, liver | Phospho-AMPK |
Xie et al. [40] | |
| Viola mandshurica W. Becker | Epididymal adipose tissue | AMPK |
Sung et al. [46] | |
| Liver | Phospho-AMPK |
The AMPK pathway is a well-known mechanism that regulates lipid metabolism.
Several medicinal plants have been evaluated to determine their ability to
regulate lipolysis through the activation of AMPK signaling in the adipose tissue
and liver of HFD-induced obese mice. Extracts of Astilbe chinensis
Franch. et Savat. promote AMPK pathways by increasing the protein levels of
phospho-AMPK, phospho-ACC, and PGC-1
Various species of medicinal plants originate from Asian countries, including
Korea, China, Japan, India, and Malaysia. Plants are generally extracted with
ethanol, and flavonoids, such as quercetin, as well as catechin, anthocyanins,
and other phenolic acids are the major components of these plants. The effects of
Asian medicinal plants on obesity have been examined through many in
vitro and in vivo studies. In this study, we found that eight types of
Asian medicinal plants, including Acer okamotoanum Nakai,
Astilbe chinensis Franch. et Savat., Cirsium setidens Naki,
Cornus kousa, Dendropanax morbifera, Moringa oleifera,
a mixture of Nelumbo nucifera L., Morus alba L., and
Raphanus sativus, and Solidago virgaurea var. gigantea, reduce
intercellular lipid accumulation and decrease the sizes and numbers of lipid
droplets during adipogenesis. These plants also inhibit lipogenesis or promote
lipolysis in 3T3-L1 cells. The major transcription factors related to
adipogenesis and lipogenesis are PPAR
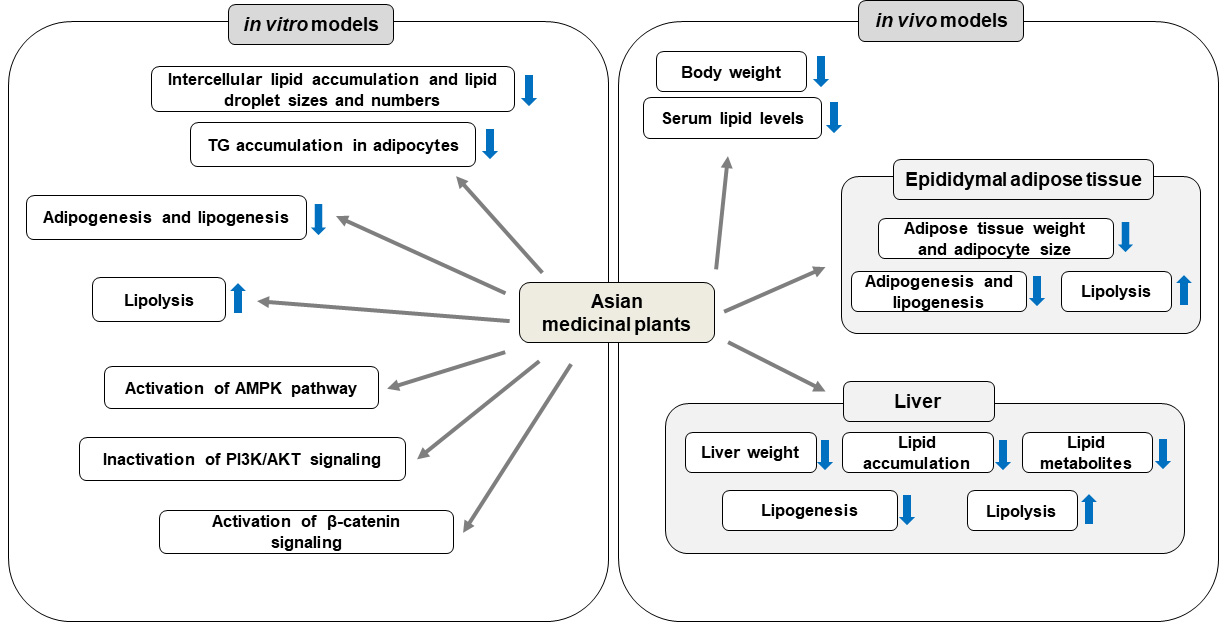 Fig. 1.
Fig. 1.Anti-obesity effects of Asian medicinal plants and their
underlying mechanisms in vitro and in vivo models. Asian
medicinal plants inhibit fat accumulation and promote lipolysis by AMPK
activation, PI3K/AKT signaling inactivation, and activation of
UCP1, uncoupling protein 1; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; C/EBP, CCAAT/enhancer binding protein; PPAR, peroxisome proliferator-activated receptor; FAS, fatty acid synthase; FABP4, fatty acid binding protein 4; aP2, adipocyte protein 2; SREBP, sterol regulatory element binding protein; ACC, acetyl-CoA carboxylase; CPT-1, carnitine palmitoyl transferase-1; LPL, lipoprotein lipase; SCD, stearoyl-CoA desaturase; HSL, hormone-sensitive lipase; AMP, adenosine monophosphate; AMPK, AMP-activated protein kinase; PGC, peroxisome proliferator-activated receptor gamma coactivator; ATGL, adipose triglyceride lipase; PI3K, phosphatidylinositol-3 kinase; mTOR, mammalian target of rapamycin; p70S6K, phosphorylation of ribosomal protein S6 kinase; HFD, high-fat diet; TC, total cholesterol; UPC, uncoupling protein.
JTH received a review invitation; SC and JTH developed the research question, and SC and SHP collected and screened the relevant articles; JHP and JTH selected the final articles and extracted the data from the articles; SC wrote the manuscript; All authors critically reviewed the manuscript and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This study was funded by a research grant from the Korea Food Research Institute (Project Number: E0210601), Republic of Korea.
The authors declare no conflict of interest.
