Atrial fibrillation is the most common symptomatic arrhythmia that is associated with stroke. Contemporary management of the disease is focused on anticoagulation to prevent stroke, coupled with catheter ablation to limit symptoms and prevent deleterious cardiac remodeling. Emerging data highlights the importance of lifestyle modification by managing sleep apnea, increasing physical activity, and weight loss. There is significant data that supports a link between the autonomic nervous system, arrhythmia development, and atrial fibrillation therapy. It is likely that lifestyle modification through these techniques that are aimed to reduce stress may also mediate atrial fibrillation development through this mechanism. This review examines how mind and body practices such as meditation, yoga, and acupuncture may influence the autonomic nervous system and mitigate atrial fibrillation progression and regression. Available evidence from molecular and anatomical levels through to clinical observations and translational clinical trials were scrutinized and a case established for these interventions as potential powerful mediators of anti-arrhythmic benefit.
Atrial fibrillation (AF) is a disease with widespread public health impact. It is one of the most common clinical rhythm abnormalities. Major clinical sequelae include cardiac dysfunction, mortality, stroke, declining quality of life, recurrent hospitalization, and increase hospitalization costs (Patel et al., 2014). Although stroke is one of the most feared complications of this arrhythmia, it is more heavily-influenced by comorbidities such as diabetes, hypertension, vascular disease, age, gender and ventricular dysfunction (Lip et al., 2010). Current therapeutic strategies such as anti-arrhythmic medication and ablation have not demonstrated a reduction in stroke where anticoagulation is the mainstay of therapy in mitigating this complication. Treatment of the arrhythmia is focused on symptomatic improvement and prevention of a cardiomyopathy from elevated heart rates.
Historically, the development of AF is likely driven via multiple etiological mechanisms, and it was accepted that there was a relentless continuum of disease (Crandall et al., 2009). Episodes of paroxysmal AF, triggered by focal electrical activity predominantly in the thoracic veins, were considered to begin erratically; and then become more frequent and prolonged until intervened. The initiating foci from the thoracic veins have been linked to the ANS via the cardiac ganglia/innervation of the heart, spurring intense interest in modulation of the ANS to prevent or limit AF and other cardiac arrhythmias (He et al., 2016; Lu et al., 2016; Madhavan., 2016).
It is now accepted that the ANS plays a fundamental role in the development of the arrhythmia, but also in its maintenance through variable feedback loops that may further perpetuate AF progression (Linz et al., 2019). Repeated episodes of AF have traditionally been considered sufficient to engender structural changes/remodeling in the left atrium through impacting atrial mechanical transport, stasis and subsequent chamber dilatation (Crandall et al., 2009). Left atrial dilatation (Blume et al., 2011) has been considered a major deleterious step towards a persistent AF phenotype with intrinsic substrate changes that create a milieu for more AF (Wijffels et al., 1995). Recently, data has emerged that contradicts this dictum whereby significant lifestyle modification - entailing weight loss and a regular exercise program - can prevent the development of AF (Pathak et al., 2014, 2015). Additionally, lifestyle intervention has also been associated with clinical improvements whereby persistent forms of AF can regress to a paroxysmal phenotype where the disease resolves entirely (Middeldorp et al., 2018). Whether additional lifestyle interventions can have a similar salutary effect on AF remains to be seen. From a mechanistic point of view, there are multiple similarities between diet/exercise and mind/body practices and how they affect the ANS, influence blood pressure, and mitigate emotional triggers for AF.
Interestingly, exercise training results in similar cardiac autonomic profiles to other lifestyle interventions such as meditation, yoga, and acupuncture (Sztajzel et al., 2008). In addition to influencing the perception and development of stress, these “de-stressing” techniques appear to modulate autonomic reflexes through sympathetic and vagal manipulation - a paradigm that is crucial to arrhythmia development. This is through suppression of sympathetic outflow to the heart that results in termination of AF episodes (Chen et al., 2014). Targeting of the ganglionated cardiac plexuses around the heart has been shown to reduce AF which further shows that selective autonomic modulation can directly trigger it (Kapa et al., 2010).
This review addresses whether current mind/body practices sufficiently influence the ANS to mediate a similar anti-arrhythmic effect (Nijjar et al., 2014; Park et al., 2014) to other lifestyle modifications and more direct autonomic stimuli. Additionally, the anatomical and physiological underpinnings of the cardiac ANS are reviewed; complex interplay between emotional stresses, the nervous system, and arrhythmia development are examined; and mind-body techniques known to influence the ANS such as yoga, meditation, and mindfulness are reviewed.
The ANS, cardiac innervation of the heart, is traditionally divided into the extrinsic nerves that connect between the central nervous system and the heart; and the intrinsic nerves that reside within pericardial sac (Witt et al., 2017).
The extrinsic ANS is divided into sympathetic and parasympathetic nervous systems, which are composed of preganglionic neurons that originate in the central nervous system (CNS) and synapse in autonomic ganglia. From this relay point, postganglionic neurons form the superior, thoracic, and inferior cardiac nerves that terminate within the cardiac plexuses in and around the heart structures. (Fig. 1) (Rosenblueth and Simeone, 1934). The sympathetic nervous system preganglionic neurons originate in the intermediolateral column of the thoracic spinal cord and synapse on the paravertebral ganglia: superior cervical ganglion (C1-C3), stellate or cervicothoracic ganglion (C7-8 - T1-2), and thoracic ganglia (down to T7). The parasympathetic nervous system preganglionic fibers originate in the nucleus ambiguus of the medulla oblongata and travel together as the vagus nerve, which divides into superior, middle, and inferior branches (Witt et al., 2017).
 Figure 1.
Figure 1.Extrinsic and intrinsic autonomic nervous system, reprinted from Witt et al., (2017) by permission of Oxford University Press
There are multiple ganglia (up to 1500) embedded on the surface of the heart that connect with extrinsic ANS branches. These ganglia are grouped into ganglionated plexuses that integrate signals from the intrinsic and extrinsic ANS. Ganglionated plexuses (GP) are clustered in specific locations within the heart. The pulmonary vein ganglia is notable for dense autonomic innervation with many GP that are fundamental to the neural/electrical development of the pulmonary vein arrhythmogenic triggers (Kapa et al., 2010).
The ANS’s influence on the heart is one of fundamental opposition at the level of target organs with sympathetic “fight or flight” and vagal “rest and digest” control mechanisms. Despite this traditional understanding of the ANS, the mechanism of this system is more complex. Under sympathetic tone, it has been observed that responses to vagal activity have a more pronounced decrease in heart rate and contractility (Rosenblueth and Simeone, 1934; Samaan, 1935).Additionally, it has been observed that the parasympathetic nervous system directly modulates sympathetic activity via vagal influences on preganglionic and postganglionic sympathetic neurons (Shen and Zipes, 2014).
The sophistication involved in autonomic innervation is evidenced in the mixed nature of sympathetic and parasympathetic fibers found in the extrinsic ANS. Immunohistochemical staining shows that most stellate ganglion cells of the sympathetic nervous system produce tyrosine hydroxylase that suggest catecholamines are the primary neurotransmitter. Detailed studies have shown that some cells within the stellate ganglion produce acetylcholine. Interestingly, similar mixed signaling has been discovered with respect to the vagus nerve (Chen et al., 2014).
Additional intricacy is recognized by the changing nature of the autonomic control of the heart. Like all nerves, there is constant remodeling in response to changing conditions, especially in disease states. For example, following a myocardial infarction atrial and ventricular nerve sprouting demonstrates how a localized infarct can lead to a more generalized change in autonomic activity with clinical implications (Chen et al., 2014).
The effect of the ANS on cellular physiology to drive atrial arrhythmias is complex and appears to converge through all arrhythmogenic mechanisms-increased automaticity, triggered automaticity, and re-entry. Increases in sympathetic or decreases in parasympathetic activity lead to increased automaticity. β2 receptor activation has been shown to increase If, also known as the phase 4 depolarizing current. In contrast, acetylcholine decreases automaticity by inhibiting If (decreases pacemaker activity) and activating IK,Ach (further mitigate the rate of phase 4 depolarization) (DiFrancesco, 2010). While this represents normal cardiac physiology, exaggerated or sustained responses may provide a substrate for triggered activity and/or allow for maintenance of AF after triggered/re-entrant initiation of AF (Chen et al., 2014). β-adrenergic activity also increases ICa-L, which lengthens the phase 2 plateau of the action potential. This can increase the risk of phase 2 early after depolarizations and trigger AF in the setting of increased automaticity (Chen et al., 2014).
Simultaneous activation of sympathetic and parasympathetic systems may also predispose to triggered arrhythmias via other mechanisms. Episodes of atrial tachycardia and fibrillation have been linked to activity within the left stellate ganglion and vagus nerve (Shen and Zipes, 2014). Sympathetic outflow can increase presence of transient intracellular calcium that leads to membrane depolarization. Concurrent vagal activity activates the potassium channel (IKACh), which enhances phase 3 repolarization, decreases action potential duration, and shortens the atrial refractory period. This combination provides a nidus for phase 3 - early afterdepolarizations that can trigger AF (Shen and Zipes, 2014). This phenomenon of AF generation due to ectopy is particularly prominent in and around the pulmonary veins making it an established target for intervention (Kapa et al., 2010).
Delayed afterdepolarizations can also trigger arrhythmogenesis via calcium leak from the ryanodine receptor. Sympathetic activity via the β-adrenergic axis leads to increased calcium loading of the sarcoplasmic reticulum, as well as increased ryanodine receptor open probability that leads to an increased risk of calcium leak. Elevated calcium levels in diastole lead to membrane depolarizations that cause delayed afterdepolarizations and triggering AF (Chen et al., 2014).
Finally, myocardial electrical reentry, also colludes as a mechanism that can trigger AF. Vagal tone can decrease the action potential duration and refractory period. This change in refractory period is variable in different regions of the heart due to baseline differences in action potential morphology as well as heterogeneous influence of vagal tone (Chen et al., 2014).Furthermore, calcium/calmodulin activity stimulated by β-adrenergic activity can cause hypertrophic/fibrotic gene activity creating even more variability in regional electrical activity. This variability along with the ectopic activity previously discussed provides the ideal substrate for triggered reentry (Chen et al., 2014).
Mental/psychological stress can have a significant effect on cardiovascular function, health and disease. It is clear that high levels of stress can adversely affect cardiovascular health from functional changes such as stress-induced cardiomyopathy (Sharkey et al., 2005) and stress-induced ischemia (Gullette et al., 1997) to decreased recovery from cardiac events (Xu et al., 2015).
From an electrophysiological standpoint, stress continues to play a significant role in contributing to arrhythmia development with predisposition toward ventricular arrhythmia (Lampert, 2010). Within the realm of AF, there is evidence to suggest that stress can be a predisposing factor (Mattioli et al., 2008). Recently, an association between negative emotion and development of symptomatic AF has been demonstrated (Lampert et al., 2014). Furthermore, patients with AF experiencing stress have been shown to have worse outcomes including greater AF symptom severity, quality of life, and recurrence (McCabe, 2010). At a cellular level, acute mental stress has been shown to influence the atria electrically suggesting that stressful situations promote adverse transient changes that engender an increased susceptibility towards AF (O’Neal et al., 2017). Similarly, stimulation of the sympathetic nervous system has been directly shown to affect the electrical refractory periods of the atrium, a fundamental described above as a precipitator for arrhythmogenesis and AF (Chen et al., 2014) and a standard feature of psychological and mental stress.
Given the significant role that the ANS plays in the development and maintenance of AF, modulation or disruption of this influence may provide some therapeutic benefit in the prevention or treatment of AF. Consequently, multiple invasive treatment modalities have been developed to alter autonomic tone for arrhythmia management. Surgical renal denervation has been shown to reduce AF in animal models (Kosiuk et al., 2015). Pulmonary vein isolation with catheter-based ganglionated plexus ablation (Kapa et al., 2010) and surgical ablation have been demonstrated to benefit and treat human patients with AF (Huffman et al., 2016).
Transvenous vagal nerve stimulation has been experimentally evaluated to determine its role in modulating the ANS. Low-level vagal nerve stimulation (LL-VNS) has been shown to be effective in reducing the potential for AF induction via rapid atrial pacing (Shen et al., 2011).Previously thought to be related to vagal nerve stimulation that inhibits sympathetic activity at pre- and postganglionic levels, LL-VNS has been shown to acutely inhibit left stellate ganglion activity and chronically potentiate an effect when active. During chronic stimulation, tyrosine hydroxylase staining is reduced, calcium-activated K channels appear to be upregulated, and choline acetyltransferase is commensurately increased (Shen and Zipes, 2014). At a cellular level, this appears to mimic a phenotype shift from sympathetic to parasympathetic within the sympathetic ganglion. This type of basic investigation is still necessary for non-invasive mind-body techniques to better define whether these practices can be utilized independently or as adjuncts in the care of patients and management of arrhythmia. Currently, these interventions provide varying levels of efficacy, and given their invasive nature, retain associated risks and potential for side effects (Ganesan et al., 2013; Huffman et al., 2016).
Psychological stress has been definitively linked with higher levels of circulating inflammatory markers such as interleukin-6, cortisol, and catecholamines (Daubenmier et al., 2011; Fischer, 2006; Jankord et al., 2010; Joseph and Golden, 2017; O’Donovan et al., 2010; Tracey, 2002).These pro-inflammatory cytokines are intimately linked with worse cardiovascular outcomes especially in patients with AF (Aulin et al., 2015), but also appear to be key to the development of the arrhythmia (Markousis-Mavrogenis et al., 2019; Van Wagoner and Chung, 2018; Yao et al., 2018). Meditation and yoga have been shown to directly reduce circulating stress hormones (Brown et al., 2012; Lim and Cheong, 2015; Nyklíček et al., 2013), and could plausibly, through this alternate mechanism, affect AF development, maintenance, and recurrence.
Excessive alcohol use has been clearly linked with the development of AF (Lowenstein et al., 1983; Voskoboinik et al., 2016). Recently, it has been shown that abstinence from alcohol in moderate drinkers (persons who consume ≥ 10 standard drinks/week) with AF have decreased AF burden and greater time to first recurrence (Voskoboinik, 2019). Both mindfulness and transcendental meditation have been shown to eliminate alcohol use, which suggest the possibility of using these techniques in risk factor management for AF development or recurrence (Carpentier et al., 2015; Gryczynski et al., 2018).
The National Center for Complementary and Integrative Health (NCCIH) uses the term “complementary health approaches” to refer to practices and products that are not conventional or originate outside standard Western medical practice. Among the most common complementary health approaches include deep breathing and mind and body practices such as Yoga/Tai Chi/Chigong, chiropractic/osteopathic manipulation, and meditation (National Center for Complementary and Integrative Health, 2011). Mind and body practices include a large group of techniques that are usually taught to participants by a trained practitioner. Examples include acupuncture, massage therapy, meditation, relaxation techniques, spinal manipulation, and yoga (Fig. 4) (National Center for Complementary and Integrative Health, 2011; Viscuse et al., 2017). The core approach of these techniques is an emphasis on focus, mindfulness, and relaxation to enhance overall wellbeing (Goyal et al., 2014; National Center for Complementary and Integrative Health, 2006). While these mind and body practices have been mainly studied in the treatment of chronic pain (Achilefu et al., 2017), depression (Cramer et al., 2017), and stress reduction (Chong et al., 2011) there is growing interest in evaluating how they can be used outside the psychological domain.
Transcutaneous stimulation to the tragus has been developed and shown to stimulate the auricular branch of the vagus nerve in animal models (Yu et al., 2011). In animal studies, low-level vagal nerve stimulation (defined as stimulation at 80% of the threshold needed to decrease heart rate) to the tragus tissue of the ear was shown to decrease the ability to clinically induce AF in dogs using rapid atrial pacing (Yu et al., 2011). A recent randomized, single blind proof-of-concept human study of about 40 patients (20 sham control and 20 intervention) similarly showed that low-level stimulation of the tragus increased the absolute effective refractory period, increased the number of attempts required to induced AF, and decreased the duration of pacing-induced AF (Park et al., 2015). Similarly, acupuncture to three established cardiac-specific acupuncture points (Neiguan, Shenmen, and Xinshu points) (Chen et al., 2014). Park et al. (2015) has been shown as a noninvasive way to decrease AF recurrence that is similar to taking amiodarone in patients with persistent AF after cardioversion (Fig. 2). A study of 80 patients with AF was studied after undergoing electrical cardioversion. Patients were divided into amiodarone reference (n = 26), Acupuncture (n = 17), sham acupuncture (n = 13), and control group without antiarrhythmic or acupuncture therapy (n = 24). The acupuncture group received weekly sessions for 10 weeks and AF recurrence was followed for one year. Cumulative AF recurrence rates were tracked and shown to be 27%, 35%, 69%, and 54% in the amiodarone, acupuncture, sham, and control groups, respectively. Mechanistically, it is suggested that sensory stimulation of those areas may influence sympathetic nerve activity as the cell bodies innervating the skin can be found in the stellate and middle cervical ganglia (also known to provide cardiac innervation) (Fig. 3) (Lomuscio et al., 2011). This has served as a foundation for a much larger multicenter, prospective, participant and assessor blinded, randomized, sham-controlled clinical trial (ACU-AF) to assess acupuncture efficacy in patients with persistent AF - the results of which are still pending (Park et al., 2015).
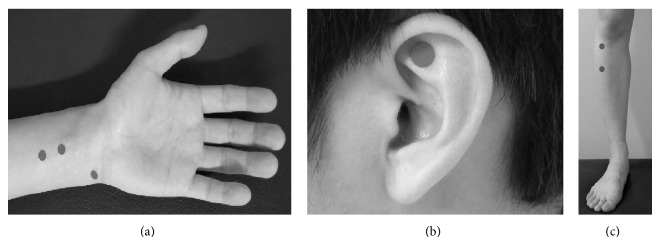 Figure 2.
Figure 2.Acupuncture sites used in ACU-AF trial (A) PC5, PC6, and HT7. (B) TF4. (C) ST36 and ST37. Reprinted from Park et al. (2015) which was published under a Creative Commons license.
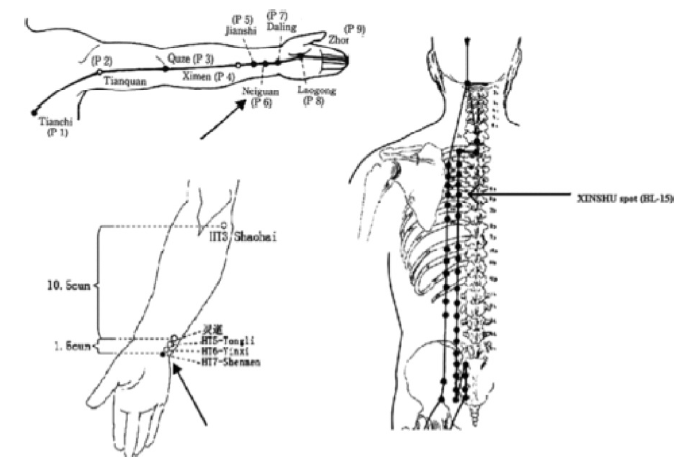 Figure 3.
Figure 3.Acupuncture needling points, reprinted from Lomuscio et al. (2011) Copyright 2010 by Wiley Periodicals, Inc. Reprinted by permission of John Wiley & Sons, Inc.
 Figure 4.
Figure 4.The five domains of complementary and alternative medicine, Reprinted from Viscuse et al. (2017) by permission of Wolters Kluwer Health, LWW. https://journals.lww.com/co-oncology/Abstract/2017/07000/Integrative_medicine_in_cancer_survivors.4.aspx
Yoga stems from ancient Indian philosophy utilizing physical movements, breathing, and meditation to improve well-being. There are a number of different schools and styles of practice that make it difficult to classify; however, the role of hatha yoga with its emphasis on physical postures has been explored as an adjunct to western medicine (Pascoe and Bauer, 2015).
In patients with symptomatic AF, a modest, but clinically significant improvement in a variety of AF parameters was recognized following a three-month yoga intervention (Fig. 5). The Yoga My Heart trial was a single center, prospective cohort study in which participants served as their own controls. A total of 49 of the original 52 that were enrolled completed the study that required a three-month control portion followed by a three-month yoga intervention phase in which they were asked to participate in a structured, 60-minute Iyengar yoga session in a studio twice-a-week. They were also encouraged to practice using a DVD at home on their own. The study demonstrated statistically significant reductions of symptomatic AF episodes, non-symptomatic AF episodes, heart rate, blood pressure, anxiety, and depression scores. Moreover, improvements in several markers of quality of life were observed. Though the notable improvement was small, this was the first example of a study that showed the effects of mind and body practices on hard, clinical endpoints in AF (Lakkireddy et al., 2013). Likewise, another 12-week intervention of mediyoga, a form of yoga adapted from Kundalini yoga specifically for patients with cardiac disease, has been associated with improvements in quality of life as measured by two validated quality of life instruments. Importantly from a mechanistic standpoint, this study showed a significant decrease in heart rate and blood pressure in the yoga group (Wahlstrom et al., 2017).
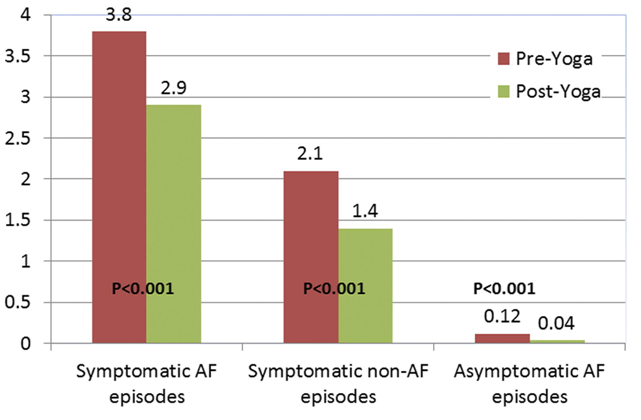 Figure 5.
Figure 5.Data from the Yoga My Heart trial, Reprinted from (Lakkireddy et al., 2013) with permission from Elsevier
It is now established that the parasympathetic axis can alter meditation (Nijjar et al., 2014; Krygier et al., 2013; Peressutti et al., 2012). Accurate quantification of functional changes in autonomic neural control requires complex invasive assessments of nerve electrical activity; hence, heart rate variability has been used as a surrogate for the underlying sympathovagal balance (Grossman and Taylor, 2007). To more carefully assess fluctuations in heart rate variability, spectral analyses can be calculated from changes in the RR interval sequence. From these spectral analyses, it is evident that high frequency fluctuations in heart rate are likely mediated by the parasympathetic nervous system, whereas low frequency fluctuations were more likely to be mediated by sympathetic and parasympathetic activity (Pomeranz et al., 1985). Using this surrogate, a variety of different forms of meditation have been evaluated including mindfulness-based stress reduction, Vipassana meditation, Iyengar Yoga, transcendental meditation, and inward attention meditation (Khattab et al., 2007; Krygier et al., 2013; Nijjar et al., 2014; Wu and Lo, 2008). These practices demonstrated that mind-body intervention increases cardiac parasympathetic nervous modulation as evidenced by changes in heart rate variability. Additionally, there is evidence to suggest that long-term treatment with meditation can induce sustained changes in the ANS. A small study was conducted on 20 subjects with meditation experience ranging from two months to 35 years to evaluate the role that the amount of cumulative meditations measured by fluctuations in heart rate variability can affect ANS. The authors suggest that overtime, meditation can shift the ANS to a more orderly operational state (Peressutti et al., 2012). This suggestion of a dose-response and shift toward organization of the ANS could have profound implications if the effect were able to be harnessed in the treatment of AF. Higher quality studies that evaluate these practices and role in AF need to be conducted before further conclusions can be drawn.
While influence of heart rate variability can be a reasonable marker of the influence of mind and body practices on autonomic activity, it is important to note that this parameter can be used as a prognostic marker for poor health outcomes. It is affected in pro-inflammatory states (Frasure-Smith et al., 2009), worsening ischemia/coronary disease (Hayano et al., 1991), and an established predictor of increased mortality after acute MI (Cripps et al., 1991; Kleiger et al., 1987).
Many of the studies that evaluated the role of mind and body practices in Atrial fibrillation treatment is small and not adequately powered, which makes it difficult to make recommendations on clinical adoption. There are methodological concerns with many of the evaluated studies and the heterogeneous nature (with respect to study design and intervention used) makes it difficult for systematic reviews or meta-analyses. These studies; however, are valuable because they raise new questions. Preliminary findings may lead to new, more promising avenues of research. Well-designed trials with adequate sample sizes, control groups, and appropriate blinding is still needed if mind-body practices are to move forward into the mainstream of anti-arrhythmic therapy.
Given the high prevalence of AF, the absence of cure and the risks of current medication and invasive treatments, there is a real need for adjunctive therapies in addition to previously identified disease-modifying lifestyle interventions. A clear role for the ANS in the initiation and maintenance of AF has been established and the biological underpinnings reviewed. Current literature has now established that the development of AF is not an endpoint; it can be altered, reduced, and even resolved. Minimizing sympathetic or increasing parasympathetic input appears to play a role in reducing the development of AF through cellular/anatomical remodeling. Acupuncture may be able to mimic some of these changes. Mindfulness-based interventions have been shown to shift the sympathovagal balance toward predominantly parasympathetic states. Symptomatic and improved quality of life have also been demonstrated. More rigorous randomized, blinded trials evaluating the biological signaling components are necessary if these complementary therapies are to be validated and adopted.
The authors acknowledge institutional support from the Mayo Clinic and from the Department of Medicine and the Department of Cardiovascular Diseases.
The authors declare that there is no conflic of interest regarding the publication of this article.
