1 Department of Neurology, Jiangbin Hospital, 530021 Nanning, Guangxi, China
Abstract
Background: Most acute cerebral infarctions (ACI) may
develop vascular dementia (VD), which involves almost all types of cognitive
impairment. Unfortunately, there is currently no effective treatment for VD. Most
patients exhibit mild cognitive impairment (MCI) before the development of VD.
N-butyl-phthalide (NBP) is used to treat ACI and improve cognitive function. The
oxygen and glucose deprivation (OGD) model of neurons is an in vitro
model of ischemia, hypoxia, and cognitive dysfunction. Methods: We
conducted clinical studies and in vitro experiments to investigate the
clinical efficacy and mechanism of action of NBP for treating ACI-induced MCI.
Patients with ACI-induced MCI were randomly divided into control (Ctrl) and NBP
groups. We assessed various indicators, such as clinical efficacy, montreal
cognitive assessment scale (MOCA), activities of daily living (ADL), and cerebral
infarct size in both groups before and after treatment. We observed the
morphology of neurons and detected the survival rate, action potentials (APs),
expression of high mobility group box 1 (HMGB1), toll-like receptor 4 (TLR4),
interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-
Keywords
- acute cerebral infarction
- mild cognitive impairment
- glucose and oxygen deprivation
- HMGB1
- toll-like receptor 4
- action potentials
The morbidity rate of cognitive impairment in patients with acute cerebral infarction (ACI) is 41% [1]. Most patients may develop vascular dementia (VD), which involves almost all types of cognitive impairment [2]. Unfortunately, no effective treatment for VD is found. Before the development of VD, most patients exhibit mild cognitive impairment (MCI) [3]. The clinical symptoms of MCI are mainly a progressive decline in memory or other cognitive functions, such as computation, orientation, attention, and executive function; however, they do not affect daily life functioning. Cognitive impairment associated with MCI is inconsistent with age and educational level, and the disease does not meet the diagnostic criteria for dementia [4, 5]. Lamb et al. [6] confirms that early treatment of patients with MCI can preserve cognitive ability and reduce the rate of progression to dementia.
The pathogenesis of ACI-induced MCI remains unclear. ACI can cause cognitive
impairment because it leads to cerebral ischemia and hypoxia. Neurons are in a
state of hypoxia and hypoglycemic metabolism, which triggers a cellular
inflammatory response [7]. The toll-like receptor 4/high mobility group box 1
(TLR4/HMGB1) pathway, containing TLR4 and HMGB1 proteins, is a
crucial mechanism and pathway of cellular inflammation and is the primary
molecular mechanism of cognitive impairment [7]. During
ischemia and hypoxia state, the TLR4/HMGB1 pathway can be activated [4], which
promotes the release of inflammatory transmitters, including interleukin-6 (IL-6)
and tumor necrosis factor-
N-butyl-phthalide (NBP) is a safe, fat-soluble drug used to treat ACI and improve cognitive function [10]. Nevertheless, it is not clear whether NBP inhibits inflammation by acting on the TLR4/HMGB1 pathway. To identify an effective therapy for MCI and to study the pathogenesis of MCI, we treated ACI patients with MCI and explored the relationship between TLR4/HMGB1 and MCI. NBP has been used to treat patients with ACI and MCI, demonstrating that it can improve cognitive function in these patients. Furthermore, we developed an oxygen and sugar deprivation (OGD) model using primary cultured neurons to investigate the effects of NBP on TLR4/HMGB1, which has been proven to be a model of ischemia hypoxia [11] and cognitive dysfunction [12]. Additionally, we explored the mechanism of HMGB1-TLT4 and the protective effect of NBP on neurons in the OGD model.
This was a case-control study. From March 2019 to March 2020, 86 patients who were hospitalized in the Neurology Department of Jiangbin Hospital and initially diagnosed with ACI-induced MCI were enrolled in our study. The diagnosis of ACI conforms to the diagnostic criteria of the Guidelines for The management of Ischemic Stroke and Transient Ischemic Attack 2008 [13]. All patients had evidence of ACI on nuclear magnetic resonance (MRI) or computed tomography (CT). Based on the results of MRI/CT, the territories of cerebral infarct were divided into three, the territory of the anterior cerebral artery (ACA), the territory of the middle cerebral artery (MCA) and the territory of posterior circulation (PC). In this study, all patients had a single territory of cerebral infarct. The diagnosis of MCI follows Petersen et al.’s criteria [14]. The exclusion criteria were as follows: patients with multiple territories of cerebral infarct; patients with diabetes mellitus, malignant tumor, metabolic disorders, recurrent stroke, or massive ischemic stroke with severe impairment of consciousness. This study adhered to the principles of the Declaration of Helsinki. Ethical approval was granted by the institutional review board of Jiangbin Hospital (No. LW-2022-09). All patients were randomly divided into the control (Ctrl) and NBP groups. Informed consent was obtained from patients or their guardians.
Patients in both groups received conventional symptomatic treatment, including blood pressure and blood glucose control, hypolipidemic, anticoagulants, and microcirculation improvement drugs, supplemented with cognitive function rehabilitation training. Patients in the Ctrl group received a placebo. Patients in the NBP group received NBP soft capsule (H20050299, CSPC-NBP Pharmaceutical Co., Ltd., Shijiazhuang, Hebei, China) (0.2 g orally thrice daily). Efficacy was evaluated after eight weeks of therapy.
The brains of the two groups were scanned by MRI (GE Signa HDXT 1.5T MR, General
Electric Company, Boston, MA, USA)/CT (SIEMENS SOMATOM Definition FLash CT,
SIEMENS, Amberg, Germany) before and after treatment, and the changes in lesions
were compared. The montreal cognitive assessment scale (MOCA)
was used to assess cognitive function in both the groups before
and after treatment. Cognitive function was evaluated based on memory, attention,
orientation, language impairment, naming ability, visual-spatial executive
ability, abstract thinking, and delayed memory. A total score of 30, 26, or above
was considered normal; 18–26, MCI; 10–17, moderate impairment; and
Neurons (CP-R107, Procell Life Science & Technology Co., Ltd., Wuhan, Hubei, China) were cultured according to the protocol described by Wang et al. [15]. Leica microscopes (DMi1, Leica, Shanghai, China) were used to observe cell morphology. OGD model was performed following the method of Tasca et al. [16]. After 24 h of exposure to OGD, neurons were replaced with normal media. The cells were divided into OGD and OGD + NBP groups. The drugs and concentrations added to each group were as follows: no drugs were added to the OGD group, and NBP was added to the OGD + NBP group at concentrations of 0.1, 1, 10, 50, and 100 µM. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit (AR1156, Boster, Wuhan, Hubei, China) was used to calculate the cell survival rate after 24 h. The optimal concentration was selected for subsequent experiments.
The patch-clamp system was used to obtain the action potentials (APs). The primary method described by Li et al. [17]. The amplitude and frequency of neuronal APs were collected and analyzed.
Protein extraction, protein concentration detection, sodium dodecyl-sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), membrane transfer, antibody
incubation, and visualization of immunoreactive bands were performed as described
by Li et al. [18]. The primary antibodies used were
anti-TLR4 antibody 1:1000 (ab8376, Abcam, Shanghai, China),
anti-HMGB1 antibody 1:1000 (ab190377, Abcam, Shanghai, China), anti-interleukin
(IL-6) antibody 1:1000 (ab259341, Abcam, Shanghai, China), anti-tumor necrosis factor-alpha (TNF-
Protein extraction, protein concentration detection, construction of antigen-antibody complexes, and immunoblotting (IB) were performed as described by Li et al. [18]. The antigen-antibody complex was constructed using an anti-TLR4 receptor antibody 1:30 (Abcam, ab252430). The primary antibodies for IB were anti-TLR4 antibody 1:1000 (Abcam, ab8376) and anti-HMGB1 antibody 1:1000 (Abcam, ab18256). The Immunoprecipitation (IP) protein band for TLR4 was used to normalize the pull-down protein levels obtained from Co-IP. The co-precipitated protein levels between each group were then compared. The original figures of Western Blot can be found in the Supplementary Materials-IP.
The fixation, penetration, and blocking of the neurons were based on the methods described by Li et al. [17, 18]. A multiplex fluorescent immunohistochemistry kit (abs50012, Absin, Shanghai, China) was used to stain neurons [18]. The primary antibodies for Immunofluorescence (IF) were anti-TLR4 antibody 1:100 (Abcam, ab8376) and anti-HMGB1 antibody 1:100 (Abcam, ab18256). A Leica 8.0 laser confocal microscope (TCS SP8, Leica, Wetzlar, Germany) was used to acquire fluorescence signals of TLR4 (green) and HMGB1 (red). The ImageJ plug-in (version 1.54b; University of Wisconsin, Madison, WI, USA) was used to calculate the Pearson’s correlation coefficients (PCCs) between TLR4 and HMGB1.
Statistical analysis was conducted using Statistical Package
for Social Sciences (SPSS, version 25.0, IBM, Chicago, IL, USA) software. The
measurement data are expressed as mean
The baseline characteristics were exhibited in Table 1. The Ctrl group included
20 male and 19 female participants aged between 43 and 78 (66.00
| Ctrl | NBP | |
| Male, n (%) | 20 (51.28) | 26 (48.72) |
| Female, n (%) | 19 (60.47) | 17 (39.53) |
| Age, years | 66.00 |
62.44 |
| Disease course, days | 5.97 |
60.9 |
| Hypertension, n (%) | 31 (79.49) | 33 (76.74) |
| Smoking, n (%) | 13 (33.33) | 16 (37.21) |
| ACA, n (%) | 12 (30.77) | 10 (23.26) |
| MCA, n (%) | 19 (48.72) | 28 (65.12) |
| PC, n (%) | 8 (20.51) | 5 (11.63) |
Abbreviation: Ctrl, control; NBP, N-butyl-phthalide; MCA, middle cerebral artery; ACA, anterior cerebral artery; PC, posterior circulation.
The NBP group comprised 26 male and 17 female participants aged between 44 and
76 (62.44
Before treatment, no significant differences in MOCA were observed between the
control and NBP groups. Table 1 presents the pretreatment composition of the Ctrl
group, including 0 normal patients, 0 patients with MCI, 13 with moderate
cognitive impairment, and 30 with severe cognitive impairment. After treatment,
there were 0 normal patients, 1 patient with MCI, 15 with moderate cognitive
impairment, and 28 with severe cognitive impairment in the Ctrl group (Table 2).
The pretreatment NBP group comprised 0 normal patients, 1 patient with MCI, 12
moderate cognitive impairment patients, and 24 severe cognitive impairment
patients. After treatment, there were 0 normal patients, 2 patients with MCI, 20
with moderate cognitive impairment, and 17 with severe cognitive impairment in
the NBP group. The Ctrl group achieved a MOCA score of 8.81
| Normal | Mild cognitive impairment (18–26) | Moderate impairment (10–17) | Severe impairment ( |
Total-population | |
| Ctrl-before treatment | 0 | 0 | 13 | 30 | 43 |
| Ctrl-after treatment | 0 | 0 | 15 | 28 | 43 |
| NBP-before treatment | 0 | 1 | 14 | 24 | 39 |
| NBP-after treatment | 0 | 2 | 20 | 17 | 39 |
| Ctrl | NBP | |||||
| Before treatment | After treatment | p value (before versus after) | Before treatment | After treatment | p value (before versus after) | |
| Memory | 0.81 |
0.86 |
0.16 | 0.64 |
1.23 |
0.00 |
| Attention | 0.81 |
0.84 |
0.32 | 0.69 |
1.85 |
0.00 |
| Orientation | 0.98 |
0.98 |
- | 0.90 |
1.18 |
0.00 |
| Language | 0.53 |
0.58 |
0.16 | 0.59 |
0.72 |
0.06 |
| Naming ability | 0.53 |
0.56 |
0.32 | 0.59 |
0.64 |
0.16 |
| Visual-spatial | 2.05 |
2.09 |
0.16 | 2.28 |
2.46 |
0.01 |
| Abstract thinking | 0.74 |
0.79 |
0.16 | 0.46 |
0.54 |
0.08 |
| Delayed memory | 2.05 |
2.12 |
0.08 | 2.28 |
2.44 |
0.03 |
| Total points | 8.51 |
8.81 |
0.00 | 8.44 |
10.79 |
0.00 |
Abbreviation: MOCA, montreal cognitive assessment scale.
| Normal (100) | Mild disorder (60–100) | Moderate disorder (42–60) | Severe disorder (20–40) | Disability ( |
Total-population | |
| Ctrl-before treatment | 0 | 12 | 17 | 4 | 10 | 43 |
| Ctrl-after treatment | 0 | 12 | 17 | 6 | 8 | 43 |
| NBP-before treatment | 3 | 4 | 19 | 2 | 11 | 39 |
| NBP-after treatment | 3 | 4 | 20 | 2 | 10 | 39 |
| Ctrl | NBP | |||||
| Before treatment | After treatment | p value (before versus after) | Before treatment | After treatment | p value (before versus after) | |
| Excrement | 5.93 |
6.05 |
0.32 | 5.26 |
5.51 |
0.16 |
| Urinate | 5.35 |
5.35 |
- | 4.74 |
4.74 |
- |
| Grooming/personal hygiene | 4.88 |
5.12 |
0.16 | 3.85 |
4.49 |
0.02 |
| Toileting | 5.35 |
5.47 |
0.32 | 4.74 |
5.00 |
0.16 |
| Eating | 4.88 |
5.12 |
0.16 | 3.46 |
3.97 |
0.04 |
| Transfer | 4.65 |
4.88 |
0.16 | 4.49 |
4.74 |
0.16 |
| Activity | 4.30 |
4.53 |
0.16 | 3.97 |
4.36 |
0.08 |
| Dressing | 4.19 |
4.53 |
0.08 | 3.59 |
3.97 |
0.08 |
| Up and down stairs | 4.65 |
4.77 |
0.32 | 4.49 |
4.74 |
0.16 |
| Bath | 3.26 |
3.37 |
0.57 | 3.59 |
3.59 |
- |
| ADL-total points | 47.44 |
49.19 |
0.00 | 42.18 |
45.13 |
0.00 |
Abbreviation: ADL, activities of daily living.
Before treatment, CT and MRI scans revealed distinct cerebral ischemic lesions
in both groups without statistically significant differences in the ischemic
area. The Ctrl group exhibited an infarct size of 276.81
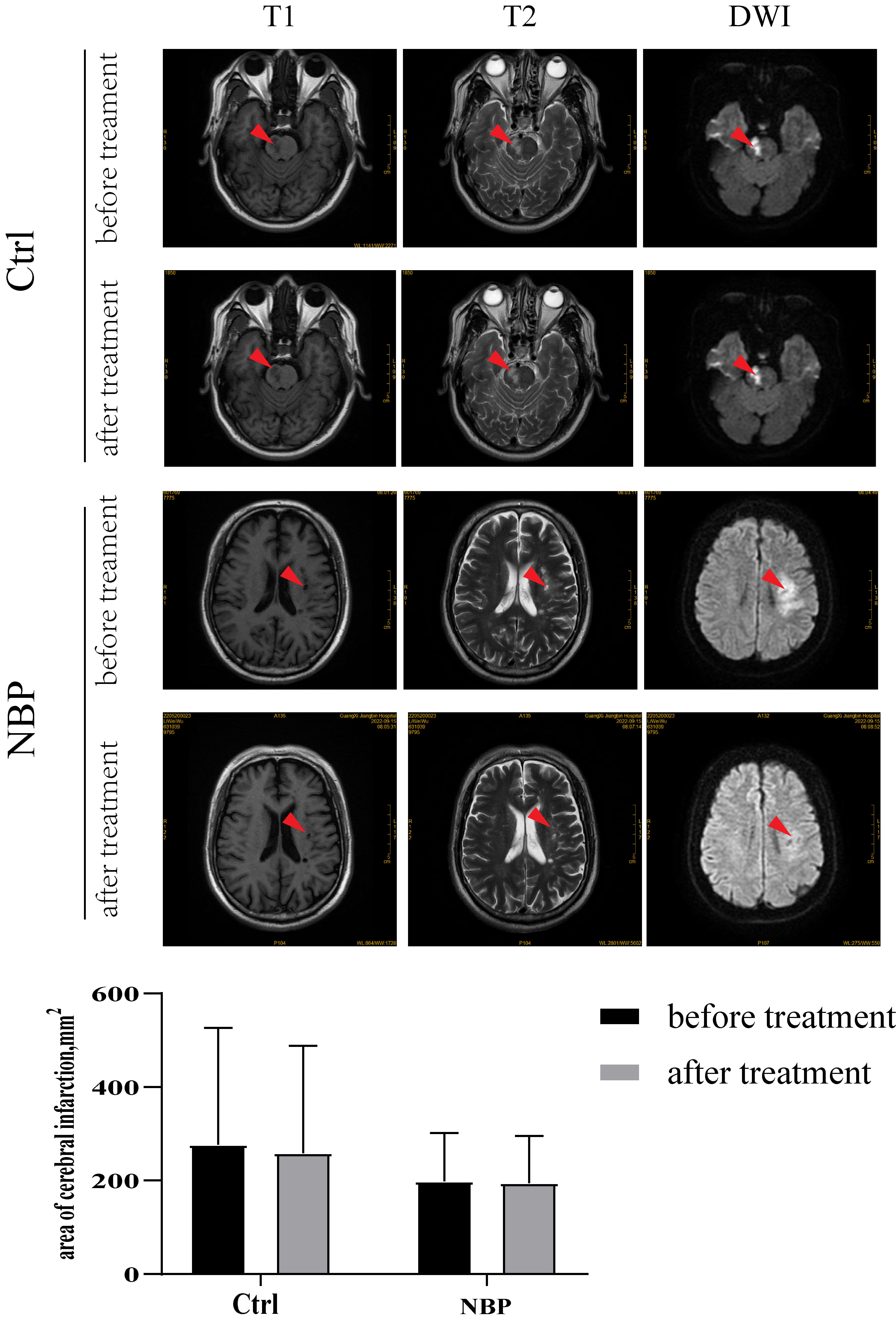 Fig. 1.
Fig. 1.
Changes in cerebral infarct size: the infarct size of the Ctrl
group was 276.81
In the OGD group, cellular edema was observed in the neurons, and their number
was also reduced. The OGD + 1 µM NBP, OGD + 10 µM NBP, and OGD + 50
µM NBP groups exhibited decreased edema and increased number of neurons
(Fig. 2A). To further clarify the protective effects of NBP on neurons, we
performed an MTT assay. The changes in neuronal survival were most significant in
the OGD + 1 µM NBP, OGD + 10 µM NBP, and OGD + 50 µM NBP groups
(versus OGD, p
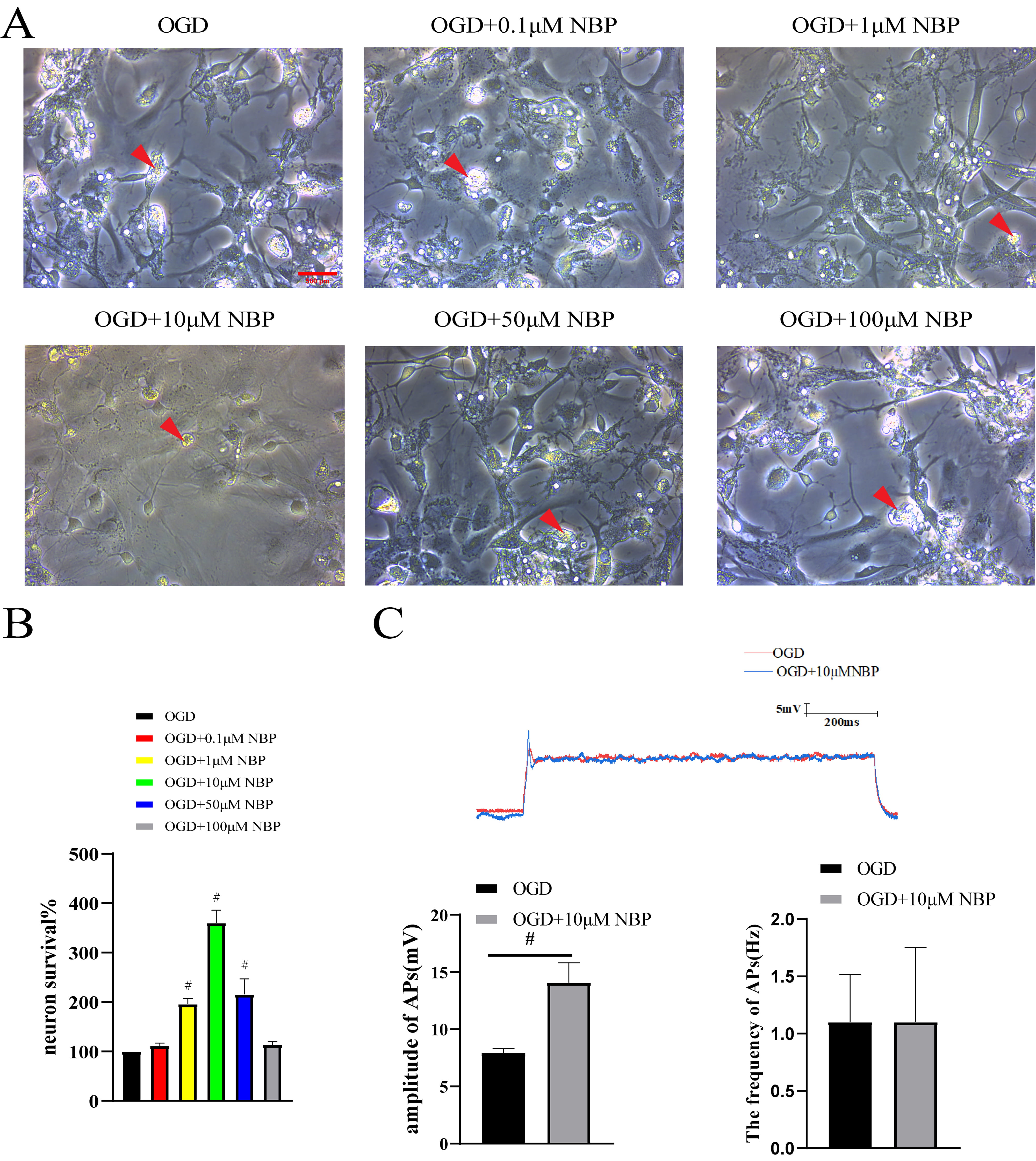 Fig. 2.
Fig. 2.
Morphology, survival and action potentials of neurons.
(A) Neuronal morphology: in the OGD group, cellular edema was observed in the
neurons, and the number was also reduced. The OGD + 1 µM NBP, OGD + 10
µM NBP, and OGD + 50 µM NBP groups exhibited decreased edema and
increased number of neurons. Red arrows: swollen and ruptured neurons. Scale Bar:
500 µm. (B) MTT assay: the changes in neuronal survival were most
significant in the OGD + 1 µM NBP, OGD + 10 µM NBP, and OGD + 50
µM NBP groups (versus OGD, #, p
We extracted total protein from neurons in the OGD and OGD +
10 µM NBP groups for the IB assay. The results demonstrated that TLR4,
HMGB1, and IL-6 expression in the OGD + 10 µM NBP group did not change
significantly. However, TNF-
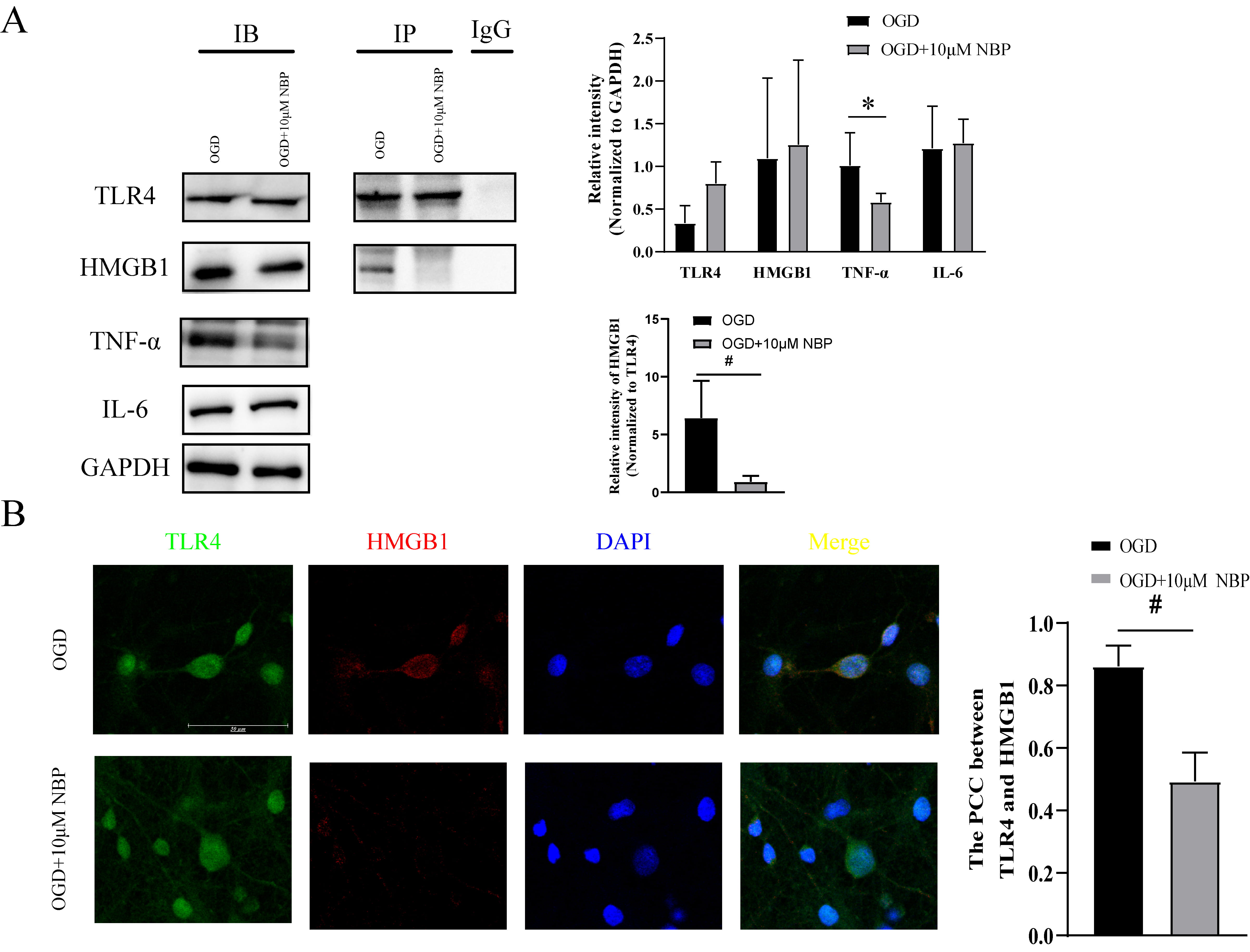 Fig. 3.
Fig. 3.
The TLR4/HMGB1 pathway was inhibited by NBP. Neuronal inflammation and the TLR4/HMGB1
pathway between OGD group and OGD+10 µM NBP group (A) The IB
results indicated that TLR4, HMGB1, and IL-6 expressions did not change
significantly in the OGD + 10 µM NBP group, but TNF-
MCI is a pathological condition that lies between normal brain aging and dementia. MCI occurs in 60% of patients with stroke, and 30% of patients ultimately develop VD. The incidence of MCI is higher in patients with ACI than in the general elderly [19]. Nevertheless, treatments for ACI-induced MCI are limited. To investigate the therapeutic effect of NBP on ACI-induced MCI, the clinical data of patients in the control group and the NBP group was analyzed. No significant difference in age, gender, disease course, the history of hypertension and smoking was found between the two groups, which could rule out the effect of these factors on the results. Arboix et al. [20] demonstrate that cerebral infarcts in the territory of the ACA have a better prognosis than infarcts in the territory of the MCA. However, no difference in the territory of ACA, MCA and PC was observed between Ctrl group and NBP group, indicating that the location of vascular cerebral topography could not influence the results.
Preventive interventions may have modest effects at the individual level. Interventions included lifestyle modifications, control of vascular risk factors (hyperlipidemia, hypertension, and diabetes), treatment of concomitant vascular disease, and proper aerobic exercise [21]. This study improved MOCA and ADL scores in the Ctrl group by controlling blood pressure, glucose, and lipids, as well as by using appropriate rehabilitation exercises. However, conventional treatment did not improve the specific functions of MOCA and ADL. After treatment, the patients in the NBP group demonstrated significant improvements in memory, attention, orientation, and delayed memory. Moreover, patients in the NBP group indicated improved grooming and eating abilities after treatment with NBP. These results suggest that NBP can enhance cognitive function and daily living activities in patients with MCI. Some researchers have suggested that the key to VD treatment is to improve brain blood supply and brain metabolism [6] and to inhibit inflammation during MCI [22]. NBP is considered an effective drug for treating ACI [23]. In addition, we also examined cerebral infarct size in patients using MRI and CT scans. However, there was no significant change in the infarct size of patients in either the Ctrl or NBP group after treatment. Imaging evidence suggests that NBP does not reduce infarct size.
To confirm the pharmacological mechanism of NBP, an in vitro model of
OGD, proven to be a model of ischemia, hypoxia [11], and cognitive dysfunction
[12]. Abnormal morphology of neurons was observed in the OGD group, which was
mainly characterized by cell edema and fragmentation. After NBP treatment, the
morphology of OGD neurons significantly improved, and edema and fragmentation
were reduced. An MTT assay was performed to confirm that NBP could effectively
protect neurons after OGD. The MTT assay revealed a significant increase in
neuronal survival. Furthermore, neuronal survival was most significant at 10
µM NBP. 10 µM of NBP may be considered the optimal concentration for
neuronal therapy. The concentration of NBP is insufficient to play a
neuroprotective role in the
The pathogenesis of ACI-induced MCI remains unclear. When the brain is in
ischemia and hypoxia state, the TLR4/HMGB1 pathway can be activated after
neuronal injury [4], which promotes the release of IL-6, TNF-
Nevertheless, several limitations should be acknowledged. Firstly, the small number of the sample is a potential limitation of the study. We will increase the sample number in future studies in order to consolidate the results. Secondly, the in vivo model of ACI-induced MCI should be constructed to explore more treatments for ACI-induced MCI. Finally, the underlying mechanisms of the connection between glial cells and neurons in ACI-induced MCI should be further investigated.
In conclusion, this study demonstrated that NBP can effectively improve the cognitive function of patients with ACI-induced MCI and effectively rescue neurons by inhibiting the inflammatory response induced by the TLR4/HMGB1 pathway.
ACA, anterior cerebral artery; ACI, Acute Cerebral Infarction; ADL, Activities
of Daily Living; AP, action potential; Co-IP, Co-immunoprecipitation; Ctrl,
control; OGD, oxygen and glucose deprivation; HMGB1, high mobility group box 1;
IB, Immunoblotting; IF, Immunofluorescence; IL-6, interleukin-6; MCA, middle
cerebral artery; MCI, mild cognitive impairment; MOCA, Montreal cognitive
assessment scale; NBP, N-butyl-phthalide; PC, posterior circulation; TLR4,
toll-like receptor 4; TNF-
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
HZ and SL conceptualized and designed the study. HZ investigated the clinical efficacy of the NBP and wrote the manuscript. SL performed the patch-clamp and wrote the manuscript. CH and YC finished the primary neuron culture and OGD model. LW and JL performed the IB and Co-IP. YL performed IF and contributed to the analysis the results of the work. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study adhered to the principles of the Declaration of Helsinki. Ethical approval was granted by the institutional review board of Jiangbin Hospital (No. LW-2022-09). Informed consent was obtained from patients or their guardians.
Not applicable.
This study was supported by grants from Guangxi Zhuang Autonomous Region Health and Family Planning Commission (Grant No. Z20201187).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.jin2308158.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



