1 College of Veterinary Medicine and BK21 FOUR Program, Chonnam National University, 61186 Gwangju, Republic of Korea
2 Department of Nephrology, Washington University School of Medicine, St. Louis, MO 63110, USA
Abstract
Sleep disorders are prevalent neurological conditions linked to neurocognitive impairments. Understanding the neuroplasticity changes in the hippocampus, which plays a central role in regulating neurocognitive function, is crucial in the context of sleep disorders. However, research on neurodegenerative disorders and the influence of sleep disorders on hippocampal neuroplasticity remains largely unclear. Therefore, this review aims to highlight the latest advancements regarding hippocampal neuroplasticity and functional changes during sleep disorders, drawing insights from clinical and preclinical research involving sleep-deprived animal models. These articles were gathered through comprehensive literature searches across databases, including Google Scholar, PubMed, Web of Science, and Scopus. Maternal sleep deprivation has been observed to cause neurocognitive impairment in offspring, along with changes in protein expression levels associated with neuroplasticity. Similarly, sleep deprivation in adult mice has been shown to affect several cognitive functions and fear extinction without influencing the acquisition of fear conditioning. While mechanistic research on neurocognitive dysfunction induced by maternal and adult sleep deprivation is limited, it suggests the involvement of several signaling pathways, including neurotrophic factors, synaptic proteins, and inflammatory molecules, which are triggered by sleep deprivation. Further studies are needed to clarify the mechanistic pathways underlying hippocampal dysfunction and synaptic alterations associated with sleep disturbances.
Keywords
- animal model
- cognition
- hippocampus
- sleep disorder
- synaptic plasticity
Sleep is crucial in maintaining overall health and well-being. Deprivation of sleep is intricately linked to several challenges across various body systems, including the endocrine, metabolic, and neurological domains [1, 2, 3]. The importance of a proper and adequate sleep in normal brain development and function has been considerably explored [4, 5]. Sleep dynamics can profoundly influence brain development throughout an individual’s lifespan and are involved in neurodegeneration [6]. During early life, which is characterized by rapid developmental changes, sleep is a fundamental brain activity that crucially contributes to healthy cognitive and psychosocial development [7]. Sleep disorders manifest in different forms, ranging from complaints of inadequate sleep to perceived excessive or abnormal movements during sleep [8, 9]. Chronic sleep disturbances are linked to neurobehavioral deficits and physiological abnormalities, often resulting in psychiatric conditions such as cognitive dysfunction and depression [10, 11]. For instance, research findings suggest that 40% and 47% of individuals experiencing insomnia and excessive sleepiness also exhibit neuropsychiatric disorders, respectively [12]. Furthermore, disturbed nocturnal sleep patterns are common among individuals experiencing depression, as revealed in clinical and epidemiological studies, where approximately 83% of patients with depression report at least one symptom of insomnia [13]. Therefore, sleep disorders, including insomnia and abnormal sleep patterns, frequently co-occur with neuropsychiatric conditions such as cognitive impairment and emotional dysregulation, highlighting the intricate interplay between sleep quality and mental well-being.
The hippocampus, extensively explored for its diverse roles in learning, cognition, memory storage, depression, and anxiety, serves as a focal point of investigation among other brain regions. Furthermore, it serves as a focal pivotal area for exploring the pathophysiology of various neurological diseases characterized by functional alterations, encompassing neurodegenerative [14, 15] and neuroinflammatory diseases [16, 17], traumatic brain injuries (TBI) [18], and sleep disorders [19]. Moreover, the hippocampus has received significant attention in studies investigating changes in neuroplasticity, particularly concerning neurological insults such as neurodegenerative diseases [15] and sleep disorders [19]. Previous research has explored hippocampal neuroplasticity based on neurodegenerative disorders [15]. However, the influence of sleep disorders on hippocampal neuroplasticity remains largely unclear.
Sleep architecture comprises two distinct stages: rapid-eye-movement (REM) (also
known as paradoxical sleep) and non-REM (NREM) sleep (encompasses deep slow-wave
sleep). Electroencephalogram (EEG) recordings facilitate the determination and
quantification of these stages [20]. REM and NREM sleep stages exhibit distinct
oscillatory rhythms in the EEG. Theta rhythms characterize REM sleep, while NREM
sleep features slow waves, spindles, and sharp wave ripples. Certain EEG
patterns, such as theta oscillations in REM sleep and high-frequency sharp wave
ripples in NREM sleep, involve approximately 10–18% of hippocampal neurons
[21]. These EEG rhythms are proposed to play crucial roles in memory
consolidation [22, 23, 24] and in transmitting information from the hippocampus to the
cortex and other brain regions [25, 26]. Moreover, the hippocampus contains a
neurogenic niche in adults, contributing to its neuroplasticity capacity.
Neurogenesis—a complex process—is implicated in hippocampal neuroplasticity
changes observed across various neurological conditions, including sleep
disorders. The hippocampus serves multiple higher-level functions, with newly
formed neurons potentially integrating into processes such as mood regulation
[27] and learning and memory [28]. The influence of sleep on adult hippocampal
neurogenesis has long been recognized. For example, total sleep deprivation
Therefore, this review aims to synthesize articles predominantly focusing on hippocampal neuroplasticity based on sleep disorders. By examining clinical and preclinical studies that focused on sleep dysregulation, these findings could shed light on potential therapeutic strategies for regulating aberrant hippocampal neuroplasticity in neurodegenerative diseases. Additionally, it seeks to identify existing gaps in comprehending hippocampal neuroplasticity within the context of neurodegenerative disorders, thereby serving as a reference point for future research endeavors.
Sleep, a dynamic and multifaceted behavioral process, can experience disruptions at different stages. The International Classification of Sleep Disorders-Third Edition (ICSD-3) outlines six primary categories [30, 31]: insomnias, sleep-related breathing disorders, central hypersomnolence disorders, parasomnias, sleep-related movement disorders, and circadian rhythm sleep disorders (Table 1).
| ICSD-3 major category | Typical symptoms |
|---|---|
| Insomnia | Difficulty initiating and maintaining sleep |
| Sleep-related breathing disorder | Excessive daytime sleepiness |
| Central disorder of hypersomnolence | Excessive daytime sleepiness, cataplexy (with narcolepsy) |
| Parasomnia | Nocturnal movements, sleepwalking |
| Sleep-related movement disorder | Urge to move legs, difficulty initiating sleep |
| Circadian rhythm sleep disorder | Difficulty initiating and maintaining sleep, early awakening, and excessive daytime sleepiness |
Abbreviations: ICSD-3, international classification of sleep disorders-third edition.
Insomnia is characterized by difficulty initiating or maintaining sleep. It results in significant daytime repercussions, including impaired functionality, decreased work performance, social withdrawal, and fatigue [32, 33]. Individuals experiencing insomnia struggle to sleep even when provided sufficient opportunity to do so in a conducive environment. It frequently coexists with other conditions, such as depression, neurological disorders, cardiovascular disease, diabetes, respiratory issues, gastrointestinal complications, and cancer [32]. Moreover, insomnia elevates the risk of developing neuropsychiatric disorders and cardiovascular disease. However, an effective treatment strategy can enhance cardiovascular and mental health outcomes [34, 35]. Research findings suggest that approximately 10% of adults experience insomnia, while up to 20% of patients in primary care report symptoms leading to functional impairment and reduced productivity [33, 36].
Studies on the hippocampal structure in insomnia have yielded varied findings. Some indicate diminished hippocampal volume among patients with insomnia [37, 38]. In contrast, others demonstrate negative associations between hippocampal volume and subjective sleep quality [39]. Conversely, other studies have reported no differences in hippocampal volume between individuals with insomnia and control groups [40, 41]. Different subtypes of insomnia may plausibly demonstrate distinct hippocampal connectivity patterns. We consider it crucial and timely to address these findings in our review to enhance our understanding of the hippocampal involvement in insomnia.
The classification system of the American Academy of Sleep Medicine categorizes sleep-related breathing disorders as obstructive apnea, central sleep apnea, and sleep-related hypoventilation [30, 42]. These nocturnal events can raise pulmonary arterial pressure during sleep and while awake [43]. However, the treatment approach for these diverse disorders often follows similar principles. Typically, alleviating the sleep-related breathing disorder involves addressing underlying medical conditions or adjusting medications.
Sleep-related breathing disorders are linked to neural injury that influences many physiological systems, including sensing chemoreception and airflow, respiratory musculature, timing circuitry for breathing pattern coordination, and blood pressure mechanisms integrated with respiration [44]. Sleep-related breathing disorders exert complex and poorly understood effects on cognition and daytime memory, involving the hippocampus. For instance, obstructive sleep apnea, observed in adults and children, is frequently associated with different levels of mood dysregulation and cognitive deficits [45]. Clinical cases have revealed decreased neural metabolites in the hippocampus and frontal cortex, leading to impaired memory, learning, and executive functions [45, 46]. Untreated neural, cognitive, and daytime functional impairments can lead to severe downstream consequences. Improving our understanding of the cognitive effects associated with these disorders and advancing the development of more efficient diagnostic assessment tools will facilitate early intervention, thereby improving the quality of life of patients.
Central disorders of hypersomnolence encompass several marked conditions characterized by excessive daytime sleepiness stemming from pathological origin. The underlying pathophysiology of most of these disorders remains elusive. While excessive daytime sleepiness is a shared characteristic among these conditions, their classification can be further aided by considering additional clinical presentations, such as cataplexy, sleep duration, and episodic or continuous nature of symptoms [47, 48].
Memory function typically remains intact in patients with central disorders of hypersomnolence, while specific subgroups may experience impairments in higher-order cognition, decision-making, and emotional processing [49]. Some patients exhibit hypermetabolism in various brain regions, including the hippocampus [50, 51]. However, studies exploring the involvement of the hippocampus in central hypersomnolence disorders remain limited.
Parasomnia is commonly classified according to the sleep state from which it originates: NREM and REM [30]. NREM parasomnia includes arousal disorders, such as confusional arousal, sleepwalking, and sleep terrors. These behaviors manifest when the cortex incompletely arouses from deep NREM sleep, often triggered by comorbid conditions that induce repeated arousal and/or promote sleep inertia. Alterations in the cyclic alternating pattern, a biomarker indicating arousal instability during NREM sleep, have been observed in sleepwalking and related disorders [52].
A significant feature of REM parasomnia is its higher prevalence among males [53, 54]. Evidence of hippocampal involvement in REM parasomnia is clear by heightened theta rhythms, indicating increased hippocampal activity during REM sleep [55]. However, the involvement of the hippocampus in REM and parasomnia is complex, involving interactions with other brain regions, such as the cortex [56]. Recognizing the role of the hippocampus in REM parasomnia highlights the importance of a comprehensive investigation of its interaction with other brain regions. Such research provides vital insights for advancing our understanding and management strategies for this complex sleep disorder.
Sleep-related movement disorders include various repetitive movements that frequently disturb sleep, resulting in insomnia, diminished sleep quality, fatigue, and excessive daytime sleepiness. This category encompasses conditions such as restless leg syndrome, periodic limb movement disorder, sleep-related leg cramps, sleep-related bruxism, and sleep-related rhythmic movement disorders [30, 57]. Despite their clinical significance, the specific brain regions involved in these disorders remain largely elusive and, unfortunately, underexplored.
Recent magnetic resonance imaging (MRI) studies investigating restless leg syndrome have provided some insights into the structural abnormalities within the brain [58, 59]. These studies revealed marked morphological alterations, particularly in the bilateral amygdala, and less pronounced changes in regions such as the hippocampus, right caudate, left globus pallidus, and left putamen. Additionally, a population-based study involving 189 individuals revealed a correlation between periodic limb movements during sleep. It reduced hippocampal and amygdala volume [60]. Cumulatively, these findings suggest the potential involvement of the hippocampus in sleep-related movement disorders. Consequently, further studies exploring the synaptic plasticity changes within the hippocampus associated with these disorders are warranted. Such investigations could provide valuable insights into the underlying mechanisms and potentially guide the development of more effective targeted treatment approaches.
Circadian rhythm sleep disorders involve disturbance of the internal clock of the body, which regulates various physiological processes, including sleep-wake cycles [30, 61]. Previous study findings have prompted discussions regarding the degree of hippocampal involvement in the circadian system [62, 63, 64, 65]. While several reviews have presented varying perspectives on this topic [66, 67, 68], the precise nature of this relationship remains subject to ongoing investigation.
It is well-established that the circadian rhythm and REM sleep significantly influence memory consolidation, a process heavily dependent on hippocampal activity [69]. A study found that rats experiencing circadian rhythm sleep disorders demonstrate decreased cognitive function alongside alterations in hippocampal neuron count and plasticity [64]. However, the direct correlation between circadian rhythm disorders and hippocampal neuroplasticity is not firmly established. Nonetheless, they are considered among several brain regions affected by these disruptions. Consequently, employing specific methodologies aimed at elucidating the precise involvement of the hippocampus in circadian rhythm sleep disorders is warranted. The findings could potentially shed light on the relationship. Furthermore, clarifying this connection could offer valuable insights into the underlying mechanisms of these disorders, thereby potentially guiding inform targeted therapeutic interventions.
While the classifications of sleep disorders delineated in the preceding chapter are consistently observed in human studies, attempts to emulate these conditions in preclinical animal models are often based on sleep disorders induced by sleep deprivation. Sleep deprivation, which is characterized by prolonged periods of inadequate sleep [70], is a primary method to evoke various sleep disturbances, as elucidated and discussed in the preceding chapter. Table 2 (Ref. [64, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97]) provides a detailed overview of recent research investigating alterations in hippocampal function and neuroplasticity across various sleep disorders and normal sleep-wake cycles. This chapter is divided into three distinct subchapters, with each focusing on a specific aspect of the interplay between hippocampal function and sleep disruptions. This structural setup aims to provide improved comprehensive insight into the hippocampal function and neuroplasticity effect on varying sleep disorders, encompassing various life stages and clinical conditions.
| Animal model/clinical study | Effect on sleep/Effect of disease model on sleep | Changes in hippocampal neuroplasticity | Reference |
| Sleep-deprived CD-1 mice (maternal) | Induced cognitive impairment | Decreased BDNF and PSD95 | [74] |
| Environmental enrichment recovered cognitive function | Environmental enrichment recovered BDNF and PSD95 levels | ||
| Sleep-deprived CD-1 mice (maternal) | Induced depression-like behavior and learning and memory impairments | Increased proinflammatory cytokines | [73] |
| Environmental enrichment improved behavioral dysfunction | Decrease in neuroplasticity-associated proteins | ||
| Environmental enrichment restored the neuroplasticity proteins | |||
| Sleep-deprived (maternal) Sprague Dawley rats | Pups born to REM sleep-deprived dams exhibited a significantly higher percentage of active sleep throughout the examination period, alongside reduced latency during postnatal days 15–21 | [75] | |
| Induced maturational delay in the sleep-wake neural networks | |||
| CD-1 mice with maternal sleep deprivation | Induced anxiety-like behavior and cognitive impairment | Decreased neurotrophic factors (BDNF and Syt-1) | [72] |
| Environmental enrichment recovered behavioral deficits | Environmental enrichment restored sleep deprivation-induced molecular changes | ||
| CD-1 mice with maternal sleep deprivation | Induced emotional and cognitive impairments | [71] | |
| Fragmented sleep deprivation exacerbated cognitive performance to a greater extent compared to continuous sleep deprivation | |||
| Sleep-deprived C57BL6 mice | Induced depression-like behavior | Reduced expression of neurotrophic factors | [76] |
| Increased cytokines in the hippocampus | |||
| Sleep-deprived C57BL6J mice | Identified 1146 DEGs: 507 upregulated and 639 downregulated genes | [82] | |
| Upregulation was associated with RNA splicing and the nucleus | |||
| Downregulation was associated with cell adhesion, dendritic localization, the synapse, and postsynaptic membrane | |||
| Sleep-deprived C57BL6J mice | Impaired object place recognition memory in young adult mice but enhances the performance in old mice | [79] | |
| Sleep-deprived C57BL6J mice | Showed 16 common DEGs in sleep deprivation and AD mice | [83] | |
| Sleep-deprived Wistar rats | Induced cognitive dysfunction | Triggered the elevation of hippocampal autophagy | [80] |
| Reduced generation of endogenous H |
|||
| Sleep-deprived Sprague Dawley rats | Affected extinction of conditioned fear, but not the acquisition of fear conditioning | Inhibited hippocampal proBDNF activity rises during the circadian cycle | [81] |
| Sleep-deprived Wistar rats | Short period ( |
[95] | |
| Increased REM sleep homeostatic drive increased phosphorylation and activation of ERK1/2 and BDNF expression in the pedunculopontine tegmentum | |||
| Sleep-deprived C57BL6J mice | Affected the contextual memory, but not cued memory | Impaired CREB activation and mTOR signaling pathways | [77, 96, 97] |
| BDPP protective against sleep deprivation-induced contextual memory impairment | BDPP exerts a protective effect through CREB activation and mTOR signaling pathways | ||
| Sleep-deprived C57BL6J mice | Sleep deprivation during hours ZT 2–5 after OPR training impairs long-term memory and LTP | [78] | |
| Sleep-deprived SD rats | Enhanced IL-1 |
[84] | |
| Decreased BDNF mRNA levels after 5 days | |||
| Wistar and SD rats simulated shift work | Simulated night shift work in rats disrupts the pathways regulating the circadian component of the translation of mRNA in the PFC | [85] | |
| SHR and WKY rats | CRD and hypertension reduced memory performance and novel object recognition and preference | Decreased fractional anisotropy values, the number of neurons and astrocytes and the expression of BDNF and synapsin 1 in the hippocampus | [64] |
| Enhanced neuron and microglia degeneration, reduced hippocampal blood flow, and increased NF- |
|||
| Female CBA/JRi mice | Synaptic Shank3 protein levels oscillated in the hippocampus and striatum throughout the day, correlating with serum melatonin fluctuations | [86] | |
| Physical activity impaired Shank3 |
|||
| Male C57BL/6J mice. 9–10 weeks (young group) or 44–52 weeks (middle-aged group) exposed to 2 days of constant darkness | 15% (214 proteins) displayed circadian rhythms in abundance in the hippocampus of young mice, while only 1.6% (23 proteins) were rhythmic in middle-aged mice | [87] | |
| Aging disrupts the circadian regulation of proteins involved in cellular functions critical for hippocampal function and memory | |||
| Focal-cerebral ischemia in male SD rats | NREM sleep duration was greater during the dark period but lower during the light period | Neurons in the hippocampal CA1 of the MCAO group were lost, atrophied, or loosely arranged | [91] |
| REM sleep duration following stroke (MCAO group) was significantly lower | PSD was significantly thinner following the stroke | ||
| Wake duration increased during the light and 24-h periods following the stroke | Combining melatonin and exercise improved the hippocampal neuroplasticity changes | ||
| Melatonin and exercise in combination improved the sleep disruption in MCAO mice | |||
| Traumatic brain injury in CD-1 mice | Disrupted sleep homeostatic drive | Reduced the amplitude and frequency of mIPSCs in dentate granule cells | [90] |
| Dual orexin antagonist (DORA-22) rescued | |||
| TBI in C57BL/6 mice + sleep fragmentation | Post-injury sleep fragmentation causes deficits in trace fear conditioning acquisition consistent with compromised dorsal hippocampus function | Sleep fragmentation increases cortical inflammation after TBI | [92] |
| Sleep fragmentation induced hippocampal CA1 neuronal activity | |||
| Post-TBI sleep fragmentation enhances ipsilateral CA1 microglia reactivity and imbalanced neuronal activity 30 DPI | |||
| Post-TBI sleep fragmentation causes persistent microgliosis lateral to the lesion area associated with increased cortical expression of glial pro-inflammatory signaling genes 30 DPI | |||
| Injury and sleep fragmentation induced Schaffer collateral deficits 30 DPI | |||
| Imaging, behavioral, and genetic datasets of 1200 participants in the age range of 22–35 years | Female good sleepers exhibited larger GMV in the right parahippocampal gyrus extending to the right hippocampus than female poor sleepers | [88] | |
| Smaller GMV in the right parahippocampal gyrus in women with poor sleep quality | |||
| Forty right-handed, healthy, adult male volunteers | Morning-to-evening increases were observed in cerebral blood flow. | [89] | |
| A night of sleep deprivation was associated with further cerebral blood flow increases. | |||
| Eighty older adults (aged |
Older adults with TBI showed a higher prevalence of OSA, insomnia, and daytime sleepiness than older adult controls. | [93] | |
| Older adults diagnosed with TBI between 2008–2014 (n = 78,044) and non-TBI controls (n = 76,107) | TBI was associated with an increased risk of insomnia | [94] |
Abbreviations: 3-MST, 3-mercaptopyruvate sulfurtransferase; AD, Alzheimer’s
disease; BDNF, brain derived neurotrophic factor; BDPP, bioactive dietary
polyphenol preparation; CA, cornu ammonis; CBS, cystathionine
The central nervous system undergoes critical developmental phases during intrauterine development growth. It is highly vulnerable to maternal sleep deprivation and other external influencing factors [98]. Recent reviews highlight various neurological and non-neurological effects of maternal sleep deprivation on their offspring [99, 100]. From these reviews and recent research, maternal sleep deprivation is shown to affect hippocampus-dependent function adversely and synaptic plasticity in offspring, especially in animal models (rodents). Maternal sleep deprivation is linked to cognitive decline [71, 72, 73, 74], emotional dysfunction, including depression- or anxiety-like behavior [71, 72, 73], and delayed maturation in the sleep-wake neural networks [75].
Additionally, maternal sleep deprivation is associated with linked to reduced levels of brain-derived neurotrophic factor, postsynaptic density protein 95, and other neuroplasticity-related proteins [72, 73, 74], alongside increased hippocampal-proinflammatory cytokine levels [73]. Furthermore, environmental enrichment has shown promise in reversing neurocognitive dysfunction and restoring synaptic protein expression in most of the studies mentioned above [72, 73, 74]. Overall, these findings underscore the substantial effect of maternal sleep deprivation on hippocampal function and neuroplasticity in offspring. Furthermore, these findings suggest the potential of environmental enrichment as a therapeutic intervention strategy to mitigate these adverse effects. However, a substantial gap exists in understanding the mechanisms underlying maternal sleep deprivation-induced hippocampal dysfunction in offspring. Thus, further studies are warranted to explore the molecular mechanisms underlying the observed maternal sleep deprivation effect on the hippocampal function of offspring and explore additional intervention and prevention strategies to protect offspring neurodevelopmental outcomes effectively.
Several preclinical studies have examined the influence of sleep deprivation in adulthood. For example, mice deprived of sleep displayed depression-like behavior [76] and experienced reduced contextual memory, while their cued memory remained unaffected [77]. Furthermore, sleep deprivation impaired object place recognition and cognitive function in young adult mice [78, 79] and rats [80]. Moreover, it enhanced cognitive performance in old mice [79]. The extinction of conditioned fear influenced sleep-deprived rats, while the acquisition of fear conditioning remained unaffected [81].
Further mechanistic studies are crucial to understanding how sleep deprivation leads to hippocampal dysfunction. Transcriptome profiling of the hippocampus in sleep-deprived mice revealed an increase in genes related to RNA splicing and a decrease in genes associated with cell adhesion, dendritic localization, and synaptic membrane function [82]. A total of 16 common differentially expressed genes exhibited similar patterns of change in both sleep-deprived mice and Alzheimer’s disease model mice [83]. Sleep deprivation resulted in decreased expression levels of neurotrophic factors and increased cytokine expression in the hippocampus [76, 84], suggesting that sleep deprivation adversely affects hippocampal neuronal function through various signaling pathways. Additionally, sleep deprivation impaired cyclic adenosine monophosphate (cAMP) response element-binding protein activation and mammalian target of rapamycin (mTOR) signaling pathways [77]. Moreover, simulated night shift work in rats disrupted pathways that regulate the circadian cycle [85]. Synaptic concentrations of Shank3 protein displayed minor oscillations during the day in hippocampal and striatal brain regions, correlating with changes in serum melatonin levels in mice [86]. It appears that aging disrupts circadian patterns of protein expression in the murine hippocampus. In young mice, 15% (214 proteins) exhibited circadian rhythms in abundance, while only 1.6% (23 proteins) displayed rhythmic in middle-aged mice [87].
Clinical studies suggest that females with good sleep exhibit a larger gray matter volume in the right parahippocampal gyrus than females without good sleep [88]. Additionally, a study involving 40 healthy adult males revealed morning-to-evening increases in cerebral blood flow, with a night of sleep deprivation associated with further increases in cerebral blood flow [89]. However, there is limited information on the hippocampal effects of sleep deprivation in adults than in preclinical studies. Thus, clinical researchers must focus more on improving and stabilizing the current understanding gained from preclinical research. Consequently, the multifaceted influence of sleep deprivation in adulthood on hippocampal function emphasizes the urgent need for additional preclinical/clinical research to clarify its underlying mechanisms and devise targeted interventions to alleviate its detrimental effects on brain health.
Sleep disorders frequently coexist with varying chronic conditions, including heart disease, kidney disease, high blood pressure, diabetes, obesity, and mental illness [24, 101, 102, 103, 104]. These disorders may be primary or secondary to other organ and/or nervous system diseases. Consequently, several recent reviews have explored the potential presence of sleep disorders in neurological disorders, such as Parkinson’s [105, 106] and Alzheimer’s disease [105, 107, 108, 109] and multiple sclerosis [110]. These prevalent neurological disorders are linked to disruptions in hippocampal neuroplasticity at different stages of the disease progression [15, 111, 112, 113, 114]. Considering the involvement of various brain regions in the pathology of neurological disorders, it is probable that sleep disorders in these conditions result from a combination of mechanisms across multiple brain regions. Moreover, therapeutic interventions administered during the course of neurological disorders have been implicated in sleep disorder development [109].
Additionally, sleep disorders frequently occur after traumatic brain injury (TBI), including conditions such as narcolepsy, posttraumatic hypersomnia, periodic limb movements in sleep, and especially obstructive sleep apnea, which can lead to significant consequences [115, 116, 117]. Preclinical animal models of TBI and ischemic stroke have shown disturbances in the sleep homeostatic drive [90, 91]. TBI has been demonstrated to decrease the amplitude and frequency of miniature inhibitory synaptic currents in dentate granule cells [90], while sleep fragmentation has been associated with heightened cortical inflammation and hippocampal cornu ammonis 1 neuronal activity post-TBI [92]. Multiple factors probably play a role in the development of sleep disturbances post-TBI. Diffuse axonal injury, for instance, can result in damage to sleep-regulating structures [118]. While direct evidence of hippocampal involvement in sleep disorders following TBI or ischemic stroke is lacking, the hippocampus may be one of the brain regions affected by these injuries [119, 120]. Clinical study findings corroborate preclinical evidence, suggesting a connection between TBI and sleep disorders. For instance, older adults with TBI demonstrate a higher prevalence of obstructive sleep apnea, insomnia, and daytime sleepiness than older adult controls [93]. A comprehensive database study showed that TBI was linked to an elevated risk of insomnia [94].
In summary, the presence of sleep disorders alongside neurological conditions highlights the complex relationship between sleep and brain health, necessitating comprehensive clinical management strategies. Further exploration of the molecular mechanisms connecting sleep disorders with neurological conditions, including the distinct role of the hippocampus, is crucial for enhancing our understanding and devising targeted interventions to enhance patient outcomes.
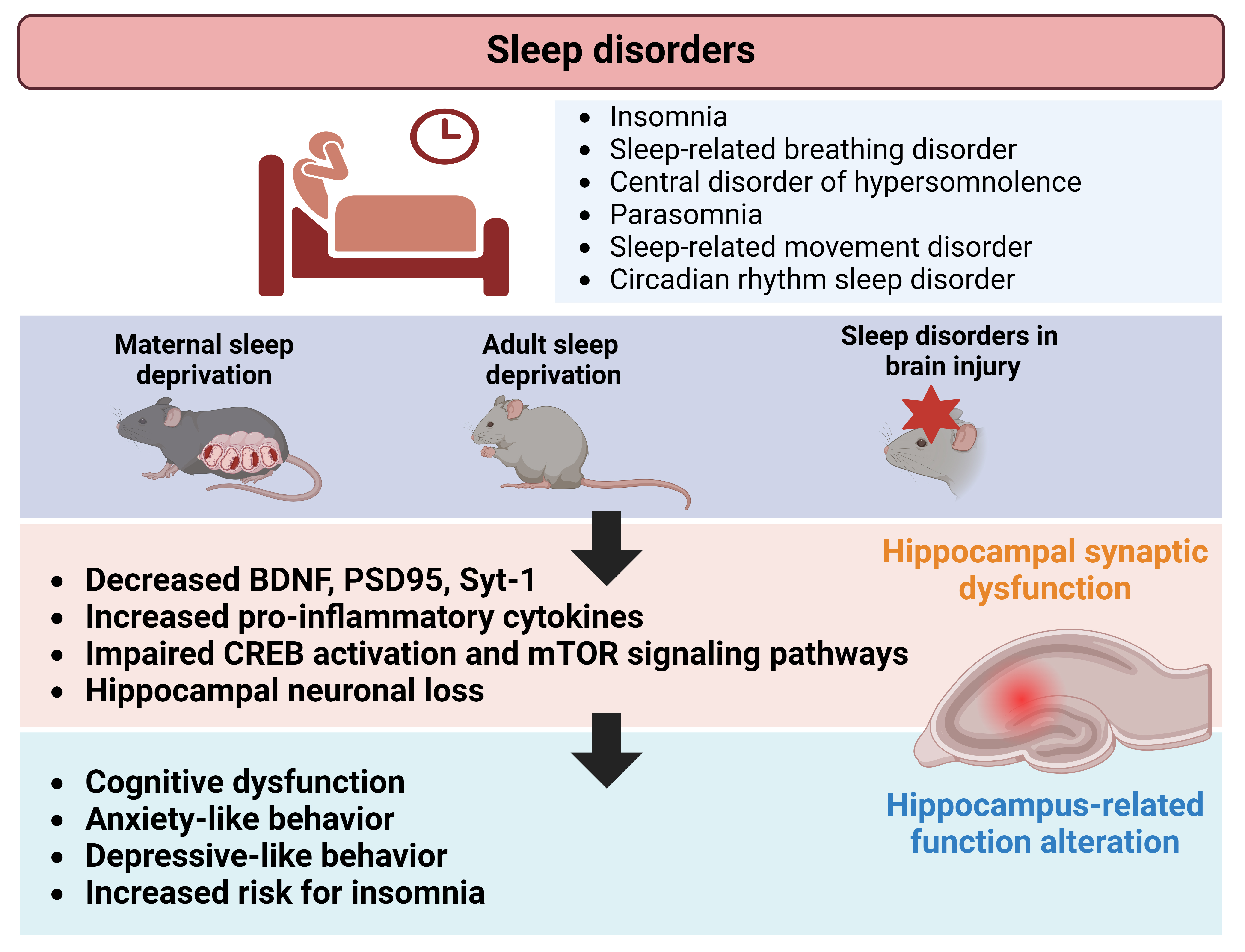
Sleep disorders, which have been tested on preclinical setting as maternal sleep deprivation, significantly affect hippocampus-dependent behaviors, such as learning, memory, anxiety, and depression. These behavioral alterations often co-occur with disruptions in hippocampal neuroplasticity (Fig. 1). This review highlights a scarcity of preclinical research examining hippocampal-structural neuroplasticity, particularly regarding specific categories of sleep disorders that are described and defined in human setting. Additionally, inconsistencies exist in the assessment strategies for hippocampal dysfunction during sleep disorders. While sleep dysregulation is also observed in animal models of brain injuries, the direct role of the hippocampus in these injuries remains uncertain owing to widespread damage across multiple brain regions. Nonetheless, molecular and synaptic changes resulting from brain injuries contribute to associated sleep dysregulation, involving various brain regions in the process. This review offers an updated overview of studies that evaluated hippocampal involvement in sleep disorders and sleep deprivation while highlighting existing gaps in comprehending hippocampal function in this context. Ultimately, this insight may stimulate future mechanistic and therapeutic research efforts in the field.
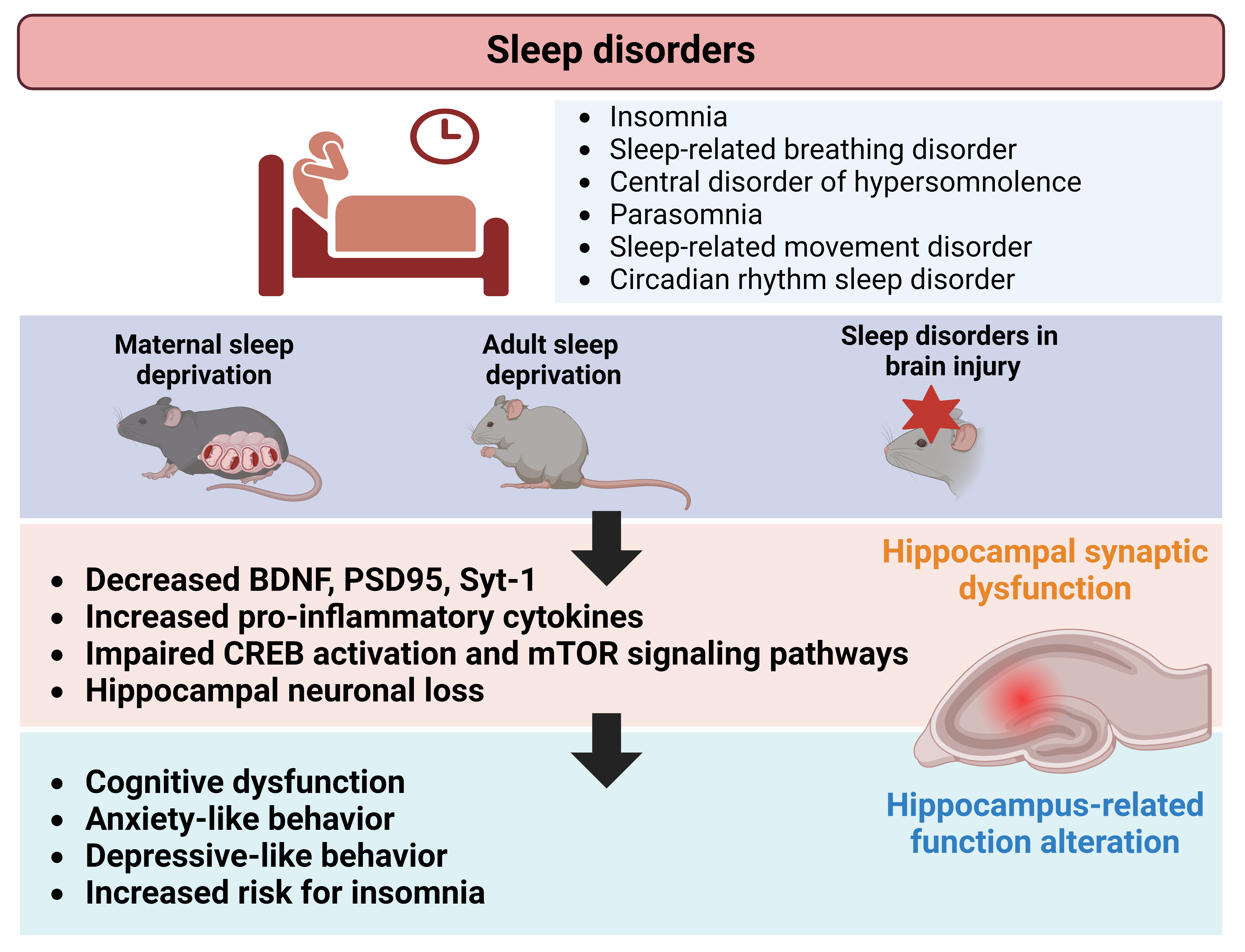 Fig. 1.
Fig. 1.
Schematic illustration depicting alterations in hippocampal neuroplasticity associated with sleep disorders. Preclinical studies investigating maternal-, adult-sleep deprivation, and brain injuries, revealed changes in synaptic proteins, neuronal growth factors, and signaling mechanisms within the hippocampus. These changes may contribute to functional and behavioral alterations, such as neurocognitive dysfunction, observed in individuals affected by sleep disorders. Figure created using BioRender.com (https://www.biorender.com/). BDNF, brain derived neurotrophic factor; CREB, cyclic adenosine monophosphate (cAMP) response element-binding protein; PSD, postsynaptic density; Syt-1, synaptotagmin 1.
PDEWM and CM conceptualized and designed the literature review. Both authors collected the literature. Both authors were involved in paper topic selection, reviewing, and editing. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was supported by a grant from the National Research Foundation (NRF) of Korea funded by the Republic of Korea Government (2022R1A2C1004022; RS-2023-00219517).
The authors declare no conflict of interest. Poornima D. E. Weerasinghe-Mudiyanselage is serving as one of the Guest editors of this journal. Changjong Moon is serving as one of the Editorial Board members and Guest editors of this journal. We declare that Poornima D. E. Weerasinghe-Mudiyanselage and Changjong Moon have no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Luigi De Gennaro.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.