Academic Editor: Gernot Riedel
Background: Cortico-cortical evoked potentials (CCEPs) have been used
to map the frontal (FLA) and parietal (PLA) cortical regions related to language
function. However, they have usually been employed as a complementary method
during sleep-awake surgery. Methods: Five male and two female patients
received surgery for tumors located near language areas. Six patients received
general anesthesia and the sleep-awake method was used for patients with tumors
located near the cortical language areas. We performed motor and somatosensory
mapping with CCEPs to identify language areas and we monitored responses during
surgery based on the mapping results. Electrocorticography was performed
throughout the surgery. Single pulses of 1 ms duration at 5–20 mA were delivered
by direct cortical stimulation using one grid at one region (e.g., FLA) and then
recording using a second gird at another area (i.e., PLA). Next, reversed
stimulation (from PLA to FLA) was performed. The charge density for electrical
stimulation was computed. Sensibility, specificity, predictive positive values,
and predicted negative values were also computed for warning alterations of
CCEPs. Results: Gross tumor resection was achieved in four cases. The
first postsurgical day showed language alterations in three patients, but one
year later six patients remained asymptomatic and one patient showed the same
symptomatology as previously. Seizures were observed in two patients that were
easily jugulated. CCEPs predicted warning events with high sensibility and
specificity. Postsurgical language deficits were mostly transitory. Although the
latency between frontal and parietal regions showed symmetry, the amplitude and
the relationship between amplitude and latency were different for FLA than for
PLA. The charge density elicited by CCEPs ranged from 442 to 1768
Cortico-cortical evoked potentials (CCEPs) are a promising technique for detecting functional connections in cortical networks, even under general anesthesia [1, 2]. This method uses single pulse electrical stimulation (SPES) applied directly to the cortex with subdural electrodes to record evoked waveforms from the remote cortex. This response has been shown to be robust and stable.
In an extraoperative setting, functional cortical networks were mapped including language networks between the frontal language area (FLA) and the temporal language area (PLA) [2, 3] and the motor-sensory system [4, 5], limbic system [6, 7], visual system [8], and seizure propagation networks [9, 10]. In addition, recent studies have demonstrated that intraoperative CCEPs are a feasible and reproducible tool for mapping language system during resection of brain tumors [2, 11, 12].
The main advantage of CCEPs is to gain access to functional connectivity in real time with good spatial resolution, unlike diffusion tensor imaging that depicts the connections between different areas, but cannot resolve the function nor direction of white matter tracts. In patients with structural lesions, the functional tract can be distorted or interrupted due to brain edema or infiltration of a tumor [13]. Also, functional magnetic resonance imaging (fMRI) provides dynamic information on cortical functions, but it does not indicate the actual dynamics of information flow.
In awake patients, the technique commonly used for functional mapping is direct
cortical stimulation (DCS) with Ojemann’s stimulation or low-frequency
stimulation, which consists of a 50–60 Hz train, 3–5 seconds in length, with a
pulse-width as high as 400
CCEPs were initially described as a neurophysiological monitoring technique for language areas in patients with brain tumors [2, 16]. In most cases, CCEPs were used during sleep-awake craniotomies. However, a good correlation between CCEPs and language function has been repeatedly observed [11] and confidence has developed for its use in patients that cannot be evaluated with an awake craniotomy.
In this report, we describe the utility of CCEPs for mapping and monitoring language associated areas in patients under general anesthesia during brain surgery performed for tumors located near language regions. Interestingly, one of the patients underwent surgery with the sleep-awake-sleep technique, which allowed us to correlate CCEPs and language functions. This patient was described in detail elsewhere [11].
We performed a prospective study on seven patients undergoing surgery for
removal of an intraparenchymal brain lesion located within or close to
language-related structures in the dominant cerebral hemisphere. The patients had
a mean age of 53.4
| Patient | Age | Sex | Location | Histology | Resection | Pre-surgery |
|---|---|---|---|---|---|---|
| 1 | 62 | M | Frontal | Astrocytoma IV | GTR | ES/dysarthria |
| 2 | 60 | F | Frontal | Abscess | GTR | ES |
| 3 | 50 | F | Fronto-parietal | Glioblastoma IV | GTR | ES/aphasia |
| 4 | 60 | M | Temporo-parietal | Glioblastoma IV | STR | ES |
| 5 | 50 | M | Frontal | Oligodendroglioma II | STR | ES |
| 6 | 60 | M | Frontal | Glioblastoma IV | GTR | ES |
| 7 | 31 | M | Temporo-parietal | Glioblastoma II | STR | ES |
| ES, epileptic seizure; F, female; M, male; STR, sub-total resection; GTR, gross total resection. | ||||||
Patients were evaluated with neurological and neuropsychological assessments using the Boston Diagnostic Aphasia Examination (BDAE) [17] before surgery. Follow-up examinations were performed on the first postoperative day and the first and six months following surgery as well as one year after surgery.
All patients were right handed and the tumors were always located in the left hemisphere. Preoperatively, all patients had a history of epileptic seizures (ES). One presented with motor aphasia and another with dysarthria due to involvement of the motor language area.
Preoperative and postoperative imaging included 1.5-T multimodal MRI (General Electric®, Fairfield, CT, USA), which included pre- and postcontrast T1-weighted, T2-weighted, FLAIR, diffusion-weighted, diffusion tensor, spectroscopic imaging, and tractography with a specific oncologic protocol.
Contrast MRI was performed after the surgery and compared with preoperative MRI results. The extent of tumor resection was defined as a gross total resection (GTR) if there was no residual enhancement in the postoperative MRI. Otherwise, the excision was classified as a subtotal resection (STR).
To identify eloquent areas, an intraoperative functional mapping was performed
with multimodal neurophysiological monitoring equipment with 32 channels (Elite,
Cadwell®, Kennewick, Washington, USA). The sampling rate was 10
kHz and the bandwidth was 1.5–1.000 Hz with the notch off. Electrocorticography
(ECoG) was used to monitor the brain responses during electrical stimulation to
identify the appearance of epileptiform patterns (post-discharges). Electrical
stimulation was performed with DCS using a grid of 4
Before placing the grids onto the language-related areas, we identified the
primary motor (PMC) and somatosensory (PSSC) cortices. After we identified the
face/tongue primary motor region, we placed the grid onto what would be the
Broca’s area or FLA. The grid that was placed onto supposedly the Wernicke’s area
or PLA was located occipital to the primary somatosensory cortex. The pre- and
postcentral gyri and the central sulcus (CS) were determined with a somatosensory
evoked potentials (SSEPs) phase reversal technique [15]. Constant-current
electrical stimulation was delivered at the right median nerve by trains of 300
pulses at 7.1 Hz, 200
DCS for identifying the PMC was performed using paired grid electrodes.
Stimulation was done using constant-current trains of 6 pulses at 500 Hz (high
frequency technique) with bi-phasic pulses of 150–200
 Fig. 1.
Fig. 1.Artifact induced by the notch filter. (A) Sham stimulation at 1 mA notch-off. (B) Sham stimulation at 1 mA notch-on. (C) Example of a true CCEP elicited at 15 mA with notch-off. All the recordings from the same patient.
For CCEP recording, two grids of 4
We started mapping by stimulating a pair of consecutive electrodes placed at the FLA that were in different orientations (parallel or orthogonal to the sulcus) and anterior to the PMC of the face and tongue, according to the results of motor mapping. To evaluate the dorsal language pathway, at least three trials were averaged separately to confirm the reproducibility of the responses on the grid located in the PLA. Recording was performed in a pseudomonopolar way, with all the grid electrodes referred to the contralateral ear-lobe. A positive response was recorded if a large N1 peak (upward directed) in the electrodes of the PLA was obtained [3, 18]. Next we used the electrodes of the PLA grid with positive responses to stimulate and record the grid located at the FLA.
Intraoperative neurophysiological monitoring (IONM) of language areas was performed throughout the surgery using the electrodes for which the higher bidirectional responses were recorded. Also, motor or somatosensory functions were monitored when the tumor was close to a primary cortices or inner capsule.
Charge density (
We offer the deduction of this formula in Appendix A.
The patients with suspected malignant tumors receive preoperatory 20 mg/kg 5-ALA
(Gliolan®). All patients were operated on under general
anesthesia, either because awake surgery was contraindicated due to deficit in
language tests or because the patient would not tolerate the awake procedure. The
anesthesia was induced with a bolus of propofol and remifentanil. They were
subsequently maintained with propofol (6.1
The craniotomy and surgery were guided with a neuronavigational system (Brainlab®, Feldkirchen, Germany). The tumor was removed using microsurgical techniques and ultrasonic aspiration guided by 5-ALA fluorescence and neurophysiology controls.
The warning criterion of CCEP was set at a reduction in amplitude greater than 20% [16]. When it appeared, the surgeon changed the resection area until a complete recovery was observed. When the changes were persistent or repeated in the same region, then the surgery was temporarily stopped and the area was irrigated with warm saline. If the recovery maneuvers did not work, the resection was stopped.
To compare latencies and amplitudes for N1 CCEP picked up at the FLA and PLA, we
averaged all the potentials obtained in one grid (e.g., FLA) after the
stimulation at the contralateral grid (PLA). Then we used a paired Student’s
t test to evaluate significance. The null hypothesis was H
Pearson’s correlation coefficient was used to determine the linear dependence
between variables. Linear regression was evaluated by means of the least-squared
method and its significance was evaluated by means of a contrast hypothesis
against the null hypothesis (H
This describes a t-Student distribution with n-2 degrees of freedom
[19]. The significance level was set at p = 0.05, and the results are
shown as the mean
Although the number of patients was quite low, we could obtain the specificity (Sp) and sensibility (Se) by building a confusion matrix. It is important to build up these matrices for early outcomes (1 day) and longer period outcomes (1 year). For the first day, we used the following criteria: presence or absence of symptomatology and CCEP alteration or not. Therefore, we had these possible variables:
• True positive (TP): CCEP alteration + symptomatic
• False negative (FN): no CCEP alteration + symptomatic
• True negative (TN): no CCEP alteration + asymptomatic
• False positive (FP): CCEP alteration + asymptomatic
where CCEP alteration can be reversible or not, because the relevance was the capacity to warn for possible lesions.
For the first year, we only considered when CCEP alterations were definite because we wanted to know the capacity for long term outcome predictions. We used the variables to obtain (Eqns. 3,4):
We used Youden’s index (J) J=Se+Sp-1 (Eqn. 5) to summarize the performance of CCEP monitoring, and the positive predictive value (PPV) and negative predictive value (NPV), which foresees the possibility of neurologic injury after CCEP alteration during IONM. To compute these values, we used the following formulas (Eqns. 6,7):
One tumor was located in the fronto-parietal lobe (Fig. 2A), four tumors were located in the frontal lobe (Fig. 2B,E,F,G), and two were in the parieto-temporal (Fig. 2C,D) lobes.
 Fig. 2.
Fig. 2.MRI of each of the patients, in which the anatomical location of the lesions is observed.
The patients with glioblastoma multiforme GBM were subsequently treated with radiotherapy and chemotherapy. Five of seven patients were asymptomatic one year after surgery (therefore, ES disappeared, although patients remained under pharmacological treatment), one persisted with ES, and one persisted with the same mild dysarthria previous to surgery but without ES. It is important to observe that none of the patients had aggravated symptomatology due to iatrogenic injury.
GTR was obtained in four of seven cases, meanwhile, in the remaining three patients, only STR was possible. In one case, the resection was stopped due to the presence of steady alterations of CCEPs; meanwhile, in the other two cases, the features of tumors (bleeding and malignity), out of IONM warnings, indicated the end of surgery. At one year of follow-up, no recurrence or tumor growth was observed (Table 2).
| Patient | IONM Warning events | Outcomes | ||||
|---|---|---|---|---|---|---|
| MEP | Reversible | CCEP | Reversible | 24 hours | 1st year | |
| 1 | Yes | Yes | Yes | No | Increased dysarthria | Mild dysarthria* |
| 2 | No | - | Yes | Yes | Asymptomatic | Asymptomatic |
| 3 | No | - | Yes | Yes | Mixed aphasia | Asymptomatic |
| 4 | No | - | No | - | Asymptomatic | Asymptomatic |
| 5 | No | - | No | - | Asymptomatic** | Asymptomatic** |
| 6 | Yes | Yes | Yes | Yes | Dysarthria | Asymptomatic |
| 7 | No | - | Yes | Yes | Asymptomatic | Asymptomatic |
| *Similar to previous; **persistence of epileptic seizures. | ||||||
We estimated a charge density between 442 and 1768
Intraoperative mapping for peri-rolandic areas was carried out as stated above, identifying initially the CS, the PMC of the face, and the hand in all cases, and PSSC for the arm and hand (Fig. 3A). Then, the grids were moved and placed at putative FLA and PLA positions for monitoring identification of CCEPs. After extensive mapping, we selected the most robust and prominent potentials for monitoring during the surgery.
No post-discharges were observed in this group of patients. However, two focal (not propagated) ES were recorded and were jugulated by cold saline irrigation (Fig. 3B).
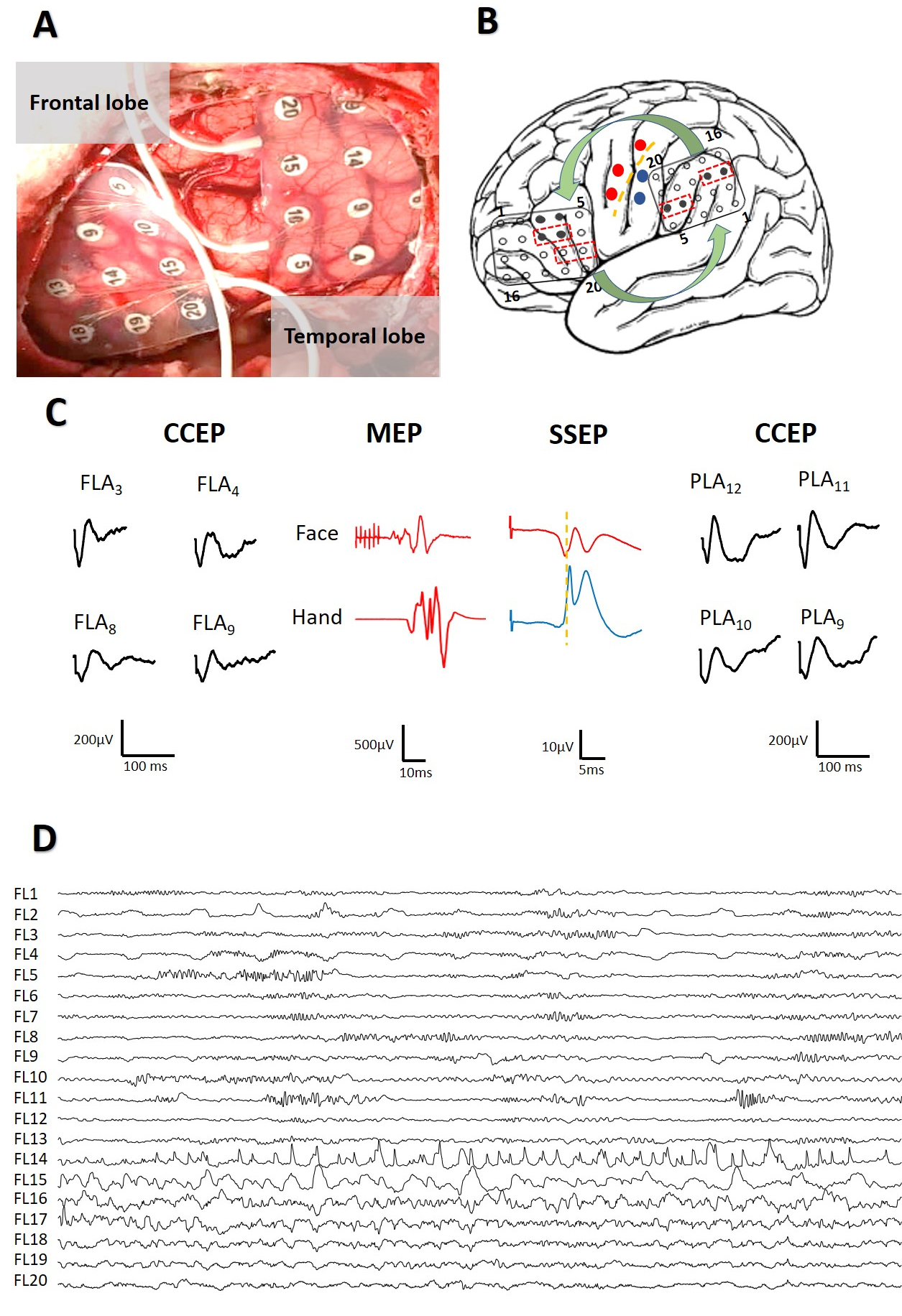 Fig. 3.
Fig. 3.Intraoperative mapping to identification of eloquent areas. (A) Placement of grids at frontal and parietal lobes. (B) Scheme of mapping showing CS (yellow), motor response (red) and somato-sensory potentials (blue). Grids are in FLA and PLA regions and show the pairs of electrodes used to DCS (red dotted lines boxes) and the electrodes were CCEPs appear (black); Curved green arrows indicate bidirectionality in the response. (C) CCEPs, MEPs and SSEPs obtained. The same color-code than in (B). (D) Start of a focal ES (restricted to 3 channels) during DCS.
In all patients we obtained CCEPs, both from the FLA and the PLA, with a clear
bidirectional response (Fig. 3B). On average, responses were obtained when
stimulating 2.3
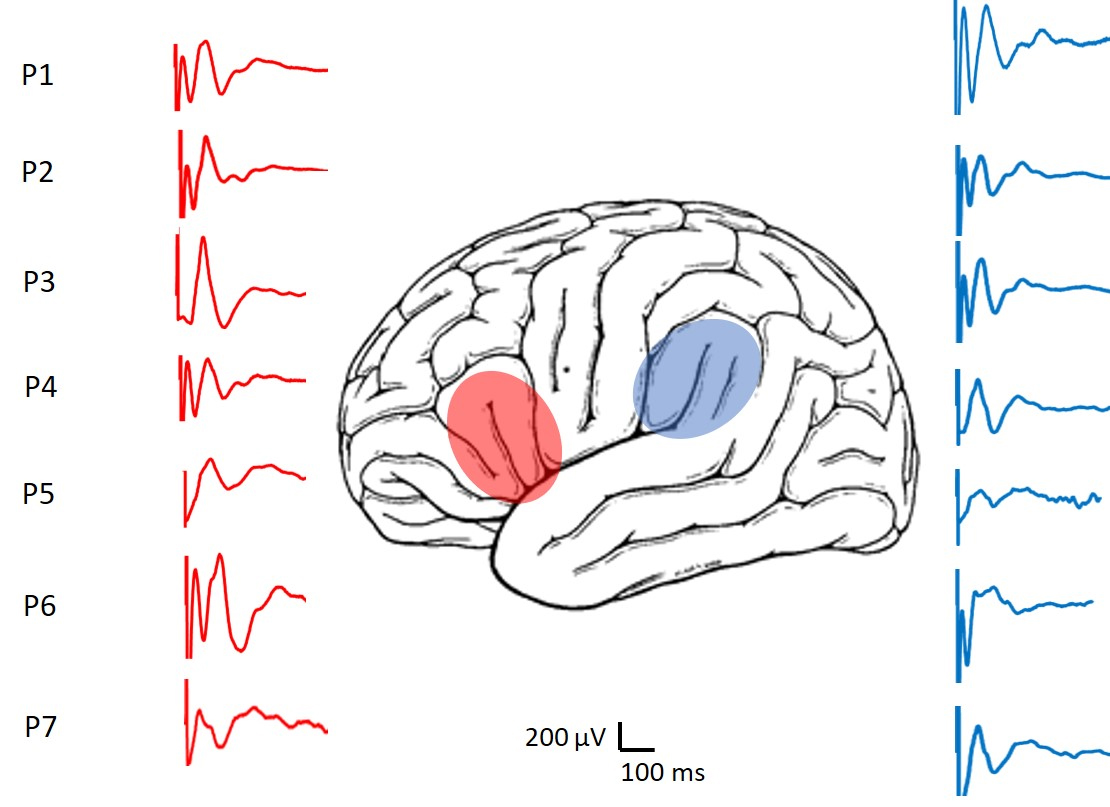 Fig. 4.
Fig. 4.Representative CCEPs recorded from the patients. In red the responses of the FLA are shown and in blue those of the PLA region.
In the FLA we found responses with an average latency for N1 of 39.0
We addressed the relationship between latencies and amplitudes for CCEPs at the FLA and PLA. Plots of these results are shown in Fig. 5.
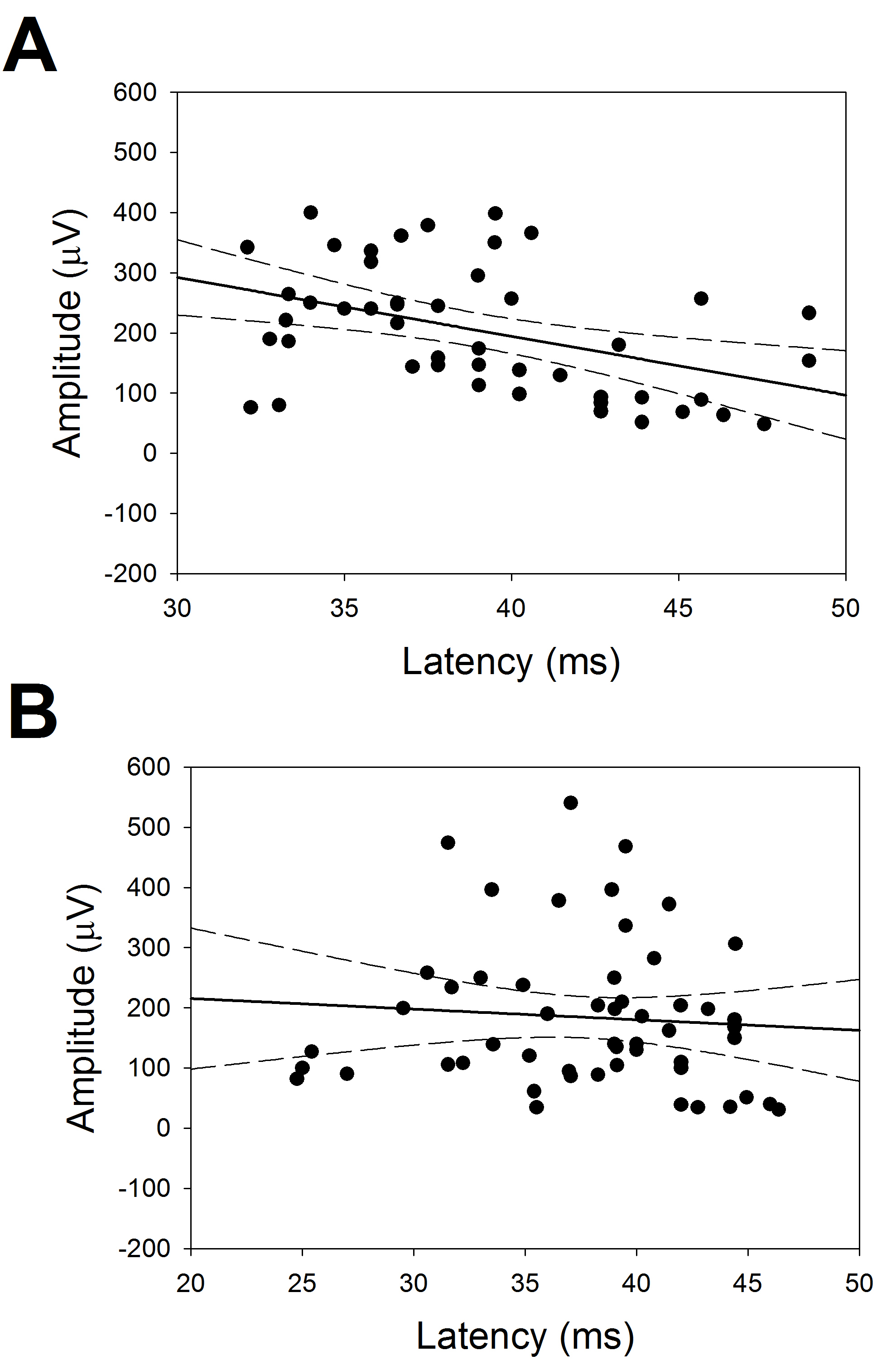 Fig. 5.
Fig. 5.Relationship between latency and amplitude of CCEPs. Linear regression between amplitudes and latencies. (A) FLA (r = 0.428) and (B) PLA (r = 0.078). Straight line indicates the regression and dotted ones the 95% interval of coefficient.
In Fig. 5, we can observe that, for FLA there was a significant linear
relationship between latency and amplitude
(
CCEPs were monitored in all cases during surgery, even in the patient that was awake [11]. In two cases, monitoring of the motor area of the face and tongue was also carried out through the electrodes of the FLA grid.
We had alarm criteria for five of the seven patients with a transitory alteration of CCEPs (Fig. 6A). Reversible alteration of tongue MEPs was also observed in one patient. In only one patient a definitely alteration in CCEPs was observed (Fig. 6B). This patient was the only one in which symptomatology persisted one year afther the surgery. In another patient, involvement of the CCEPs and MEPs of the face and mouth were also observed, occurring at different times during surgery, and both changes (Fig. 6C) were reversible. The patient presented with transient dysarthria in the early postoperative period.
 Fig. 6.
Fig. 6.CCEPs (black) and Motor (red) responses during IONM. (A) Reversible CCEP alteration in patient without postsurgical deficit. (B) Definitive CCEP alteration in a patient with established post-surgical dysarthria. (C) Reversible and alternative change of CCEP and MEP. Transient dysarthria in the early post-op.
The remaining patients did not develop new neurological deficits in the postoperative period (Table 2). At the one year follow-up visit, five patients were asymptomatic, one of them still had mild dysarthria, and another still had ES. Symptomatology was similar for one, six, and twelve months after surgery. Although the number of patients in this study was quite limited, we have computed several variables regarding Se and Sp for the early and late post-surgery periods. These results are shown in Table 3.
| Variable | 1st day | One year |
|---|---|---|
| Se | 1 | 1 |
| Sp | 0.5 | 1 |
| Youden’s index (J) | 0.5 | 1 |
| PPV | 0.6 | 1 |
| PNV | 1 | 1 |
The Se and Sp were high enough to assure confidence, as indicated J. More relevant, the PPV and NPV were also high, which means that the presence of CCEP alterations during IONM predicts the existence of postsurgical language deficits with high probability. On the contrary, the absence of warning events during IONM are associated with a high probability of a language function that is similar to function previous to surgery.
In this study, we showed that CCEPs can reliably identify cortical regions related to language function. CCEPs can also be used to monitor patients under general anesthesia because they are easy to obtain and reliable for predicting postsurgical outcomes of language function.
Intraoperative CCEP monitoring for language function is usually performed with the patient awake as a complementary technique. Therefore, this is one of the first works addressing its usefulness in an group patients under general anesthesia with most of them not awake. Giampiccolo et al. [20], recently described the usefulness of CCEP monitoring in patients under general anesthesia with results very similar to those obtained in this study.
Obviously, an unconsciouss patient has a great risk to suffer iatrogenic injuries, especially these patients in which the surgery is performed in extremely complex regions near the FLA, PLA, PMC, PSSC and subcortical structures such as the inner capsulae, thalamo-cortical radiations, or arquate fascicle. Further, most of these patients (all of them in our study) suffered from ES. Therefore, it is of great importance to monitor all of these structures and functions with a comprehensive neurophysiological approach. Consequently, MEP, SSEP, and maybe others, as cortical evoked potentials are mandatory. By the same reasoning, ECoG can precociously prevent or identify the presence of ES [21].
Understanding the neural connectivity between cortical eloquent areas and white matter pathways (e.g., cortical and association fibers related to language or motor functions) is extremely important to preserve brain function when surgically treating brain lesions. Some of them have been extensively identified in conscious patients [22]. Further, in the last two decades, it has been suggested that CCEP connectivity can be recorded between the FLA and PLA, such as in awake anesthetized patients [16, 18].
The reliability of this technique for identifying language areas had been previously described [11, 16, 18, 23, 24]. In our study, we performed mapping and monitoring of the language areas in anesthetized patients with excellent clinical results. The PPV and NPV were high enough to confirm the intense correlation between the presence of CCEP alteration and the appearance of new language deficits. On the contrary, the absence of CCEP warning was highly correlated with the absence of new language deficits. Nevertheless, as we recognized above, the number of patients was small and we must be cautious in the acceptance of these values. This high correlation between changes in CCEPs and post-op functions was demonstrated in the patient operated on with the sleep-awake technique [11] in which transitory changes in CCEPs were strictly associated with transitory language deficits.
Although the mechanism underlying CCEPs remains unclear, an accepted theory is that one of the transmission mechanisms could be cortico-cortical propagation directly conveyed through white matter fibers [25]. We have observed some anisotropy in the responses of FLA and PLA areas. Although no differences in latencies have been observed, it was suggested that long neural connections share some symmetry from the FLA to PLA, and in the reversal direction, amplitudes were higher for CCEPs obtained from the PLA compared to the FLA. Potentials are the result of extracellular currents from the underlying cortex. Therefore, the cortical structure, including the number and types of neurons and synapses, as well as its synchronization would be responsible for the amplitude and dynamics of the potentials. Therefore, it is conceivable that cortical regions with different cyto-architecture (Brodmann’s area 40 for Broca’s area and for Wernicke’s area) give rise to different potentials. Nevertheless, much work is needed to understand the sources of these potentials.
CCEPs have proven to be an effective technique for the identification and monitoring of language areas, so their use in anesthetized patients should not be limited to those who cannot tolerate awake surgery. An important matter to note is that, in awake surgery, 4–23% of postoperative deterioration of speech function has been described, despite negative intraoperative cortical mapping [26, 27]. Besides the presence of language deficits, the limitations of awake surgery must be considered seriously [21]. It is a stressful situation for the patient. Hence, patients must have both adequate cognitive function and emotional maturity. In fact, the Japan Society for Awake Surgery Guidelines limits the target patient population to those ranging from 15–65 years of age, although with some limitations, awake craniotomy can be used in the pediatric population [28]. Nevertheless, use in mentally handicapped patients remains problematic or impossible. In addition, although it is currently accepted that the intracranial pain-sensitive structures are limited to the dura mater and its feeding areas, and pain can be adequately controlled by topical anesthesia of the skin, bone, and dura, it has been observed recently that the pia and small cerebral vessels are also pain sensitive, inducing sharp, intense, and briefly painful events [29].
CCEP monitoring in sleeping patients could be an adequate alternative, since it would also have four added advantages over awake surgery, (i) it is better tolerated, (ii) it is not subject to subjectivity, such as the intraoperative evaluation of verbal responses, (iii) it can be performed in patients with preoperative language deficits, in whom an intraoperative neurological evaluation cannot be carried out, and (iv) can be monitored constantly, not only at some time points before or between different stages of tumor removal.
The disadvantages of this technique are the necessity of an extensive craniotomy
to allow adequate placement of the grid and also the increase in surgical times.
It could be argued as well that the charge density during SPES to obtain CCEPs is
high and could be harmful because it is several times higher than 30
Finally, we would like to pose a technical question not previously mentioned in the literature. The use of a notch filter gives rise to spurious waveforms quite similar to the true CCEPs. This can be misleading to the neurophysiologist, considering the presence of a positive response when in fact there is not. This bias can have dramatic results if there is erroneous identification of the functional areas. Therefore, it is extremely important to be conscious of this phenomenon.
Perhaps the most surprising fact used to justify awake surgery is that no differences were observed in the immediate postoperative motor status extent of resection between IONM in anesthetized patients and stimulation during awake craniotomy [25, 26, 31], although no detailed evaluation has been performed for the different techniques or surgeries. If there is no clear difference in the postsurgical outcomes, it is difficult to understand why it is necessary to stress the patient and the medical team when, instead, a systematic, calm and reliable mapping and monitoring of most brain functions, including language, can be done.
We hope that in the near future the CCEP technique will be widespread and equally accepted as motor or somatosensory evoked potentials for the assessment of motor and somatosensory pathways.
CCEPs have proven to be a reliable neurophysiological technique for mapping and monitoring the regions associated with language function in a small group of anesthetized patients. The high correlation between warning events and postsurgical outcomes suggests high sensitivity and specificity. Nevertheless, the small number of patients studied to date suggests these results should be considered cautiously.
CCEP, cortico-cortical evoked potentials; DCS, direct cortical stimulation; DTI, diffusion tensor imaging; ECoG, electrocorticography; ES, epileptic seizure; FLA, frontal language area; FN, false negative; FP, false positive; GTR, gross total resection; fMRI, functional magnetic resonance imaging; IONM, intraoperative neurophysiological monitoring; MRI, magnetic resonance imaging; PLA, parietal language area; PMC, primary motor cortex; PNV, predictive negative value; PPV, predictive positive value; Se, sensibility; Sp, specificity; SPES, single pulse electrical stimulation; STR, subtotal resection; TN, true negative; TP, true positive.
These should be presented as follows: JP and RGS designed the research study. JP and LVZ performed the research. PP and RGS provided help and advice on surgery and data recording. JP and LVZ analyzed the data. Initials JP and LVZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study observed the principles of the Helsinki’s Declaration and was approved by the Ethics Committee of the Hospital Universitario de la Princesa (Ref. 3109), and each patient provided wrote informed consent before surgery.
Not applicable.
This work was financed by a grant from the Ministerio de Sanidad FIS PI17/02193 and was partially supported by FEDER (Fonds Europeen de Developpement Economique et Regional).
The authors declare no conflict of interest. JP is serving as one of the editorial board members of this journal and the guest editor of the special issue on “Brain Stimulation and Neuroimaging”. We declare that JP had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GR.
Computation of charge density (
From the first equation, we can obtain
In our case, we have (in International System units)
Now, we must convert to more useful units. We know that
Therefore, the expression searched will be (Eqn. 1)
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
