†These authors contributed equally.
Academic Editor: Chul-Kyu Park
Background: Current data indicates the incidence of neuropathic pain after surgical nerve injury is as high as 50%, thus representing a major problem for patients and for the medical system. Triptolide, a traditional Chinese herb, has anti-inflammatory effects on various neurodegenerative and neuroinflammatory diseases. This agent also reduces peripheral nerve injury-induced neuropathic pain, although the mechanism underlying this effect is still unknown. Materials and Methods: The effects of triptolide on spinal nerve ligation (SNL) injury-induced neuropathic pain was studied in an animal model using behavioral, morphological and molecular biological methods. Results: Repeated administration of intrathecal triptolide was found to alleviate SNL- or Poly(I:C) (toll-like receptor 3 agonist) injection-induced mechanical allodynia without any motor impairment. The mechanism by which triptolide reduces SNL- and Poly(I:C) injection-induced microglial activation appears to be via the inhibition of OX42 expression, which is a microglial-specific marker. Intrathecal triptolide also suppressed SNL- and Poly(I:C) injection-induced expression of spinal TRIF. TRIF transmits signals from activated TLR3 and is the downstream adaptor of TLR3 in microglia. In addition, intrathecal triptolide inhibited the expression of spinal pro-inflammatory IL-1
Neuropathic pain is a typical postoperative chronic pain that has attracted considerable attention. Recent data from 2019 showed the incidence of chronic pain following surgical nerve injury was as high as 50% [1]. For many years, the research focus on neuropathic pain has been “neuron centered”. However, more attention is now being directed towards glial cells, and especially microglial cells [2]. Microglia are very sensitive to changes in their external environment. They can be rapidly activated after spinal nerve injury and participate in the pathological process by releasing cytokines that act on neurons and astrocytes, as well as on themselves. Therefore, microglia could play a decisive role in the development and progress of neuropathic pain [2].
Toll-like receptors (TLRs) exert critical roles during the inflammatory process in the central nerve system and are expressed by microglia, especially after peripheral nerve injury. Previous research indicated that L5 spinal nerve ligation (SNL) induced the up-regulation of toll-like receptor 3 (TLR3) in spinal microglia. The inflammatory response also increases after L5 SNL. Intrathecal administration of TLR3 agonist significantly increases microglial activation, whereas L5 SNL-induced microglial activation was apparently suppressed after TLR3 knockdown. Studies have suggested that TLR3 plays an important role in spinal microglia activation and in the development of SNL-induced neuropathic pain [3]. In addition, a previous study showed that inhibition of TLR3 signaling by spinal microglia contributes to the alleviation of neuropathic pain triggered by SNL. Therefore, targeting of TLR3 may be an effective way to relieve neuropathic pain induced by peripheral nerve injury.
Triptolide, a diterpenoid triepoxide, was first isolated and characterized from Tripterygium wilfordii Hook F (TWHF). It has demonstrated pharmacological activity against inflammation, neurodegeneration and neuropathic pain [4]. Previous studies also showed that repeated administration of triptolide could inhibit SNL-induced neuropathic pain in a dose-dependent manner. Triptolide inhibits SNL-induced activation of spinal dorsal horn astrocytes and microglia, reduces SNL-induced phosphorylation of MAPKs, and decreases the up-regulation of inflammatory cytokines after neuropathic pain. Therefore, triptolide exhibits potent pharmacological activities against SNL-induced neuropathic pain by modulating glial activation in the spinal dorsal horn. However, the direct target site of triptolide, such as a specific receptor, has yet to be identified.
Premkumar et al. [5] first observed that triptolide suppressed the Poly(I:C) (TLR3 agonist)-induced expression of COX-2 and iNOS in mouse macrophages. This suggests that triptolide might offer protection against inflammation by inhibiting the TLR3 pathway in macrophages. However, it is not known whether microglial TLR3 is involved in the anti-inflammatory effects of triptolide reported in previous studies.
In view of the above observations, the aim of the present research was to explore the effects of triptolide on the microglial TLR3 pathway in SNL-induced neuropathic pain.
Male Sprague–Dawley rats (200 g–220 g) were obtained from the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). Food and water were freely available and the light/dark cycle was 12:12 hr, with a 22–25 °C ambient temperature. Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Xi’an Jiaotong University (Xi’an, China) (No. 2017-225). Ethical guidelines for experimental pain in conscious animals were rigorously followed [6], and all efforts were made to minimize animal suffering and the number of animals used.
Intrathecal implantation for drug delivery was performed, by inserting polyethylene tubing into the subarachnoid space of the lumbar enlargement. Briefly, under pentobarbital anesthesia (45 mg/kg, i.p.), a midline incision (3 cm) was made at the level of the thoracic vertebrae of the rat. Polyethylene-10 tubing (inner diameter 0.28 mm, outer diameter 0.61 mm) of premeasured length was passed caudally from the T8 to the L3 level of the spinal cord, with 2 cm of tubing left exposed in the upper thoracic region. Animals were left to recover for 5 days before further study. Animals used for the subsequent procedures were neurologically normal and showed complete paralysis of both hind legs and tail following administration of intrathecal lidocaine (2%, 10
After anesthesia, the left L6 transverse process was cut to reveal the L4 and L5 spinal nerves. The L5 spinal nerve was carefully separated and tightly ligated using 6-0 silk thread [7]. All operative procedures in the sham groups were identical to the experimental group (SNL), but without ligation.
The Poly(I:C) ligand for TLR3 was purchased from Invivogen (San Diego, CA, USA). Triptolide (Sigma, St. Louis, MO, USA) was dissolved in 5% dimethyl sulphoxide (DMSO, Sigma, St. Louis, MO, USA). The doses of triptolide used in the present study were based on previous reports in the literature [8] and on preliminary experiments. Triptolide (10
Experimental animals were adapted to the behavioral test environment for 3 days in order to determine baseline levels. Following this, animals were placed on an elevated iron mesh floor and each animal was separated by an upside-down plastic box (30
After deep anesthesia (pentobarbital, 60 mg/kg, i.p.), animals were perfused with 100 mL of 0.9% normal saline through the ascending aorta, and then with 500 mL of 0.1M phosphate buffer (Pb, pH 7.3) containing 4% paraformaldehyde and 2% picric acid. The target tissue was then harvested, fixed for another 2–4 hours, and then frozen at 4 °C for 24 hours in 0.1 M Pb containing 30% sucrose. A cryostat (Leica cm1800; Heidelberg, Germany) was used to obtain transverse frozen spinal sections in three plates (30
Following animal sacrifice, the L5 dorsal horn was quickly harvested and immediately frozen on dry ice. The dorsal horn of the spinal cord was dissected using the open book method [9]. The selected area was homogenized with a hand-held pestle in SDS sample buffer (10 mL/mg tissue), containing a mixture of protease and phosphatase inhibitors. The specimens were then heated for 5 minutes at 100 °C and loaded onto a 10% SDS polyacrylamide gel with standard Laemmli solution (Bio-Rad laboratory, CA, USA). Following electrophoresis, proteins were electro-imprinted onto polyvinylidene fluoride membranes (PVDF, immobilon-p, Millipore, Billerica, MA, USA). The membrane was placed for 1 h in a blocking solution comprised of Tris-buffered saline containing 0.02% Tween (TBS-T) and 5% non-fat dry milk. It was then incubated with the primary antibody (rabbit anti-TRIF, 1:1000 dilution; Sigma, St Louis, MO, USA) or with mouse anti-
Animals were sacrificed following anesthesia, and Trizol (Gibco/BRL Life Technologies Inc, Rockville, MD, USA) was then used to collect total RNA after harvesting the samples as described previously [10]. Superscript™ III Reverse Transcriptase for RT-PCR (Invitrogen) was used to synthesize complementary DNA (cDNA). Amplification was then performed and repeated three times. The level of target cDNA was estimated from the threshold amplification cycle (CT). GAPDH was used as a control.
The rotarod test was used to evaluate whether the administration of triptolide affected the results of the behavior tests. Rats that had not previously been exposed to the test were placed on the rotarod accelerator treadmill (Ugobasile, Varese, Italy) and trained for 1–2 minutes at an interval of 30–60 minutes. After training, the animals were placed on the rotarod at a constant speed of 25 RPM. When the animal grasps the drum, the accelerator mode on the treadmill is selected so that the drum rotation speed increases linearly at 20 RPM. The time from the beginning of the acceleration period to the time the animal falls off the drum was measured, with a deadline of 30 seconds.
Two researchers who were unaware of the experimental design were responsible for data collection. Data was expressed as the mean
Immunofluorescence data was analyzed with ANOVA followed by calculation of the least significant difference. All positively stained cells were evaluated using a computer-assisted image analysis program (Metamorph 6.1) by setting the low and high thresholds for the immunofluorescent intensity as a signal. The same configuration was applied to measure cell areas in all groups, which were then automatically exported to Excel for statistical analysis. Metamorph 6.1 is calibrated to provide standardization of area measurements. Data was presented as changes relative to the DMSO-sham operation group or to the DMSO-saline group.
Behavioral results were evaluated by repeated measure ANOVAS and Fisher’s protected least significant difference post-hoc comparisons. One-way ANOVA followed by the least significant difference test was used to analyze the data from Western blots and RT-PCR.
SPSS
The experimental protocols used are shown in Table 1.
| Experimental protocols | |
| POD -5 | Intrathecal intubation |
| POD -4 | Recovery |
| POD -3 | Recovery and adapt to experimental environment |
| POD -2 | Recovery and adapt to experimental environment |
| POD -1 | Recovery and adapt to experimental environment |
| POD 0 | Baseline value assessment |
| Intrathecal drug | |
| SNL/sham operation or Poly(I:C)/saline injection | |
| POD 1 | Intrathecal drug |
| POD 2 | Intrathecal drug |
| POD 3 | Intrathecal drug |
| Rotarod and behavioral test | |
| IF, WB and RT-PCR | |
The allodynia behavioral test as indicated by the paw withdraw response may be influenced by motor impairment. Intrathecal triptolide or DMSO did not appear to alter motor function (Fig. 1). This confirms that triptolide does not affect motor function once there is an effect from SNL-induced allodynia.
 Fig. 1.
Fig. 1.
Effects of intrathecal triptolide or DMSO on rotarod test results. Triptolide or DMSO was injected intrathecally to normal control animals. The rotarod test was carried out at POD0 and POD3. No statistical difference was observed following intrathecal triptolide or DMSO compared to the baseline value.
After evaluating the baseline of mechanical allodynia, triptolide was injected intrathecally before SNL or Poly(I:C) injection on POD0, and then once a day for the next 3 days. An effect of triptolide on neuropathic pain induced by SNL or by Poly(I:C) injection was observed at POD3.
Compared to the DMSO-sham and DMSO-saline groups, the DMSO-SNL and DMSO-Poly(I:C) groups presented an obvious allodynia (Fig. 2A,B). Intrathecal triptolide suppressed mechanical allodynia in both the triptolide-SNL and triptolide-Poly(I:C) groups (each p
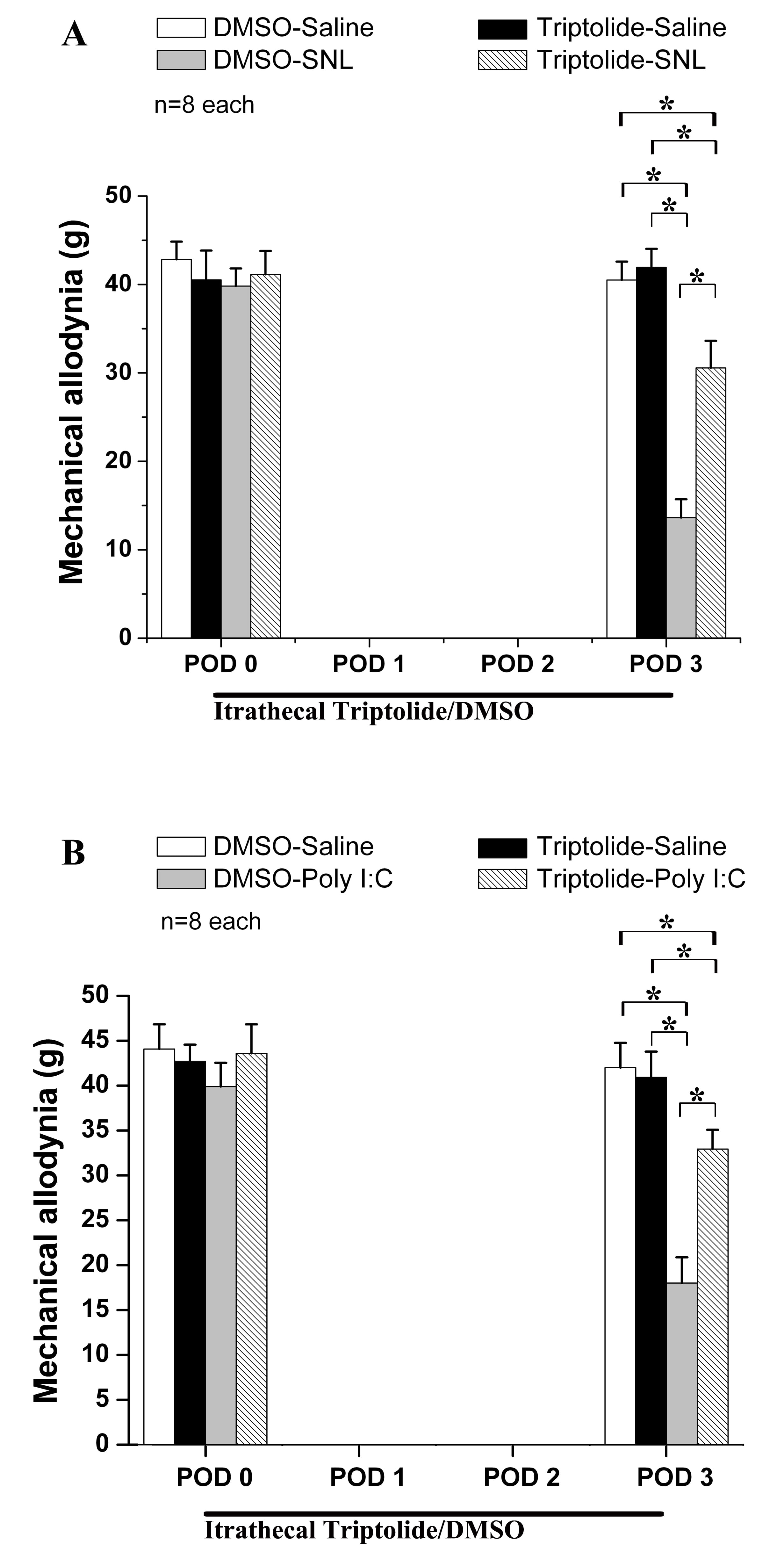 Fig. 2.
Fig. 2.
Effect of intrathecal triptolide on Poly(I:C) injection and on spinal nerve ligation (SNL). Behavioral tests showed that Poly(I:C) injection (B) and SNL (A) both induced mechanical allodynia on POD3. Intrathecal triptolide had a significant effect on neuropathic pain following Poly(I:C) injection (B) and SNL (A). * p
Up-regulation of OX42 expression in the spinal dorsal horn indicates that SNL and Poly(I:C) injection both induced microglial activation (Fig. 3). Moreover, spinal microglia in the DMSO-sham and in the DMSO-saline groups did not change, while triptolide did not alter OX42 expression in the triptolide-saline and triptolide-sham groups. In addition, intrathecal triptolide markedly decreased the up-regulation of OX42 induced by SNL or by Poly(I:C) injection (Fig. 3). These results suggest that triptolide can inhibit microglial activation induced by SNL or by Poly(I:C) injection during the development of neuropathic pain.
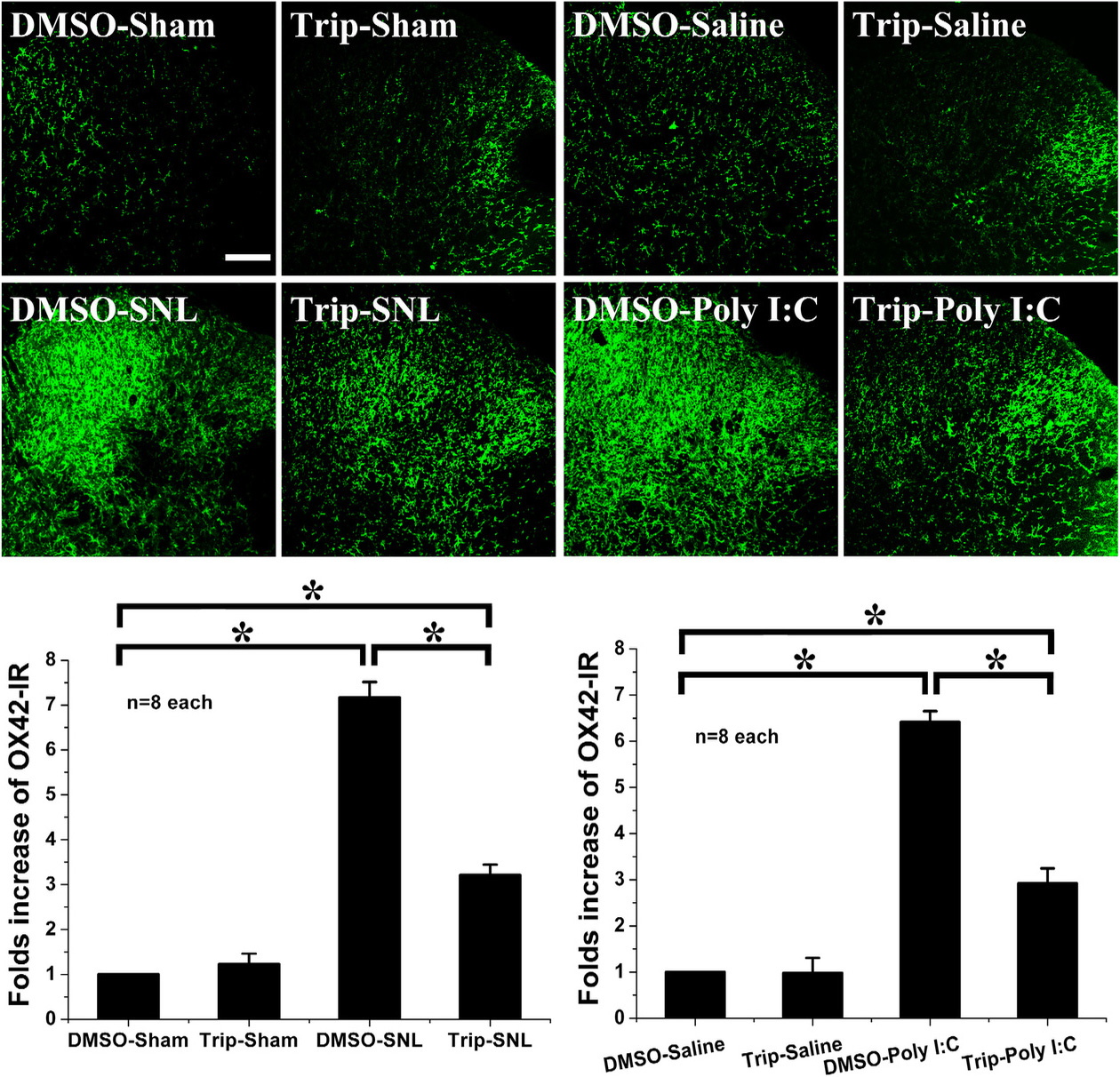 Fig. 3.
Fig. 3.
Effect of intrathecal triptolide following Poly(I:C) injection or SNL. Poly(I:C) injection and SNL induce microglial activation with marked up-regulation of OX42. Following intrathecal triptolide administration, microglial expression was not altered in the triptolide-sham group or in the triptolide-saline group. However, intrathecal triptolide suppressed the up-regulation of microglial OX42 induced by Poly(I:C) injection or by SNL, as compared with DMSO-SNL or DMSO-Poly(I:C). * p
Hence, the suppression of Poly(I:C) injection-induced spinal microglial activation concurs with the anti-allodynic effect of triptolide on SNL-induced neuropathic pain.
SNL and Poly(I:C) injection both induced high TRIF expression in the ipsilateral spinal dorsal horn (Fig. 4A,B). However, intrathecal triptolide markedly decreased spinal TRIF expression in the Triptolide-SNL and Triptolide-Poly(I:C) groups compared to the DMSO-SNL and DMSO-Poly(I:C) groups. Intrathecal triptolide did not affect spinal TRIF expression in the triptolide-saline or triptolide-sham groups. Inhibition of SNL- or Poly(I:C) injection-induced spinal TRIF activation, which is downstream of TLR3 in the microglia, might therefore be the underlying mechanism by which triptolide alleviates neuropathic pain.
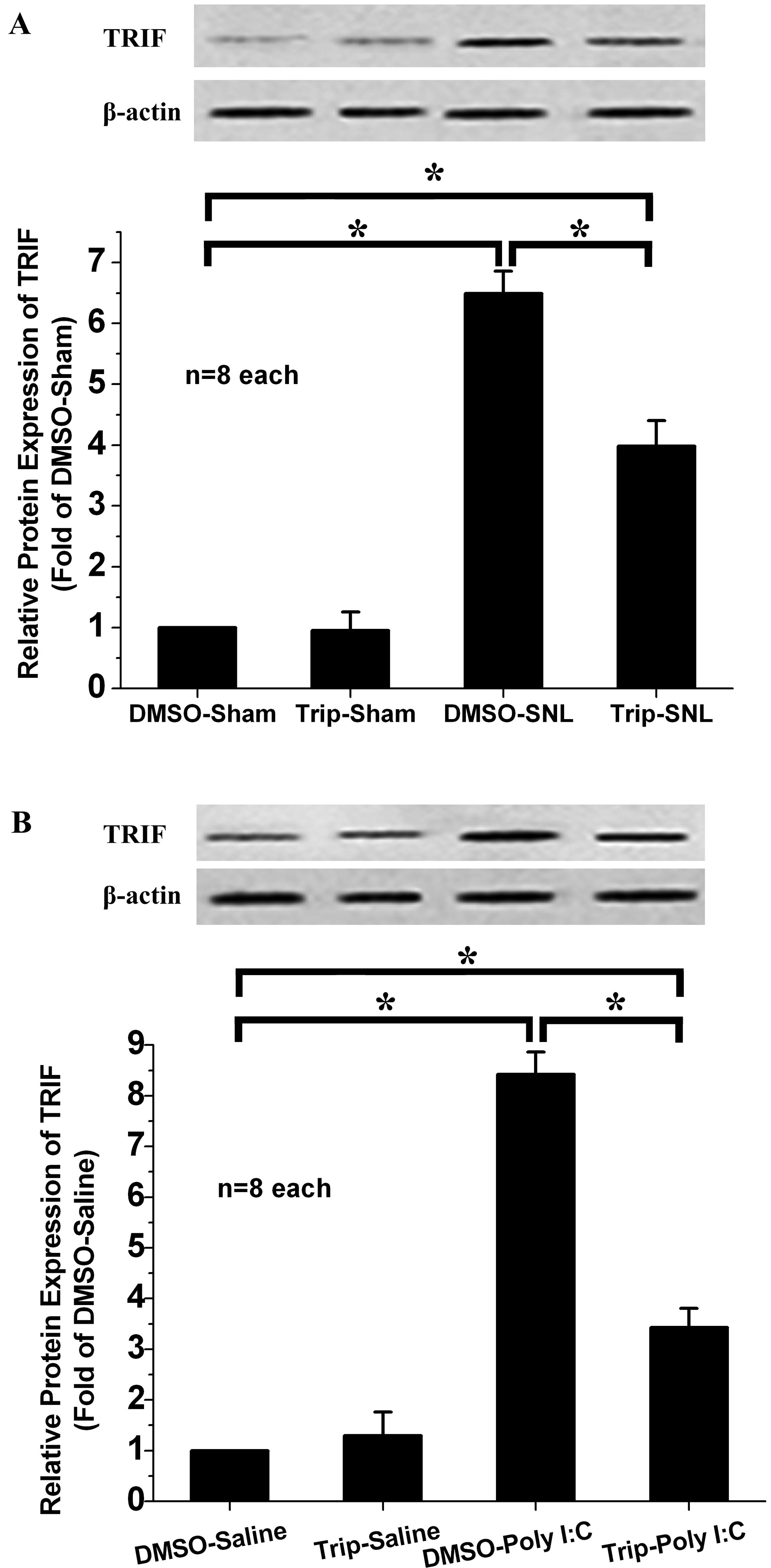 Fig. 4.
Fig. 4.
Effect of intrathecal triptolide on spinal TRIF expression. Poly(I:C) injection or SNL induced a striking increase in TRIF expression in the DMSO-SNL (A) and DMSO-Poly(I:C) (B) groups compared to the DMSO-sham group and DMSO-saline group. Intrathecal triptolide suppressed the up-regulation of TRIF expression following SNL (A) or Poly(I:C) (B) injection, without influencing TRIF expression in the triptolide-sham or triptolide-saline groups. * p
The RT-PCR results showed that IL-1
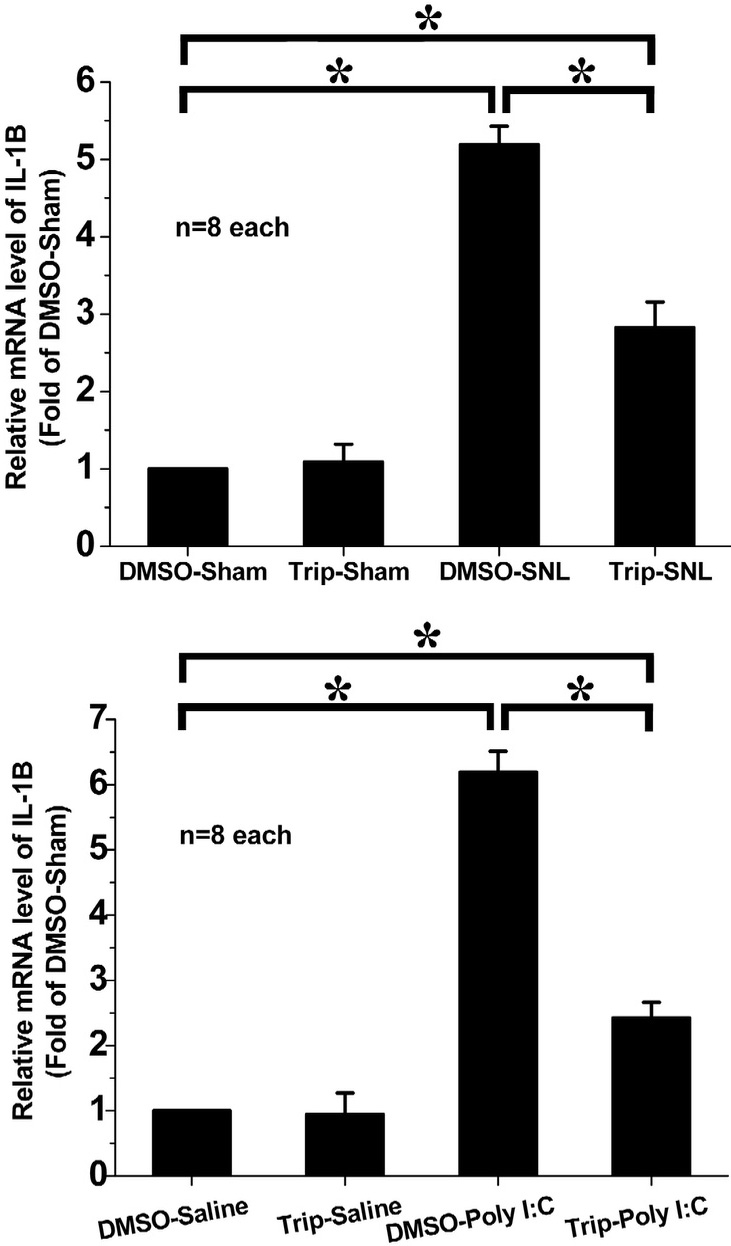 Fig. 5.
Fig. 5.
Effect of intrathecal triptolide on IL-1
This study examined the effect of intrathecal triptolide on mechanical allodynia associated with SNL and with Poly(I:C) intrathecal injection. The tactile allodynia induced by SNL was found to be partially reversed by triptolide injection. Moreover, intrathecal triptolide also attenuated the mechanical allodynia induced by intrathecal Poly(I:C), a TLR3 agonist. This result suggests that TLR3 might be involved in the anti-allodynic effect of intrathecal triptolide on neuropathic pain. Results from the rotarod test suggest the anti-allodynic effects of intrathecal triptolide did not imply there was motor impairment. In addition, SNL and Poly(I:C) intrathecal injection induced microglial activation via OX42 up-regulation. Intrathecal triptolide could suppress microglial activation, as indicated by the decreased expression of OX42 in the triptolide-SNL and triptolide-Poly(I:C) groups. These results suggest that inhibition of microglial activation may be involved in the alleviation of neuropathic pain by intrathecal triptolide. SNL and Poly(I:C) intrathecal injection also increased the expression of TRIF, which is downstream of TLR3. Moreover, intrathecal triptolide attenuated the increased expression of TRIF. This result also suggests that a TLR3-related pathway is involved in the observed effect of intrathecal triptolide on peripheral nerve injury-induced neuropathic pain. Furthermore, SNL and Poly(I:C) intrathecal injection caused increased expression of IL-1
Triptolide is one of the major active ingredients from the herb Tripterygium wilfordii Hook F. It has been used in traditional Chinese medicine to treat various diseases because of its well-known anti-inflammatory, immunosuppressive and anti-cancer properties [11]. Previous studies have shown that triptolide could exhibit anti-allodynic effects on neuropathic pain following SNL, although the underlying mechanism is unclear. The present research suggests that intrathecal triptolide exerts anti-allodynic effects by inhibiting activation of the microglial TLR3-related inflammatory pathway, which is a known cause of neuropathic pain after peripheral nerve injury. Triptolide can diffuse directly into the subarachnoid space via intrathecal injection, without going through the blood-brain barrier. The intrathecal route is especially suitable for the treatment of neuropathic pain since higher drug concentrations are obtained in the cerebrospinal fluid, leading to better efficacy. The current results suggest a new approach by which triptolide could be used to treat neuropathic pain after nerve injury.
In accordance with the present work, a recent study using a neurodegenerative model of Parkinson’s disease reported that triptolide could inhibit the activation of microglia and subsequent release of the proinflammatory cytokine IL-1
TLR3 is expressed by spinal microglia and has been confirmed to have a pivotal role in regulating the inflammatory response associated with neuropathic pain after peripheral nerve injury [3]. It was found that SNL induced TLR3 expression in microglia, resulting in microglial autophagy dysfunction that contributed to inflammatory activation. The intrathecal TLR3 agonist Poly(I:C) significantly induced microglial autophagy, whereas TLR3 knockdown markedly suppressed SNL-induced microglia autophagy activation. Intrathecal Poly(I:C) promotes the up-regulation of proinflammatory factors, whereas the inhibition of autophagy reduced the secretion of Poly(I:C)-induced proinflammatory mediators. Autophagy inhibition further decreased TLR3-mediated mechanical allodynia after SNL. These observations suggest that suppression of the TLR3/autophagy pathway helps to alleviate SNL-induced neuropathic pain. In line with this, the present work showed that TLR3 was involved in suppressing neuropathic pain by triptolide via modulation of the microglial proinflammatory mediator response. It is unclear, however, whether autophagy contributes to the effect of triptolide on neuropathic pain.
MyD88 is the intercellular adaptor for almost all TLRs, but not for TLR3 which uses TRIF as an adaptor for signaling [13, 14]. Extracellular ligands, as well as the synthetic analog poly(I:C), can bind to TLR3 and thereby recruit the critical downstream adaptor protein TRIF [15]. Activated TRIF dissociates from TLR3 and builds a speckle-like structure that acts as a platform for the recruitment of downstream complexes. This in turn leads to the activation of transcriptional factors such as IRF3 and NF-
A previous study showed the Poly(I:C) administration resulted in up-regulation of IL-1
Several limitations of the present study should be noted. Given the limited experimental time and sample size, this research could not evaluate whether intrathecal injection of triptolide caused any adverse reactions. This study also did not explore whether triptolide has the same therapeutic effect on other types of neuropathic pain besides SNL-induced, such as cancer, diabetes, and chemotherapy. Furthermore, it is unclear whether the regulation of autophagy is another mechanism by which triptolide can alleviate neuropathic pain. Although Poly(I:C) was used to investigate the effect of triptolide on a TLR3-induced pathway, this may not be a sufficiently direct approach. Considering the overall workload, it may be better to design TLR3 knockout experiments and other methods to allow more direct study of the effect and mechanism of triptolide action on neuropathic pain caused by peripheral nerve injury.
Based on the present research, some new perspectives should therefore be applied in future work. Firstly, the sample size should be expanded to investigate the effect of different times of triptolide administration on its efficacy, and to investigate whether this efficacy is different before and after injury. Secondly, the observation time following intrathecal triptolide administration should be prolonged in order to determine whether serious adverse effects can occur. Thirdly, different animal models of neuropathic pain should be established to compare the efficacy and mechanism of triptolide administration on different types of neuropathic pain. Finally, appropriate clinical studies should be carried out to further explore the use of triptolide for practical applications.
Taken together, the present research findings indicate that intrathecal triptolide can suppress activation of the microglial TLR3/TRIF/IL-1
SNL, spinal nerve ligation; Poly(I:C), Polyinosinic,polycytidylic acid; TRIF, Toll/IL-1 receptor domain containing adaptor inducing beta-interferon; TLRs, Toll-like receptors; TLR3, Toll-like receptor 3; IL-1
ZQZ and SMJ performed the experiments. LYY contributed to the data collection and analysis. XPM designed and wrote the manuscript. All authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the Institutional Animal Care and Use Committee of the Xi’an Jiaotong University (Xi’an, China) (No. 2017-225).
The authors thank for the critical scientific opinions by Jerome Staal from the Menzies Institute for Medical Research and University of Tasmania and for language polishing by Ejear Editing (SE2022060842).
This work was supported by the grants from the National Natural Science Foundation of China (30901400, 81270016), the Natural Science Foundation of Shaan-Xi Province (2018JM7101), the Clinical Research Award of the First Affiliated Hospital of Xi’an JiaoTong University (XJTU1AF-CRF-2018-018), the Fundamental Research Funds for the Central Universities (xjj2017128/0816-1191329727) and the Foundation of Chinese Medical Association (220160900005, CSDE012017120009).
The authors declare no conflict of interest.
