†These authors contributed equally.
Academic Editors: Pasquale Calabrese and Pietro Caliandro
Background: Although an extensive body of literature is trying to
verify the acute effects of exercise, findings are highly contradictory due to
many different study protocols. The number of studies using an intermittent
exercise (IE) protocol is limited, especially with regard to comparison across
the life span. We examined whether the effects of a HIIE protocol on performance
in a perceptual-cognitive task (NeuroTracker® (NT)) differed
between children, young adults, and older adults to address this gap.
Methods: A total of 36 participants participated in the present study:
12 children (CH, 6 females, 9.83
Physical exercise positively impacts the body [1] and has several well-documented neurobiological effects, such as augmentations of brain vascularization and increases in proteins and neurotransmitters, which promote neurogenesis, neuronal survival, and angiogenesis overall brain volume enhancement [1, 2]. High-intensity intermittent exercise (HIIE) has recently been shown to be an effective alternative to aerobic exercise programs, with positive effects on cognitive functions, such as information processing and response inhibition [3, 4].
Although a substantial body of literature attempts to verify the acute effects of exercise on cognitive performance in children, young or older adults, findings are highly contradictory. For example, there are studies showing positive effects of exercise on cognitive performance [5, 6, 7, 8], while other studies show a negative effect of exercise [9, 10, 11, 12, 13]. In addition, some studies demonstrated no effect of acute exercise (e.g., [13, 14, 15]) on cognitive performance. In terms of cognitive functions, acute exercise primarily improves executive functions (EF) that are associated with the prefrontal cortex, including attention, working memory, problem solving, cognitive flexibility, verbal fluency, decision making, and inhibitory control [16]. These positive changes have been shown to occur at very low to very high exercise intensities [17], with effects lasting up to two hours after exercise [18]. These effects depend on several factors, such as the exercise protocol (intensity and duration), the timing of cognitive testing (during versus post-exercise), the complexity of the tasks, and the fitness level of the participants [10, 17, 19]. Typically, acute effects of exercise are measured on a variety of simple cognitive tasks that target, for example, attention/perception (e.g., reaction time tests) or EFs such as working memory and inhibition (e.g., Flanker task, Stroop Color task, n-back task [20]). The consensus from the literature on acute exercise effects on cognitive performance is a small but positive effect of moderate HIIE, particularly in older adults [21, 22, 23]. However, Etnier et al. [24] noted that we are not dealing with a strong effect.
In general, all the exercises used in these studies increased heart rate, which may influence exercise-induced psychological arousal levels and play an important role in improving EF, possibly by increasing neural activity in the brain [10]. The mechanisms underlying the discussed effects of acute exercise on cognition are still under debate, and different neurophysiological explanations, such as the reticular-activating hypofrontality model [25] or the catecholamines hypothesis [26], emerged to explain these findings. However, there is evidence that the positive effects on cognition are caused by changes in the concentration of specific extracellular neurochemicals (neurotransmitters, neurotrophins, and neuromodulators; [27, 28]). These neurotransmitters (e.g., dopamine, norepinephrine, serotonin, acetylcholine, GABA, and glutamate), neurotrophins such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF), and neuromodulators (e.g., endogenous opioids and endocannabinoids) in turn lead to increased plasticity along with altered synaptic transmission and induction of brain vascularization during physical exercises, particularly affecting prefrontal cortices, which are associated with thinking, decision making, and behavior (executive function hypothesis; [29, 30]). Although increases in various neurochemicals have been confirmed, it is not entirely clear how these neurochemicals are involved in the changes in cognitive function induced by acute exercise [31]. For example, the effect of BDNF on plasticity or vascularization does not immediately lead to changes in cognitive performance. Instead, changes in the factors mentioned above are acute or chronic effects, which are changes that can occur as a result of exercise over a certain duration. Furthermore, vascular remodeling is not an acute process, nor is synaptogenesis. However, the modulation of a neurotransmitter or activation of its receptor on an endothelial cell, which causes vasodilation, are acute effects that may have an immediate impact on cognitive function.
There is a wide variation in the protocols used to deliver acute exercise
intervention [32]. Most studies examining the effect of exercise on cognitive
function focused primarily on continuous forms of exercise such as running or
cycling at a constant intensity (low, moderate, and/or high). The number of
studies using an intermittent exercise (IE) protocol is limited [33], although
acute HIIE protocols tend to increase concentrations of certain extracellular
neurochemicals more significantly than low-intensity acute exercise protocols in
healthy young adults [31, 34, 35] and late- and middle-aged older adults [36]. In a
prospective randomized controlled trial, Winter et al. [37] demonstrated
that a HIIE protocol (2
The question remains whether or not the benefits of HIIE exercise on cognition, particularly EF, occur in children. Several studies demonstrated positive effects of HIIE on cognitive function in children [8, 38, 39, 40]. Cooper et al. [38] stated that these findings in children are consistent with the literature for adults, suggesting that HIIE has a particularly positive effect on cognitive function. A recent systematic review summarized the existing evidence on the chronic effects of exercise interventions on cognitive function across the life span [41]. This review concluded that there is moderately strong evidence that moderate to high exercise intensities lead to improvements in cognition, especially processing speed, memory, and EF. According to this review article, the most substantial evidence is observed in children between 6 and 13 years and older adults over 50 years. Similar to this review, a meta-analysis by Chang et al. [17] investigating the effects of acute exercise on cognitive performance demonstrated that acute effects may have contributed to larger effects in children and older adults. Since these age groups are vulnerable to exercise, exercise’s acute and chronic positive effects may be observed. However, the meta-analysis by Chang et al. [17] highlighted the difficulty in comparing the intensity and duration of acute physical activity due to different physiological developmental processes in children, young adults, and older adults. Overall, the results of this meta-analysis showed that moderate exercise had a positive effect on cognitive performance during and immediately after exercise and after exercise with a delay. Limitations in the experimental literature on children are that many of the studies have not described the experimental procedure of the exercise interventions (e.g., intensity) in sufficient detail, are often characterized by low methodological rigor, and show procedural differences that are likely due to the usual classroom-based research approaches to exercise and cognition in children (i.e., Tomborowski et al. [42]).
However, acute exercise is likely to affect complex cognitive tasks that engage the executive control system due to the interaction between executive tasks and brain function and exercise-induced stress [43]. The decline in performance is particularly evident during moderate-intensity [44] and high-intensity exercise [45]. Tasks that require EF are related to cognitive flexibility, inhibitory control, and working memory [32] and are more involved in prefrontal cortex functions than other simple tasks such as recall or short-term memory, visual search, or simple and choice reaction time [46]. Such simple tasks require focusing on identifying relevant stimuli and then responding to comparatively simple, predetermined responses [47] and show limited involvement in the function of the prefrontal cortex [48]. In contrast, multiple object tracking (MOT; [49]), in which multiple moving targets are tracked simultaneously, requires continuous task performance with higher-level cognitive functions. Also, MOT tasks resemble various everyday tasks that require tracking multiple objects, such as playing or watching various team sports, crossing a busy street, or driving in traffic. The Multiple Object Tracking (MOT) paradigm, first developed by Pylyshyn and Storm [49], has been used extensively to study MOT in a laboratory setting. Faubert and Sidebottom [50] introduced a perceptive-cognitive training program for athletes called NeuroTracker (NT; CogniSens Athletic, Inc., Montreal, Quebec, Canada). This training program is of great importance for processing information that requires attention and visual-spatial working memory in a dynamic context [50]. It stimulates many brain networks that must work together during exercise, including complex motion integration, dynamic, sustained, and distributed attention processing, dynamic visual-spatial processing, and working memory [51]. 3D-MOT tasks are now widely used in research to investigate dynamic visual attention in different groups of individuals (elderly, healthy controls, and children with neurodevelopmental disorders). For example, a recent study demonstrated a positive relationship between 3D-MOT ability and task performance in elderly participants tested with the NeuroTracker and two driving simulator scenarios. Better NeuroTracker performance was significantly associated with fewer crashes and lane deviations [52]. In another study, older participants performed worse than younger participants on MOT tasks [53]. Pothier et al. [54] tested MOT while walking simultaneously with young and older adults. They found a decrease in performance on the MOT with increasing complexity of the MOT task. An age-related decrease in MOT and walking performance was found, with older adults’ performance impaired under conditions of high attentional load. Similar results were observed in children with neurodevelopmental disorders, in whom repeated 3D-MOT training resulted in better overall attentional performance on the Conners Continuous Performance Task [55].
In summary, much of the literature examining the exercise-cognition relationship focuses on young adults. However, there is a lack of information on children [56, 57], especially compared with young and older adults. In addition, most studies measure cognitive performance at rest but rarely during exercise, and only few studies use HIIE protocols. Although our previous study investigated perceptual-cognitive performance using the NT during physical exercise in terms of a dual-task (DT) paradigm [58], studies examining this during acute exercise across the lifespan are still lacking. To address these gaps, we focus on the effect of a HIIE protocol on perceptual-cognitive performance during exercise in children (CH), young adults (YA), and older adults (OA). In a review by Basso and Suzuki [31], acute exercise was defined as a single bout of physical activity, such as the HIIT protocol used in this study. Therefore, we examine whether the effects of a HIIE protocol on performance in a perceptual-cognitive task differ between CH, YA, and OA. We expected that (1) all groups improve their perceptual-cognitive task performance throughout the HIIE protocol and that (2) YA, in particular, demonstrate better performance in the perceptual-cognitive task than OA and CH at rest and during exercise.
This study employed a repeated-measures, within-subjects design.
Thirty-eight physically active participants participated in the present study: 12 children (8 to 12 years), 12 young adults (18 to 30 years), and 14 older adults (60+ years; see Table 1 (Ref. [59, 60, 61]) for group characteristics). This is based on a power analysis using G-power. We determined that a total sample size of at least 27 participants would suffice to detect between/within-factor interactions for repeated measures ANOVA, assuming a moderate effect size (f = 0.25) with alpha set at 0.05 and power at 0.8. Considering a 30% dropout rate, the sample was estimated to be 12 participants in each group, for a total of 36 participants. Recruitment occurred via a convenience-based, non-probability sampling approach (flyers at the University Sports Center and classroom announcement at a school in the Rhein-Neckar area containing the objectives and procedures of the experiment).
| CH | YA | OA | stat. analyses | |
| (n = 12) | (n = 12) | (n = 12) | ||
| Age (years) | 9.83 |
23.5 |
66.9 |
F(2, 33) = 1042***, |
| Sex (n male) | 6 | 6 | 8 | |
| Education (years) | 3.83 |
14.4 |
13.1 |
F(2, 33) = 80.5***, |
| Weight (kg) | 34.1 |
68.2 |
70.9 |
F(2, 33) = 67.9***, |
| Height (cm) | 1.48 |
1.76 |
1.73 |
F(2, 33) = 38.5***, |
| BMI (kg/m |
15.5 |
21.9 |
23.5 |
F(2, 33) = 74.8***, |
| HRmax |
201 |
186 |
161 |
F(2, 33) = 277***, |
| Sports activity/week (min) | 279 |
323 |
215 |
F(2, 33) = .891 |
| Bruce protocol |
8.90 |
13.2 |
5.60 |
F(2, 33) = 51.9***, |
| VO |
33.3 |
49.9 |
20.6 |
F(2, 33) = 74.0***, |
| MoCA | n.a. | n.a. | 29.6 |
n.a. |
| Note: ***p | ||||
They were excluded if they reported (a) musculoskeletal disorders such as arthrosis affecting running, central or peripheral neurological diseases (e.g., previous stroke), (b) recent acute illness or surgery, (c) taking psychiatric drugs that may affect cognitive performance, and/or psychiatric disorders, (d) wearing glasses, as the use of 3D glasses was required. The following inclusion criteria were used to recruit participants: normal or corrected-to-normal vision and hearing, ability to walk independently, and the ability to follow instructions for testing. Two participants with pre-existing myocardial conditions were excluded after an initial preliminary examination. Only healthy, active, and inactive participants were included.
The young and older adults were asked for their consent and willingness to participate in the study, and the children’s legal guardian/next of kin provided written informed consent to participate in this study. The participants or the legal guardians of the children did not receive any financial compensation or incentive for taking part in the study. All assessments were conducted in accordance with ethical rules for research in human subjects following the Declaration of Helsinki [62].
Participants’ demographic information was collected, their height and weight
were measured, and the body mass index (BMI, kg/m
We used a questionnaire validated in our laboratory [63, 64]. It records the
types of sports and the frequency of weekly sports activity (frequency/week and
duration/exercise session) with the following questions: What sports do you do in
the club (or recreational). How many training sessions do you currently complete
per week in the club? How long does a training session last on average? Then, the
total sports participation (h/week) was calculated as follows:
(frequency_activity1
In the present study, the Bruce protocol [66] was performed on a treadmill
(model: h/p/cosmos pulsar® 3p, Nussdorf-Traunstein, Germany) to
determine the maximum oxygen uptake (VO
The 3D-MOT (3D Multiple Object Tracking) is a perceptual-cognitive training program under the NeuroTracker® system licensed by the University of Montreal (NT; CogniSens Athletic, Inc., Montreal, Quebec, Canada). The participants stood or ran on a treadmill with 3D glasses; their position was chosen to move at an angle of 45 degrees in front of a 3D TV (Samsung, 65 inches).
To complete the task, participants must track the target objects in Core mode while ignoring the distraction objects. At the beginning, participants are presented with eight yellow balls for 2 seconds, four of which briefly light up orange to signal which balls they must track. Then, all eight balls return to their original yellow color and move in the 3D space across the screen for eight seconds. Next, participants track the four target balls as all balls move while ignoring the four distraction balls. Once all eight balls stop moving, participants select the four balls they think are the target balls by naming the corresponding numbers. If the participant correctly identifies all four target balls, the speed of the next trial increases. However, if the participant does not correctly identify all four balls, the speed of the next trial decreases. In this way, a session-specific speed threshold is calculated (staircase method; [69])), which is then used as the average visual pursuit speed (achieved speed (cm/s) after 20 repetitions; NT score). Both the score and the hit rate (proportion of target balls correctly identified) of each session are included in the analysis. The individual phases of the task are shown in Fig. 1 (Ref. [70]). Each session lasted between six and eight minutes. The NT score has proven to be a valid indicator of high-brain cognitive function [51, 71].
 Fig. 1.
Fig. 1.3D-MOT task. (a) Presentation of the randomly positioned objects in a virtual volumetric space. (b) Target objects to be tracked during the trial. (c) Movement of all objects with dynamic interactions. (d) Observer’s response by identifying the target objects. (e) Feedback is given to the observer (with permission from the author; [70]).
The HIIE treadmill protocol used in the present study consisted of eleven
30-second intervals at 90% VO
All measures were conducted in the Institute for Sports and Movement Science laboratory at the University of Stuttgart. The overall test procedure per participant comprised two test dates, each lasting 90 minutes, two weeks apart. On the first day of testing, participants (in the case of children and their legal guardians) were informed about the purpose of the study and signed an informed consent form. A questionnaire was used to collect demographic information and sports biography. The MoCA was used to screen older adults for cognitive impairment. After 15 minutes of rest, perceptual-cognitive performance was assessed with the NeuroTracker® software (baseline1: one session with 20 trials) while standing, followed by assessing aerobic endurance on a treadmill using the Bruce protocol. On the second day of testing (2 weeks later), the perceptual-cognitive task was again performed while standing (baseline2: one session with 20 trials) and during the HIIE protocol (HIIE condition: three sessions with 20 trials each at HIIE-5 min, HIIE-15 min, and HIIE-25 min). All measures on the first and second day of testing were conducted in the morning (see Fig. 2).
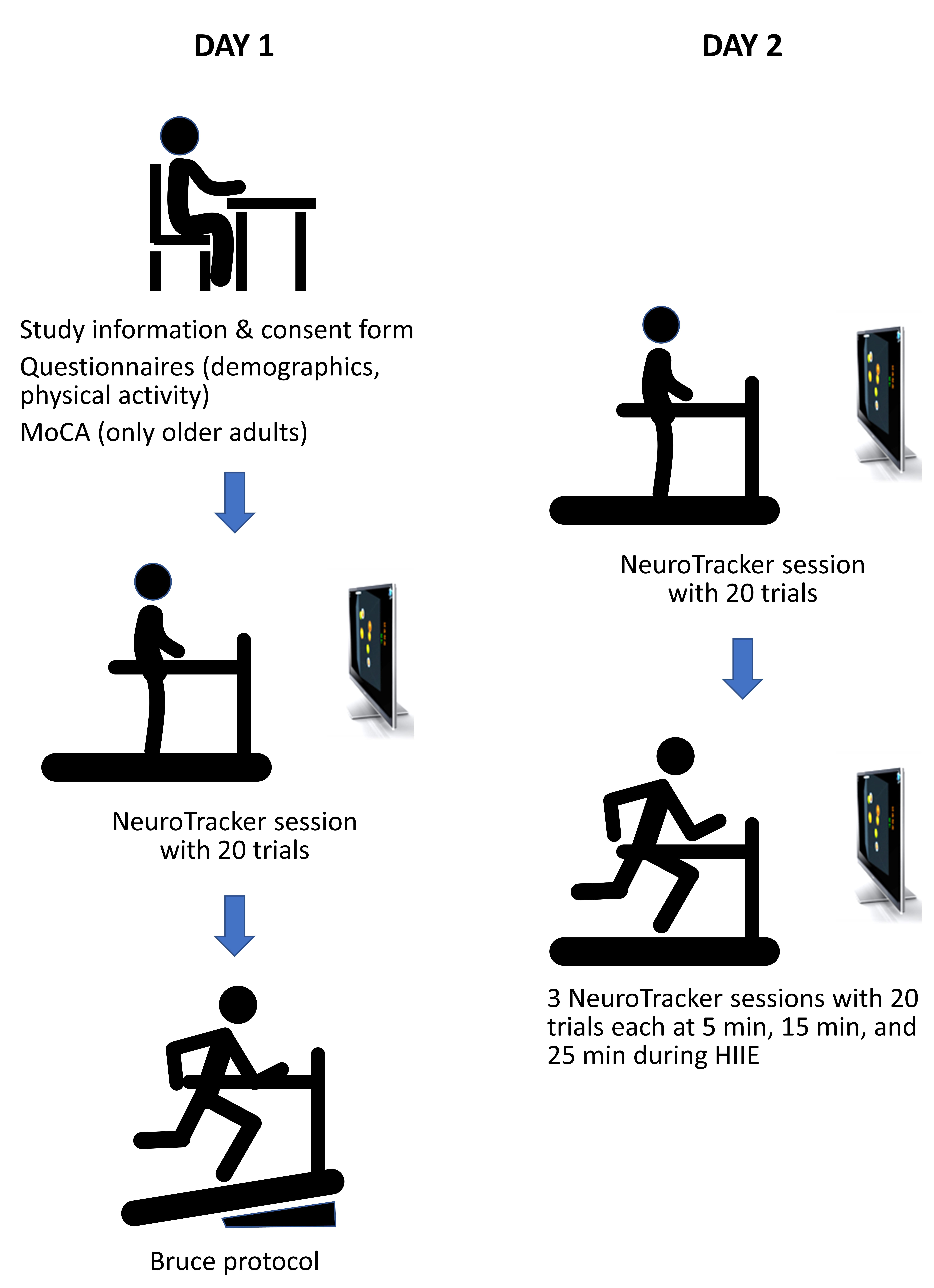 Fig. 2.
Fig. 2.The experimental design of this study.
Data were analyzed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA). First, we explored all dependent variables to examine missing values, normality of distributions as a prerequisite for calculating the ANOVAs (tested by Kolmogorov–Smirnov tests), and the presence of outliers. An alpha level of 0.05 was used for all statistical tests. Group comparison with respect to the three age groups was analyzed for continuous variables (age, BMI) using repeated measures ANOVAs, and sex as categorical demographic variables was analyzed using the Chi-Square test.
The course of perceptual-cognitive tasks (speed threshold) was analyzed using both absolute (unprocessed speed thresholds) and normalized values (processed speed thresholds; [55]). Normalized values represent performance relative to the mean of baseline1. Thus, in terms of normalized progressions, baseline1 is the zero value, and improvements/declines at subsequent time points (baseline2, HIIE-5 min, HIIE-15 min, and HIIE-25 min) vary based on participants’ performance at baseline1. We divided the data obtained in each session (baseline1, baseline2, HIIE-5 min, HIIE-15 min, and HIIE-25 min) by the data obtained in baseline1 to calculate changes in the progression.
Each possible predictor variable was first mean-centered, where each individual
score was subtracted from the average value to determine the correlations between
NT performance and age, BMI, education, exercise, and VO
A 3 (group: CH, YA, and OA)
If the result of the ANOVAs were significant, post-hoc tests (Bonferroni
correction) were used to test which factor levels (group) were significantly
different from each other. Effect sizes for all ANOVAs were expressed using the
partial Eta
Table 1 shows the characteristics of the sample. All participants can be
classified as normal-weighted according to the current WHO criteria. On average,
adults have a high level of education. None of the older adults had a MoCA score
that would categorize them as cognitively impaired. Fitness is at least adequate
in all participants as measured by VO
There were no significant correlations between NT performance and BMI and the
weekly sports activity. However, moderate correlations were found for NT
performance and VO
| NT performance | |||||
| Baseline1 | Baseline2 | HIIE-5 min | HIIE-15 min | HIIE-25 min | |
| Children | |||||
| BMI | 0.393 | 0.366 | –0.087 | 0.096 | 0.086 |
| Sports activity/week | 0.342 | 0.176 | 0.282 | 0.365 | 0.095 |
| VO |
–0.590* | –0.501 | –0.441 | –0.628* | –0.507 |
| Education | 0.529 | 0.453 | 0.672* | 0.491 | 0.745* |
| Young adults | |||||
| BMI | 0.039 | 0.005 | 0.121 | 0.321 | 0.384 |
| Sports activity/week | –0.122 | –0.019 | 0.154 | 0.082 | 0.302 |
| VO |
0.360 | 0.485 | 0.352 | 0.543 | 0.656* |
| Education | 0.186 | 0.108 | –0.263 | –0.426 | –0.292 |
| Older adults | |||||
| BMI | 0.124 | –0.278 | 0.124 | 0.155 | –0.213 |
| Sports activity/week | 0.215 | –0.092 | –0.055 | 0.176 | –0.294 |
| VO |
0.011 | –0.014 | 0.051 | 0.213 | 0.002 |
| Education | –0.415 | –0.073 | –0.284 | 0.153 | –0.395 |
| Note: *p | |||||
Repeated measures ANCOVA for absolute NT scores controlled for VO
 Fig. 3.
Fig. 3.Absolute perceptual-cognitive task progression.
Regarding progression during HIIE, we saw a significant main effect time,
F(2, 64) = 6.12, p = 0.004,
 Fig. 4.
Fig. 4.Normalized perceptual-cognitive task progression.
Repeated measurement ANCOVA for normalized NT scores controlled for VO
This study aimed to examine the performance of CH, YA, and OA on a perceptual-cognitive task during an HIIE. Considering baseline measures, the group of YA performed best and achieved significantly higher absolute NT scores than CH and OA.
Legault et al. [63] reported similar results on young and older adults in a previous study. This pattern is also consistent with Kennedy et al. [73], who observed an age-related decline in tracking ability and suggested that these findings may indicate a decline in higher-level cognitive functions. In addition, Trick et al. [74] noted that, on average, YA could track four targets at once, whereas OA can track around three targets (see also Alvarez and Franconeri [75]). These results may be related to the development of functions thought to be involved in MOT, mainly working memory [76] and attention [77, 78]. In MOT tasks, participants must continuously monitor (i.e., track) the changing spatial positions of targets and actively maintain target representations over time, which requires visual working memory, another process involved in MOT. Drew et al. [79] showed neural activity, indicating that two separate mechanisms are involved in tracking: an indexing mechanism closely associated with visual working memory and a mechanism that tracks target locations. There appears to be a high degree of inter-individual variability in this tracking threshold, which may link to MOT expertise. For example, the ability of humans to track multiple objects simultaneously increases with age [80]. Various examples from daily life include monitoring children on a playground or in a swimming pool, tracking multiple vehicles and pedestrians while driving, or watching a soccer match. Oksama and Hyönä [81] examined individual differences in MOT performance and found that visual-spatial short-term memory capacity of 2 to 6 items was a significant predictor of MOT, indicating a role for memory in tracking. The well-documented performance limitations (e.g., speed, distances, number of targets) also suggest that a limited attentional resource is available to support tracking. In particular, increasing the difficulty of a MOT task can deplete the attentional resource to the point where a second object cannot be tracked [82].
Since both attention and working memory can be affected by age, it seems reasonable to expect age-related decrements in performance on MOT tasks [83]. Furthermore, the significant difference between CH and YA and OA in the present study is consistent with previous examinations of MOT performance in childhood and across the lifespan [53, 74]. Moreover, Trick et al. [74] and Harris et al. [84] suggest that regular exposure to certain real-life activities, such as action sports and videogames, is associated with higher MOT performance. Therefore, CH likely performs better than OA because they experience such gaming situations and complex dynamic scenes more frequently in their everyday lives. However, Legault et al. [85] pointed out that further experiments will be required to identify which functions may be affected by aging since 3D-MOT is a complex task sensitive to many factors.
Considering NT performance during HIIE, the present study includes DT conditions (i.e., running while MOT) that target participants’ ability to flexibly allocate attentional resources and switch between the goals of the tasks performed in parallel (cognitive flexibility). There is strong evidence that OA demonstrate greater DT costs than YA [54, 86] as they require more time to switch between two different tasks [87]. Thus, these decreases with increasing age and demands of the NT could explain the differences in the absolute task progression between YA and OA. The reticular-activating hypofrontality model (RAH; [48, 88]) hypothesizes that acute exercise shifts the brain’s metabolic resources away from specific regions such as the prefrontal cortex and instead favors structures that support exercise, such as the reticular formation and motor cortices. This process would facilitate sensory and motor task performances, whereas the associated hypofrontality would impair EF. Therefore, the effects of shifting metabolic resources can be greater in OA than CH, resulting in less MOT task progression, particularly in OA. The significant improvement in absolute NT scores in YA during HIIE seems consistent with the notion that acute exercise has a small positive effect on cognitive performance [5, 7]. Notably, since performance in CH and OA remains relatively constant, this may be indicative of age-related differences in potential effects of acute HIIE.
However, the present findings are inconsistent with learning effects in MOT
tasks, as previous studies demonstrated continuous task progression in different
populations [89], including YA and OA adults [85], concluding that both have a
similar ability to improve with training. In the absence of a significant
interaction time
In conclusion, the present study confirms previous findings on age-related differences in NT performance. Results show that YA perform better than CH and OA at baseline and during HIIE. However, in all three groups, we saw an improvement in NT performance for the absolute scores. Beneficial effects of acute HIIE on perceptual-cognitive performance may be confounded with learning effects and cannot be inferred from mere task progression; therefore, future studies should include a control group without HIIE but with perceptual-cognitive training. Based on the present findings, the effects of different exercise protocols (e.g., continuous vs. intermittent) seem to be a worthwhile subject for future investigations. Normalized speed thresholds should best capture improvement differences between groups to compare results across studies better, as pre-test values are taken as the baseline.
CH, children; HIIE, high-intensity intermittent; exercise; IE, intermittent exercise; MOT, multiple object tracking; NT, NeuroTracker; OA, old adults; YA, young adults.
TJK—Formal analysis, Writing-Original draft, Visualization; SYP—Conceptualization, Methodology, Investigation, Data Curation, Writing-Original draft; VB—Investigation, Writing-Reviewing and Editing; NS—Conceptualization, Methodology, Writing-Reviewing and Editing, Visualization, Supervision.
The study was conducted according to the guidelines of the Declaration of Helsinki. After consultation of the Ethics Committee of the University Stuttgart, the anonymous analysis of data of our participants, which were collected as part of intervention, needed no guidance after the Professional Code for Physicians in Germany (§15 (1)). There were no concerns of the commission about collecting, processing and publishing such data. Also, regarding the guidelines of the German Research Foundation (DFG), no ethics application was required to conduct this study. All younger and older adults and the children and their parents/caregivers gave their written informed consent.
The authors thank the volunteers who participated in the study.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
