Ischemic stroke is an acute cerebrovascular disease and the third most common cause of death after ischemic heart disease. Increasing attention is being paid to finding effective treatments through traditional medicine. Thus, studying the traditional medicine for the treatment of ischemic stroke is of great importance. Traditional medicine in China includes traditional Chinese medicine (TCM) and other ethnic medicines, which is rich in variety and resources. This review first introduces the treatment mechanisms associated with ischemic stroke, such as antioxidant nitrification, antiexcitotoxic, antiapoptotic, anti-inflammatory, antiplatelet and anticoagulation mechanisms. Then, we calculated the frequency of prescription use for ischemic stroke and summarized the treatments for ischemic stroke by investigating 13 drug monographs and standards. We found 192 prescriptions from the traditional medical system for ischemic stroke, including Angong Niuhuang pill, Qishiwei Zhenzhu Pills, Ginkgo biloba leaf, and other traditional Chinese patent medicines and national medicines. There were 398 kinds of traditional medicine, including 301 kinds of plant-based medicines, 54 kinds of animal-based medicines, 28 kinds of mineral-based medicines, and 15 kinds of other medicines. We introduced the names, families, medicinal components, traditional uses, phytochemical information, and pharmacological activities of the commonly used Chinese patent medicines and TCMs. In addition, some chemicals were introduced. These medicines may be potential candidates for the treatment of ischemic stroke. This work provides a reference for the research and clinical use of new drugs for ischemic stroke.
Stroke is a kind of acute cerebrovascular disease. Brain tissue damage or dysfunction caused by the sudden rupture or blockage of cerebral vessels prevents blood flow into the brain. Stroke is also the third most common cause of death (4.5%) after ischemic heart disease (6.1%) [1]. According to the statistics of the World Health Organization (WHO), 16.9 million people suffer from stroke every year, and the incidence of stroke is 1.5 times higher in males than in females. According to an epidemiological survey, the number of stroke survivors will continue to increase to 77 million by 2030 [2]. Stroke can be divided into hemorrhagic and ischemic types. In traditional Chinese medicine (TCM), hemorrhagic stroke is caused by wind yang harassing the upper body and internal disturbances of the phlegm fire, which results in Qi and blood disorders. In addition, head trauma or brain tumors may also cause hemorrhagic stroke. Ischemic stroke, also known as cerebral infarction, refers to a clinical syndrome caused by the lack of blood supply in the brain and results in local brain ischemia and hypoxic necrosis, along with corresponding neurological deficits. Ischemic stroke is the most common type of stroke and accounts for 60% to 80% of cases [3].
Ischemic stroke is a common complication of hypertension, heart disease, and diabetes. Symptoms include limb weakness, sensibility deficits, facial palsy, ataxia, speech problems (aphasia or dysphagia), and visual disturbances [4]. In recent years, the diagnosis of ischemic stroke has depended on computed tomography. Cerebral infarction, local edema, and congestion of surrounding tissue occur after cerebral thrombosis. The diseased area develops swelling and ischemic necrosis and softens after several hours to several days. Historically, the first approved treatment for ischemic stroke in 1996 was thrombolysis by intravenous administration of tissue plasminogen activator [5].
Traditional medicine in China is divided into Chinese material medicine and ethnic medicine. Chinese material medicine includes substances used to prevent, treat, and diagnose diseases and mediate rehabilitation and health care under the guidance of TCM theories. Ethnic medicine refers to medicines that are guided by the theory and practice of the traditional medicine of specific ethnic groups, including Tibetan, Mongolian, and Uygur medicine. At present, the treatment of ischemic stroke involves surgical and drug treatments. In addition to thrombolytic drugs and anticoagulants, Ligusticum chuanxiong, safflower, musk, and other traditional medicines play important roles in the treatment of ischemic stroke by promoting blood circulation, removing stasis, and improving hemodynamics. In addition, traditional the Chinese patent medicine Angong Niuhuang pill, the Tibetan medicines Qishiwei Zhenzhu Pills and Ershiwuwei Zhenzhu Pills, and the Mongolian medicine treasure pill have good therapeutic effects. Many kinds of traditional medicine can be used for the treatment of ischemic stroke. Chinese material medicine and ethnic medicine can also be classified into plant, animal, and mineral medicines. A summary of these traditional medicines can provide a reference for the development of new traditional medicines for clinical use in the treatment of ischemic stroke.
Vascular endothelial cell injury leads to atherosclerosis, which leads to
cerebral artery occlusion and then ischemic stroke. Neurons in the central
ischemic area release and activate the immune response. Astrocytes and microglia
are activated and release a series of inflammatory factors, such as tumor
necrosis factor-
In ischemic stroke, mitochondria produce a large amount of highly reactive free radicals, such as ROS, through a variety of enzymatic reactions [7]. The excessive accumulation of ROS in the body can cause lipids and proteins to peroxidize and irreversibly damage nucleic acids and sugars, which lead to oxidative stress and apoptosis, ultimately damaging brain tissue [8]. Antioxidants can be divided into endogenous and exogenous antioxidants. Exogenous antioxidants, such as vitamin C, uric acid, bilirubin, albumin, and mercaptan, are hydrophilic and lipophilic [9].
Endogenous antioxidants refer to antioxidants produced by the human body, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX). SOD removes ROS generated in the body through a disproportionation reaction. GSH-PX can catalyze the reaction of hydrogen peroxide and reduce glutathione (GSH) to generate water, reduce the level of oxidative stress, and ultimately reduce the production of the lipid peroxidation product malondialdehyde (MDA) to protect cells and tissues from damage [10].
Nitric oxide synthetases, including neuronal NOS, inducible NOS (iNOS), and
constitutively expressed endothelial NOS, are present in neurons [11]. NOS
produces nitric oxide (NO) by reducing nicotinamide adenine dinucleotide
phosphate [12]. NO induces nitrosative stress via O
 Fig. 1.
Fig. 1.General pathway of ischemic stroke-induced cell oxidation.
In recent years, some components of Chinese material medicines have been widely studied as antioxidants that scavenge free radicals. These medicines play an antioxidant role by inhibiting lipid peroxidation, reducing MDA levels, increasing the total antioxidant defense system, and reducing the levels of reactive nitrogen species and ROS. Safranal, a constituent of Crocus sativus (saffron), also has some protective effects on different markers of oxidative damage in the hippocampus of rats. Pretreatment with p-coumaric acid ameliorates brain oxidative stress by reducing MDA while increasing CAT and SOD levels [17].
Nuclear factor erythroid 2-related factor 2 (Nrf2) has increasingly been recognized as a crucial transcription factor that mediates protection against electrophiles and oxidants and enhances cell survival in many tissues. Nrf2 is a key regulator of cellular oxidation at the transcriptional level by controlling the concentration of SOD, CAT, GSH, and heme oxygenase-1 (HO-1). Mangiferin (MF) is a natural glucosyl xanthone found in Chaenomeles sinensis (Thouin) Koehne. The results indicated that MF exerted a certain antioxidant effect and improved the level of oxidative stress induced by ischemia-reperfusion in rat brain tissues. The tetramethylpyrazine analog Z-11 [18] has a protective effect against cerebral ischemia. Nrf2, HO-1, SOD, and GSH levels in ischemic brain tissue were significantly reduced after Z-11 treatment in the context of cerebral ischemia-reperfusion injury (CI/RI). Z-11 may regulate the Nrf2/NOX2 antioxidant defense pathway and play a role in neuroprotection [19].
Too many excitatory neurotransmitters, especially glutamate, are released into
the synaptic space during ischemia [20]. The accumulation of glutamate results in
the overstimulation of glutamate receptors, such as NMDA, alginate, and
Some traditional medicine ingredients can reduce glutamate release, inhibit glutamate receptor stimulation, and reduce cellular calcium overload. Cactus extract and astragaloside have neuroprotective effects against NMDA- and KA-induced neurotoxicity [24].
Apoptosis is initiated by external signals or through internal mitochondrial signaling. Apoptosis is characterized by distinctive morphological changes in the nucleus and cytoplasm, such as chromatin cleavage at regularly spaced sites and the endonucleolytic cleavage of genomic DNA [25].
Apoptosis induced by cerebral ischemia generally occurs through two pathways (Fig. 2). (a) The mitochondria-mediated apoptotic pathway plays a key role in neuronal apoptosis after ischemic stroke [26]. The increase in the intracellular calcium activator bid (tBid); the interaction between tBid and apoptosis proteins, such as Bad and Bax, on the mitochondrial membrane [7]; the increase in mitochondrial membrane permeability and the decrease in membrane potential; the release of cytochrome c and apoptotic protein-activating factor-1; and the activation of procaspase-9, caspase-9, and caspase-3 lead to apoptosis. (b) In the extrinsic cascade, extracellular Fas ligand binds to Fas death receptors and activates caspase-8 [17].
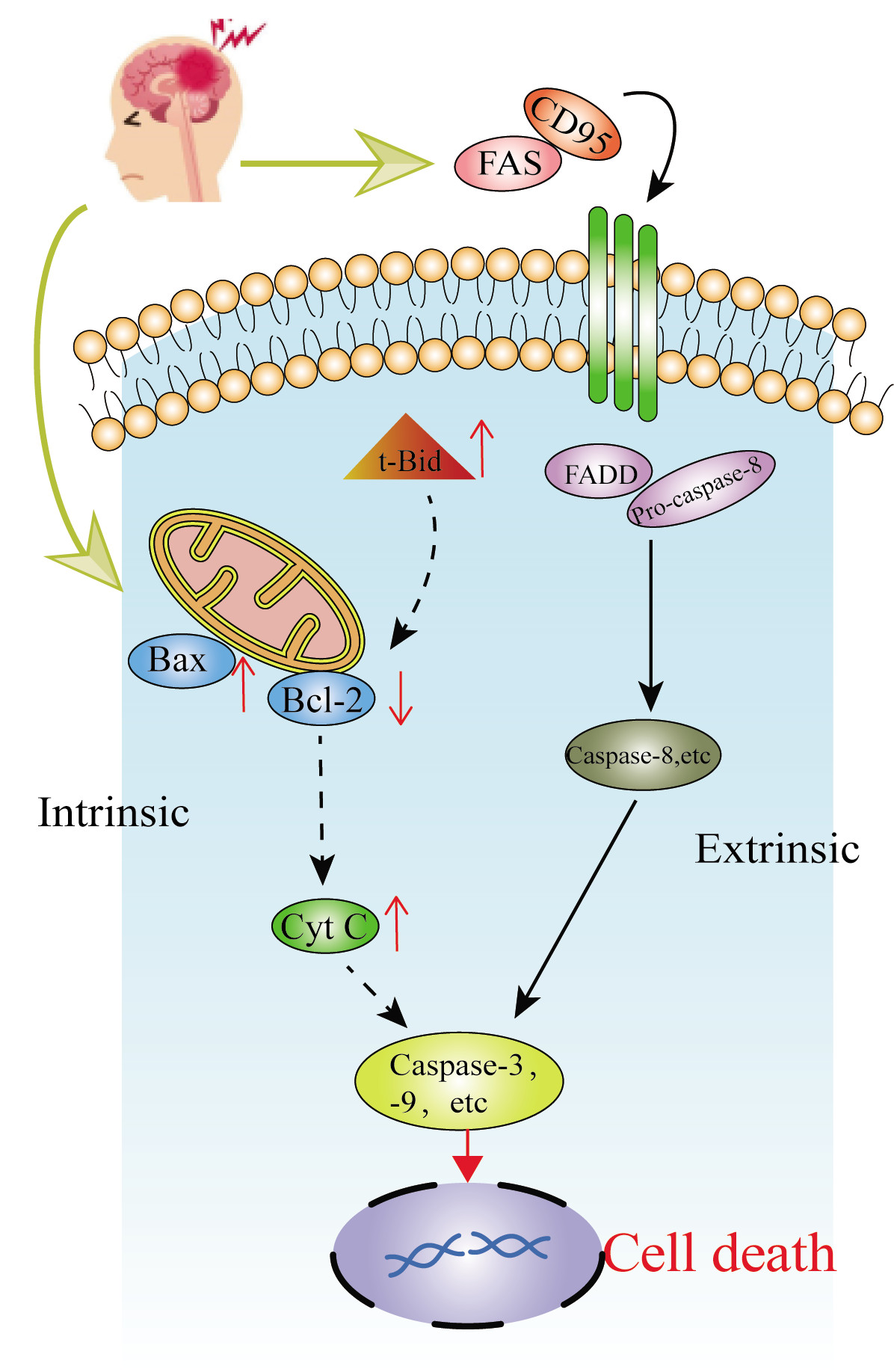 Fig. 2.
Fig. 2.The general way of ischemic stroke-induced apoptosis.
Some components of traditional medicine can inhibit the internal or external apoptotic pathway. For example, astragaloside IV, which is isolated from Astragalus propinquus Schischkin [27], can promote the binding of hexokinase and mitochondria to protect neurons from apoptosis and DNA damage. Astragaloside IV can inhibit various cell death pathways of neurons injured by hypoxia and glucose deficiency, reduce damage to mitochondrial function, and protect neurons. The water-soluble component of Salvia miltiorrhiza, magnesium lithospermate B (MLB), inhibits the activation of caspase-3 after CI/RI, and up-regulates p-Akt in the ischemic hemisphere, thereby protecting CI/RI [28].
Inflammation is a series of complex interactions between inflammatory cells and
molecules with neurotoxic or neuroprotective effects [29]. Cerebral ischemia can
lead to a substantial increase in the levels of proinflammatory cytokines, such
as TNF-
The bioactive components of many traditional medicines have remarkable
anti-inflammatory effects. Xanthanol, the active component of Cnidium
monnieri (L.) Cusson., has neuroprotective effect on CI/RI after cerebral
ischemia by inhibiting the production of proinflammatory mediators such as
IL-1
Cerebral thrombosis is the most common cause of cerebral ischemia, and platelets
play an important role in the process of thrombosis. When the vascular
endothelium is damaged, specific receptors on the surface of vascular endothelial
cells, such as glycoprotein, P-selectin and integrin, bind to subendothelial
proteins such as hemophilia-related factor, fibronectin, vitronectin and
thrombospondin, facilitating platelet adherence to damaged endothelial cells.
After the adherent platelets are stimulated, they cause small platelet actin
rearrangement, platelet pseudopodia formation, cell body extension,
Thrombin is the final product of the blood coagulation cascade. Thrombin acts on fibrinogen in the circulation and produces fibrin, which leads to thrombosis. Thrombin can induce platelet aggregation, induce the expression of P-selectin in endothelial cells, and increase the permeability of endothelial cells. Thrombin combined with fibrin can prevent the inactivation of plasma anticoagulant factor and heparin and prolong the action time of these factors [36].
At present, most antiplatelet and anticoagulant drugs are Western medicines,
such as aspirin, clopidogrel and prasugrel. Some Chinese herbal medicines also
have antiplatelet and anticoagulant effects. Xu used an arteriovenous shunt
thrombosis model and an adenosine 5’-diphosphate (ADP)-induced acute pulmonary
embolism model to observe the antithrombotic effect of supercritical CO
Autophagy is a degradation process by which cells maintain cell homeostasis by lysosomal-mediated degradation of biomacromolecules, damaged organelles or pathogenic microorganisms in the cytoplasm. Under physiological conditions, autophagy is very low in cells. In the nervous system, moderate autophagy is considered to have neuroprotective effects [39]. During autophagy, the components to be degraded in the cytoplasm are isolated in double-membrane vesicles to form autophagosomes, and then the autophagosome and lysosome fuse to produce a single-membrane autophagic lysosome, which is degraded by lysosomal hydrolase [40]. LC3 and beclin-1 are two marker proteins of autophagy, and LC3 is present in the form of cytoplasmic LC3 (LC3-I) and membrane LC3 (LC3-II). The ratio of LC3-I and LC3-II can reflect the level of autophagy. The Bcl-2/beclin-1 complex is considered to be a molecular switch that regulates the formation of autophagic membranes [41]. However, when autophagy is overactivated, autophagy can not only mediate cytoprotection but also degrade the nucleus and cause cell death [42].
Many components of traditional medicines can achieve neuroprotective effects by activating moderate levels of autophagy, such as Schisandrin A and Oxymatrine. The rats were given Schisandrin A through lateral ventricle after 90 minutes of ischemia [43]. The results showed that Schisandrin A upregulated the autophagy proteins beclin-1 and LC3-II, and immunofluorescence double-labeling showed that autophagic activity was mainly present in neurons. These results suggest that the neuroprotective effect of schisandrin A is related to the enhancement of autophagy in the ischemic penumbra. Oxymatrine was injected intraperitoneally 60 minutes before cerebral ischemia [44], measured cell apoptosis by the TUNEL method, the expression of P53, Bax, dissociated Caspase-3, LC3 and P62, and evaluated autophagy. It was found that oxymatrine treatment reduced tissue damage in I/R rats, inhibited cell apoptosis, and promoted autophagy.
In recent years, the concept of the neurovascular unit (NVU) has been proposed, which has changed the goal of cerebral protection after ischemia from protection at the level of individual neurons in the past to the structural protection of nerve cells and blood vessels. The neurovascular unit is composed of microvessels, astrocytes, neurons and their axons, microglia, oligodendrocytes and other supporting cells. Traditional Chinese medicine-mediated protection of the neurovascular unit of ischemic stroke mainly manifests as neuroprotection, vascular protection and glial protection. The neuroprotective mechanisms include antioxidation, inhibiting neuronal apoptosis, inflammation, and excitatory neurotoxicity [45].
The blood-brain barrier (BBB) is the core structure of the neurovascular unit. The BBB can prevent a variety of substances from entering the brain but allow nutrients and metabolites to pass and maintain the relative stability of the internal environment of the nervous system [46]. During cerebral ischemia, the blood-brain barrier is destroyed, and the permeability is increased, which then causes edema and hemorrhage, further exacerbating the ischemic condition. Ligustrazine may restore tight junction function by upregulating claudin-5 expression and reducing BBB permeability after cerebral ischemia-reperfusion in rats, thus reducing brain edema after ischemia-reperfusion [47]. Tanshinone can also upregulate the expression of claudin in the blood-brain barrier. Citrulline, paeoniflorin and other components in Gualou Guizhi granule can enter brain tissue through the BBB and have neuroprotective effects on rats with cerebral ischemia-reperfusion injury [48].
Glial cells can secrete a variety of cytokines and neurotrophic factors, which
play important roles in maintaining the survival of neurons. Under physiological
conditions, the increase in the number of glial cells plays a beneficial role in
supporting active neurons, which is beneficial to the learning and memory
function of the brain [49]. When cerebral ischemia occurs, glial cells undergo
excessive proliferation, and proliferative glial cells produce and release
neurotoxic substances such as tumor necrosis factor and NO and promote the
development of injury. It has been reported that Scutellaria barbata
flavonoids can inhibit
We manually searched 13 drug monographs and standards (Table 1), including “new Chinese patent medicine”, “Pharmacopoeia of the People’s Republic of China (Part 1 of the 2015 edition)”, “Ministry-promulgated Standards”, “Tibetan Medicine Standards”, and “Ethnic Medicine Prescriptions”, to identify the functions and indications of prescriptions in the context of “cardiovascular and cerebrovascular embolism, cerebral infarction, cerebral thrombosis, ischemic stroke, stroke, paralysis, hemiplegia, constriction, skewed mouth and eyes, unclear speech, white pulse disease, and black and white pulse disease”. We collected the indications for these prescriptions and the names, original species, families, and medicinal parts of the traditional medicines in these prescriptions. The active components and mechanisms of these traditional medicines in the treatment of ischemic stroke were collected from databases (i.e., Wanfang, Weipu, CNKI, ISI Web of Science, and Science Direct). The botanical names of the original plants were obtained from references and verified through the “Flora of China (http://www.iplant.cn/frps)” database based on their Chinese names.
| No. | Monograph | Author | Publication date of the monograph | The type of the monograph | The number of prescriptions for ischemic stroke extracted from the monograph in our paper |
| 1 | Newly compiled Chinese medicine application guide | Yang, S. S. | 1993 | Print edition | 113 |
| 2 | Pharmacopoeia of the People’s Republic of China | Health Bureau | 2015 | Print edition | 69 |
| 3 | Drug standards issued by the Ministry | Health Bureau | 2015 | Print edition | 18 |
| 4 | Tibetan Medicine Standards | Health Bureau of Tibet, Qinghai, Sichuan, Gansu, Yunnan, and Xinjiang | 1979 | Print edition | 7 |
| 5 | Waitai Miyao | Wang, T. | 618-907 AD | Print edition | 6 |
| 6 | Dai medicine prescription | Jia, K. L., Zhao, Y. H. | 2007 | Print edition | 5 |
| 7 | Xianshou Lishang Xuduan Mifang | Lan, D. R. | 618-907 AD | Print edition | 3 |
| 8 | Shaolin Temple Secret Recipe Collection | Teaching by De,C. written by De,Q. | 1986 | Print edition | 3 |
| 9 | Emei Shenxiao Yanfang | Wang, F. Q. | 1992 | Print edition | 3 |
| 10 | Prescription preparation of ethnic medicine | Song, M. X. | 2014 | Print edition | 2 |
| 11 | Zhulingsi Nvke Quanshu | Bamboo forest monk | 1636-1912 AD | Print edition | 1 |
| 12 | Dianxue Mijue | Xue, D. | 1938 | Print edition | 1 |
| 13 | Jingshi Zhenlei Beiji Bencao | Tang, S. W. | 960-1127 AD | Print edition | 1 |
We identified 398 kinds of traditional medicines (Supplementary Table 1) among the 192 prescriptions we collected. The traditional medicines were distributed among 132 families. The most common families were Leguminosae (4.5%), Umbelliferae (3.5%), Compositae (3.5%), Labiatae (3.5%), Rutaceae (3.2%), Zingiberaceae (3%), Ranunculaceae (2.5%), Liliaceae (2.5%), Rosaceae (2.0%), and Araceae (2.0%), as shown in Fig. 3. Among these traditional medicines, botanical medicinal materials accounted for 75.6%, animal medicine accounted for 13.6%, mineral medicine accounted for 7.0%, and other traditional medicines accounted for 3.7%. Botanical medicinal materials were the main source of these traditional medicines. Root (20.5%), stem (14.7%), fruit (12.4%), whole grass (8.4%), and seed (8.1%) were the most commonly used plant parts (Fig. 4).
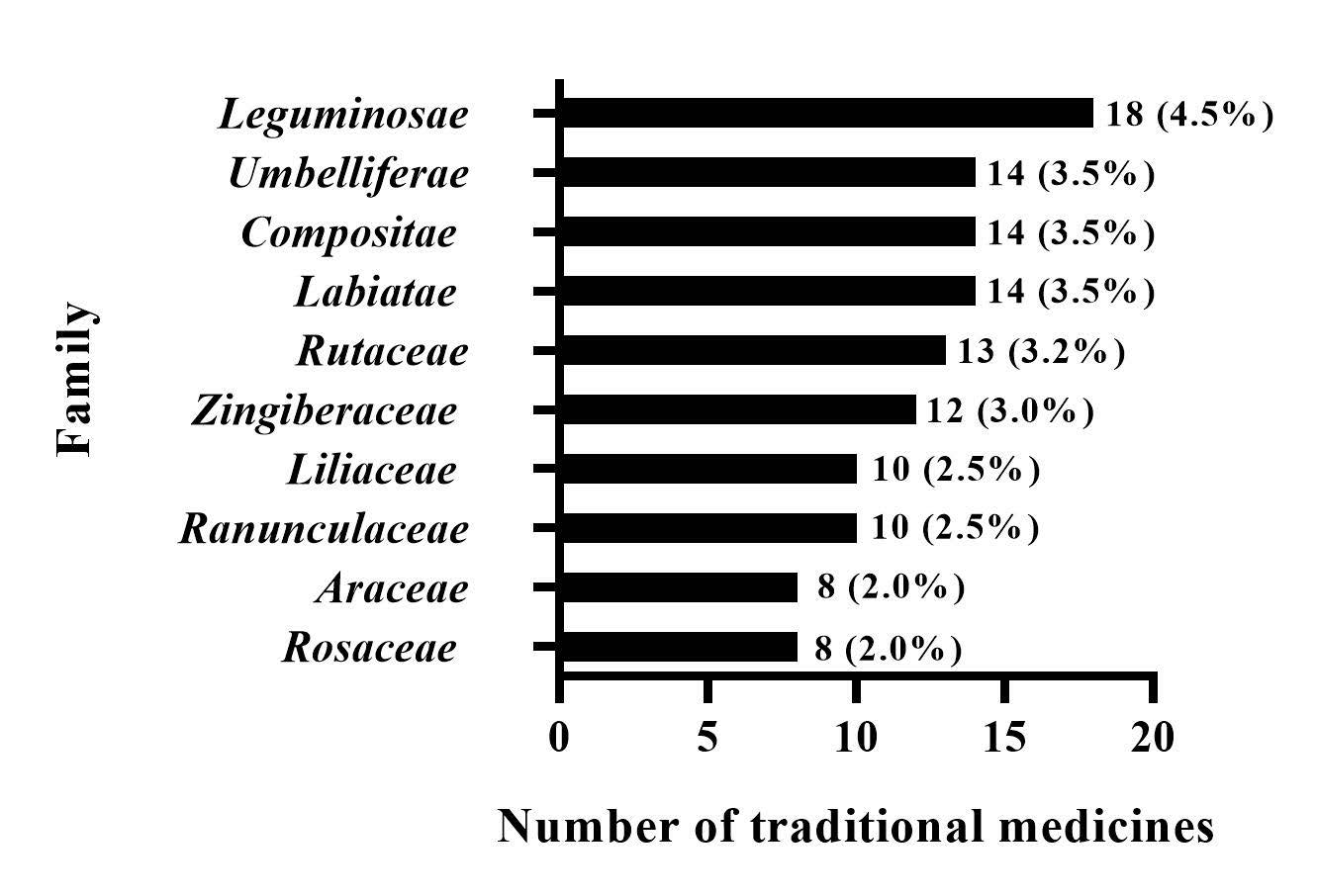 Fig. 3.
Fig. 3.Family distribution of traditional medicines for ischemic stroke.
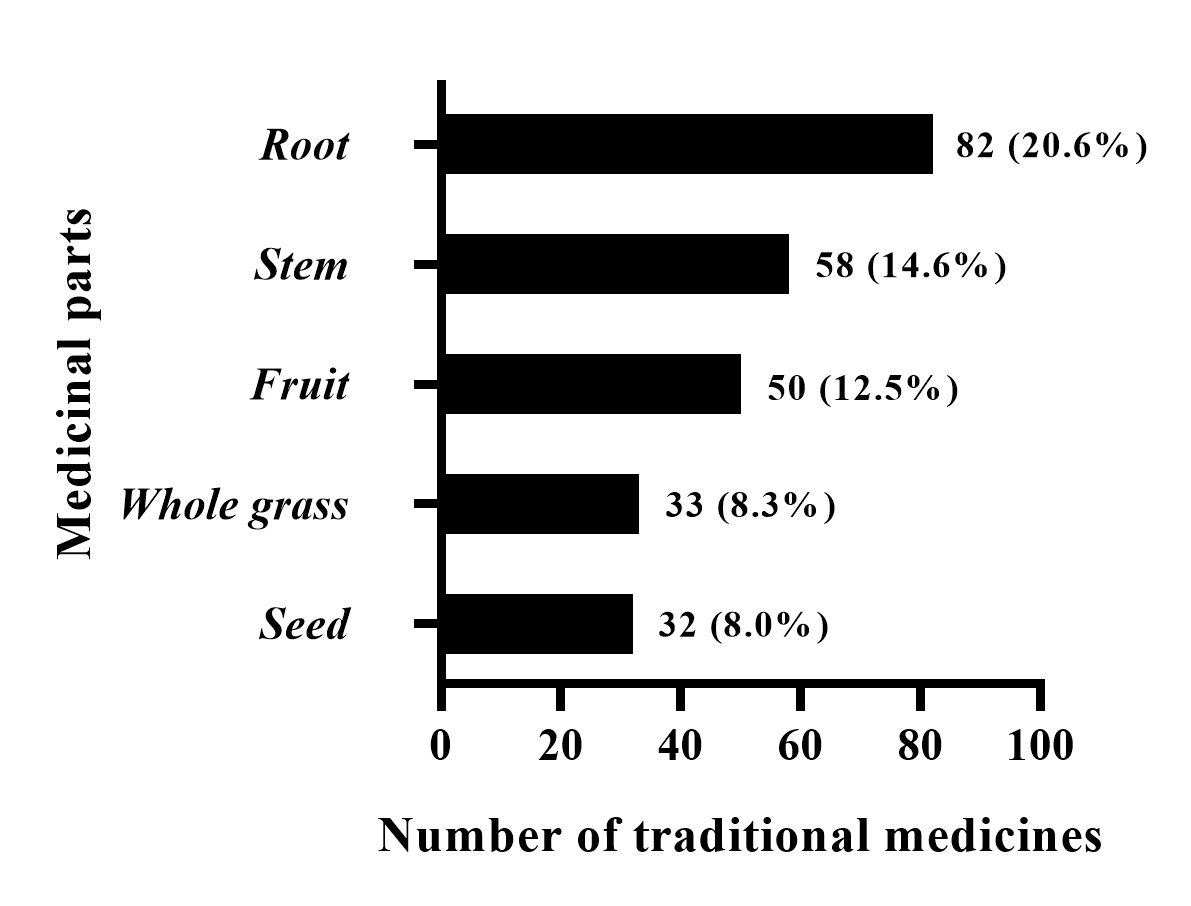 Fig. 4.
Fig. 4.Parts of traditional medicines used in the treatment of ischemic stroke.
Traditional Chinese patent medicines are Chinese material medical products made of raw Chinese material medical components processed into a specific form according to the prescription and preparation process under the guidance of TCM theory. Traditional Chinese patent medicine is convenient to transport, easy to consume, and stable in efficacy and thus has occupied a very important position in the pharmaceutical industry. Many of the 192 prescriptions we studied have been made into very common Chinese patent medicines. We selected five of these as a detailed introduction (Fig. 5).
 Fig. 5.
Fig. 5.Common Chinese traditional patent medicines for ischemic stroke. (A) Angong Niuhuang Pill. (B) Qishiwei Zhenzhu Pills. (C) Ginkgo biloba leaf. (D) Huatuo Zaizao Pill. (E) Xueshuan Xinmai Ning.
Angong Niuhuang pill is the most famous emergency TCM. This pill originated from the plague debates by Wu Jutong, a plague expert in the Qing Dynasty. The prescription consists of 11 kinds of Chinese materia medicines [51]: bezoar (Bos taurus domesticus Gmelin), Curcuma aromatica Salisb., Rhinoceros unicornis L., musk (Moschus), pearl (Pernulo), Gardenia jasminoides Ellis, Coptis chinensis Franch, Scutellaria baicalensis Georgi, cinnabar, realgar, and borneol. The rhinoceros horn in the modern formula of Angong Niuhuang pill is replaced with a buffalo horn, and the natural bezoar is replaced with an artificial bezoar. This medicine clears away heat, detoxifies, alleviates convulsions, and opens up the mind [52]. This pill has been used as a key drug for treating febrile convulsions, stroke and coma [53].
Yang [54] reported that Angong Niuhuang Pill could significantly reduce the level of NO and the activity of NOS in brain tissue. Liu [55] reported that Angong Niuhuang pill could improve blood and plasma viscosity and substantially increase the platelet aggregation rate and erythrocyte aggregation index. In addition, Fu [56] and Yu [57] used Angong Niuhuang Pill in the treatment of stroke, and the total effective rates were 86% and 100% respectively.
The realgar and cinnabar in Angong Niuhuang pill contain 90% As
Qishiwei Zhenzhu Pills [60], one of the most representative and precious medicines, is composed of Pernulo, Santalum album L., Dalbergia odorifera T. Chen, C. sativus L., B. taurus domesticus Gmelin, Moschus, and more than 70 flavor components. This medicine is now included in the Pharmacopoeia of the People’s Republic of China (2015 edition). This preparation harmonizes qi and blood, awakens the brain, and opens the mind. This medicine is used for the treatment of “black and white pulse disease”, “dragon blood disorder”, stroke, paralysis, hemiplegia, epilepsy, cerebral hemorrhage, concussion, heart disease, hypertension, and neurological disorders [61].
Qishiwei Zhenzhu Pills [62] is rich in essential trace elements, especially zinc, cobalt, copper, manganese, and vanadium, which are closely related to the treatment of cardiovascular diseases. Modern pharmacological studies [63] have shown that Qishiwei Zhenzhu Pills can counteract the convulsions induced by thiosemicarbazide and improve the learning and memory deficits induced by ethanol and the memory acquisition induced by anisodine in mice. This treatment can also increase the cerebral blood flow of rabbits by clearing dampness. Qishiwei Zhenzhu Pills induces sedation, inhibits convulsion, improves memory, microcirculation and cerebral blood circulation, inhibits thrombosis, and reduces blood pressure. An [64] used Qishiwei Zhenzhu Pills to treat 50 patients with paralysis and 16 patients with epilepsy. The total effective rate was 100%; the effective rate was 91% for 22 patients with hypertension and 79% for 14 patients with concussion. Wu [65] used Qishiwei Zhenzhu Pills to treat ischemic cerebral obstruction. This treatment can alleviate the behavioral disorders caused by ischemic cerebral obstruction, reduce platelet aggregation and adhesion, reduce infarct size, and improve the efficacy of treatment for cerebral arteriosclerosis.
Ginkgo biloba leaf [66] is made into a film-coated tablet by boiling and extraction. Ginkgo biloba leaf contains total flavonoids and terpene lactones, activates blood circulation, removes blood stasis, and dredges collaterals. This medicine can be used for chest pain, heart pain, stroke, hemiplegia, stiff tongue, sluggish speech, stable angina pectoris, and cerebral infarction caused by blood stasis. The standard extract of Ginkgo biloba leaves [67] has been clinically used in Europe to treat the symptoms of brain dysfunction in patients with primary degenerative dementia syndrome. A large number of in vivo and in vitro studies have supported this clinical application.
Wang [68] reported that Ginkgo biloba extract can significantly prolong the
survival time of mice with acute cerebral ischemia induced by bilateral common
carotid artery ligation, and reduce the damage of hippocampal subcellular
structure after reperfusion. Yuan [69] reported that Ginkgo biloba leaves can
improve the learning and memory function of vascular dementia rats caused by
multiple cerebral infarction, significantly increase the activity of SOD in
hippocampus, inhibit the activity of ache, and significantly reduce the level of
MDA. Clinically, there were significant differences in blood viscosity, plasma
viscosity, hematocrit, erythrocyte sedimentation rate and blood flow velocity of
middle cerebral artery before and after treatment in 180 patients with ischemic
cerebrovascular disease treated with Ginkgo biloba (p
Huatuo Zaizao pill is composed of L. chuanxiong Hort, Evodia rutaecarpa (Juss.) Benth., borneol, and other medicines. This treatment has been used for more than 200 years in clinical practice and is included in the Chinese Pharmacopoeia. This treatment activates blood circulation, removes blood stasis, resolves phlegm, dredges collaterals, activates qi and alleviates pain. The Huatuo Zaizao pill is often used to treat stroke paralysis, constriction, numbness, deviation of the mouth and eyes, and unclear speech [71, 72].
Strychnos nux-vomica L., borneol, Schisandra chinensis, Radix ginseng rubra, and other active ingredients in the pill contain volatile oils [73], which can excite the central nervous system, increase the oxygen supply in the brain, improve the working ability of the cortex, and promote the functional recovery of brain cells. Liu [74] reported that Huatuo Zaizao pill can inhibit thrombosis in vitro, prolong thrombosis time in vivo, reduce blood viscosity, inhibit platelet aggregation, and improve cerebral blood supply, oxygen supply and pia mater. Huatuo Zaizao pill activates blood circulation, removes blood stasis, and antagonizes thrombosis, as observed by changes in microcirculation. Hence, this treatment is an ideal drug for treating the sequelae of cerebral thrombosis [75].
Xueshuan Xinmai Ning [76] is composed of Ligusticum chuanxiong Hort.,Salvia miltiorrhiza Bge., Whitmania pigra Whitman, and other medicines. This medicine has the function of aromatic resuscitation, activates blood circulation, and removes blood stasis. Clinically, this medicine can be used to treat many cardiovascular and cerebrovascular diseases because it can improve the blood supply of the cardiovascular and cerebrovascular systems by expanding blood vessels and protecting endothelial cells. Modern pharmacological studies have shown that the protection of vascular endothelial cells may be achieved through modulating the expression of genes related to multiple signal transduction pathways in vascular endothelial cells [77].
Han [78] used high-performance liquid chromatography and ultraperformance liquid chromatography coupled with electrospray ionization and tandem quadrupole time-of-flight mass spectrometry to preliminarily identify 63 components in Xueshuan Xinmai Ning capsules, namely, 20 saponins, 4 flavonoids, 15 phenolic acids, 8 sterols, 4 bile acids, 10 hydroquinones, and 2 other compounds. Wang [79] divided 66 patients with transient cerebral ischemia into three groups according to the transcranial Doppler method: an anterior cerebral circulation group, a posterior cerebral circulation group, and a mixed circulation group. Xueshuan Xinmai Ning capsules were taken orally, and the different systemic insufficiencies in blood supply were compared with those of the 20 cases in the oral nimodipine group. The results showed that the Xueshuan Xinmai Ning group had the most improvement in dizziness caused by insufficient blood supply in the three groups, and Xueshuan Xinmai Ning was better than nimodipine in improving tinnitus. Yang [80] reported that Xueshuan Xinmaining can significantly reduce the infarct size of rats with acute myocardial infarction within 24 hours and 7 days after. Thus, Xueshuan Xinmai Ning capsules have a protective effect on acute myocardial ischemia.
The data of the traditional medicines in the prescriptions were gathered to determine the frequency of use of each medicine in the prescription and understand the most commonly used medicines for the treatment of ischemic stroke. As a result, 192 prescriptions for the treatment of ischemic stroke were collected from the drug monographs and standards. The medicines with a frequency of use that was higher than 1% are shown in Table 2 (Ref. [28, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100]). The top five were Chuanxiong Rhizoma (frequency: 70 times), safflower (60 times), Angelica sinensis and musk (53 times each), and Salvia miltiorrhiza (49 times). The following sections provide a detailed review of the names, original plant types, traditional uses, active ingredients, and biopharmacological activities of these five medicines (Figs. 6,7).
| No. | Chinese name | Latin name | Family | Life form | Used part | Reported stroke related bioactivity | Dose | Pharmacological effects related to stroke | Mechanism of action related to stroke | Used frequency | Reference |
| 1 | Chuan-xiong | Ligusticum chuanxiong Hort. | Umbelliferae | Herb | Rhizome | Ferulic acid, ligustrazine | 100 mg/kg; i.g. | Antioxidant, Inhibiting inflammation and Inhibiting apoptosis | Inhibition of superoxide radicals, ICAM-1 and NF-κB expression and production of microglia, inflammatory cells and MCP-1 | 70 (2.77%) | [97] |
| 2 | Hong-hua | Carthamus tinctorius L. | Compositae | Herb | Flower | Hydroxysafflower yellow a, Kaempferol-3-O-rutinoside, kaempferol-3-O- glucoside | 0.4 g/kg; i.g. | Anti-nitrification, Inhibiting inflammation and Inhibiting apoptosis | Reduced nitrotyrosine, inhibited STAT3 and NF-κB, JAK2/STAT3 pathways | 60 (2.38%) | [98] |
| 3 | Dang-gui | Angelica sinensis (Oliv.) Diels. | Umbelliferae | Herb | Root | Angelica essential oil | 0.25, 0.5, 1 g/kg; i.g. | Inhibiting apoptosis | Decrease the expression of 4-hydroxy-2-nonenal, cytc and lyse Caspase-3, and inhibit the apoptosis of hippocampal CA1 cells | 53 (2.1%) | [81] |
| 4 | She-xiang | Moschus berezovskii Flerov., Moschus sifanicus Przewalski., Moschus moschiferus Linnaaeus. | Moschus | Animal | Secretion | Muscone | 0.9 µm; i.g. | Inhibiting apoptosis | The protective effect of Muscone on cerebral ischemia depends on the activation of PI3K/Akt signaling pathway | 53 (2.1%) | [99] |
| 5 | Dan-shen | Salvia miltiorrhiza Bunge | Labiatae | Herb | Root and rhizome | Salvianolic acid, Tanshinone | 10 mg/kg, i.p. | Inhibiting inflammation and Inhibiting apoptosis | Increase or maintain endogenous inflammatory cytokines (IL-4 and IL-13) to protect neuron death caused by ischemia in CA1 | 49 (1.94%) | [100] |
| 6 | Shao-yao | Paeonia lactiflora Pall. | Ranunculaceae | Herb | Root | Paeoniflorin | 10, 15, 20 mg/kg; i.v. | Inhibiting inflammation and Inhibiting apoptosis | Reduce the levels of ED1, IL-1 |
47 (1.86%) | [82] |
| 7 | Mu-xiang | Aucklandia lappa Decne | Compositae | Herb | Root | Costunolide | 2.7%; i.g. | Inhibiting apoptosis | Regulate mitochondrial cyto-c pathway and increase the expression of Bcl-2 protein | 46 (1.82%) | [83] |
| 8 | Huang-qi | Astragalus membranaceus (Fisch.) Bge | Labiatae | Herb | Root | Astragaloside A | 12.5, 25, 50 mg/kg; i.g. | Inhibiting apoptosis | It inhibited the mRNA expression of Fas, FasL, Caspase-8 and Bax/Bcl-2 and the protein levels of caspase-8, Bid, cleaved caspase-3 and Cyto C. | 43 (1.7%) | [28] |
| 9 | Bing-pian | Cinnamomum camphora (L.) Presl | Lauraceae | Compound | Synthetics | L-borneol, R-borneol | 0.2, 0.6 g/kg; i.g. | Inhibiting apoptosis and Inhibiting inflammation | Increase serum VEGF level, decrease serum TNF- |
42 (1.66%) | [84] |
| 10 | Gan-cao | Glycyrrhiza uralensis Fisch. | Leguminosae | Herb | Root and rhizome | Liquiritigenin | 10, 20, 40 mg/kg, i.g. | Antioxidant and Inhibiting apoptosis | Increase GSH/GSSG ratio and SOD, CAT, GSH PX activity, decrease 8-OHdG and TUNEL positive cells. | 41 (1.63%) | [85] |
| 11 | Di-long | Pheretima aspergillum (E.Perrier)., Pheretima vulgaris Chen., Pheretima guillelmi (Michaelsen)., Pheretima pectinifera Michaelsen | Megascolecidae | Animal | Whole body without the viscera | Pheretima aspergillum | 0.25, 0.5, 1.0 g/kg, oral | Inhibiting apoptosis | Decrease neuron death, GFAP proliferation and S100B protein expression | 39 (1.55%) | [86] |
| 12 | Niu-huang | Bos taurus domesticus Gmelin | Bovidae | Animal | Bile-cyst,bile duct or the stones in the liver tube | Taurine, Ursodeoxycholic Acid, tauroursodeoxycholic acid | 20 g/kg, i.p. | Antioxidant and Inhibiting apoptosis | Inhibit excitatory amino acid neurotoxicity and endoplasmic reticulum stress | 36 (1.43%) | [87] |
| 13 | San-qi | Panax notoginseng (Burk.) F.H Chen | Araliaceae | Herb | Root | Panax notoginseng polysaccharide | 100, 300 mg/kg, i.g. | Antioxidant and Inhibiting inflammation | Increase the activity of GSH Px, SOD and the content of IL-10, decrease the content of MDA, TNF- |
36 (1.43%) | [88] |
| 14 | Niu-xi | Achyranthes bidentata Blume | Amaranthaceae | Herb | Root | Achyranthes bidentata polypeptide | 0.1, 0.2, 1.0 mg/kg, i.v. | Inhibiting apoptosis | Regulate the expression of apoptosis related genes, restore mitochondrial membrane potential, regulate mitochondrial dysfunction, reduce the release of mitochondrial apoptosis factors, and inhibit the production of ROS in cells | 33 (1.31%) | [89] |
| 15 | Ge-gen | Pueraria lobata (Willd.) Ohwi | Leguminosae | Vine | Root | Pueraria flavonoids | 50, 100, 200 mg/kg, i.g. | Antioxidant | Improve the activity of SOD, reduce the content of MDA and resist the damage of oxygen free radical | 31 (1.23%) | [90] |
| 16 | Chen-xiang | Aquilaria sinensis (Lour.) Gilg | Thymelaeaceae | Tree | Wood contains resin | Water extraction | – | Antioxidant | Improve energy metabolism and reduce energy and oxygen consumption | 30 (1.19%) | [91] |
| 17 | Tian-ma | Gastrodia elata Bl. | Orchidaceae | Herb | Rhizome | Gastrodin | 15, 30, 60 mg/kg, i.g. | Inhibiting apoptosis | Down regulating the expression of caspase-3 mRNA and reducing the apoptosis of brain neurons | 28 (1.11%) | [92] |
| 18 | Tao-ren | Prunus persica (L.) Batsch., Prunus davidiana (Carr.) Franch | Rosaceae | Tree | Seed | Peach kernel decoction | 2, 4 g/kg, i.g. | Inhibiting apoptosis, Antioxidant | Increase the activity of neurons and decrease the water content of brain tissue | 27 (1.07%) | [93] |
| 19 | Di-huang | Rehmannia glutinosa Libosch. | Scrophulariaceae | Herb | Tuberous root | Rehmannia polysaccharide | 5, 10, 20 mg/kg, i.p. | Antioxidant | Enhance the activity of SOD, Na |
27 (1.07%) | [94] |
| 20 | Ding-xiang | Eugenia caryophyllata Thunb. | Myrtaceae | Tree | Bud | Eugenol | 200 mg/kg, i.p. | Inhibiting inflammation,Inhibiting apoptosis | By up regulating the expression of BDNF protein in brain, we can regulate the function of brain area and improve the injury after ischemia | 27 (1.07%) | [95] |
| 21 | Shui-zhi | Whitmania pigra Whitman., Hirudo nipponica Whitman., Whitmania acranulata Whitman. | Hirudinidae | Animal | Whole body | Hirudo polypeptide | 20, 40, 80 mg/kg, i.p. | Antioxidant | Increase SOD activity, decrease MDA content, inhibit lipid peroxidation and increase antioxidant enzyme activity. | 26 (1.03%) | [96] |
 Fig. 6.
Fig. 6.Common traditional medicines for ischemic stroke. (A) Chuanxiong Rhizoma. (B) Angelica sinensis. (C) Safflower. (D) Musk. (E) Salvia miltiorrhiza.
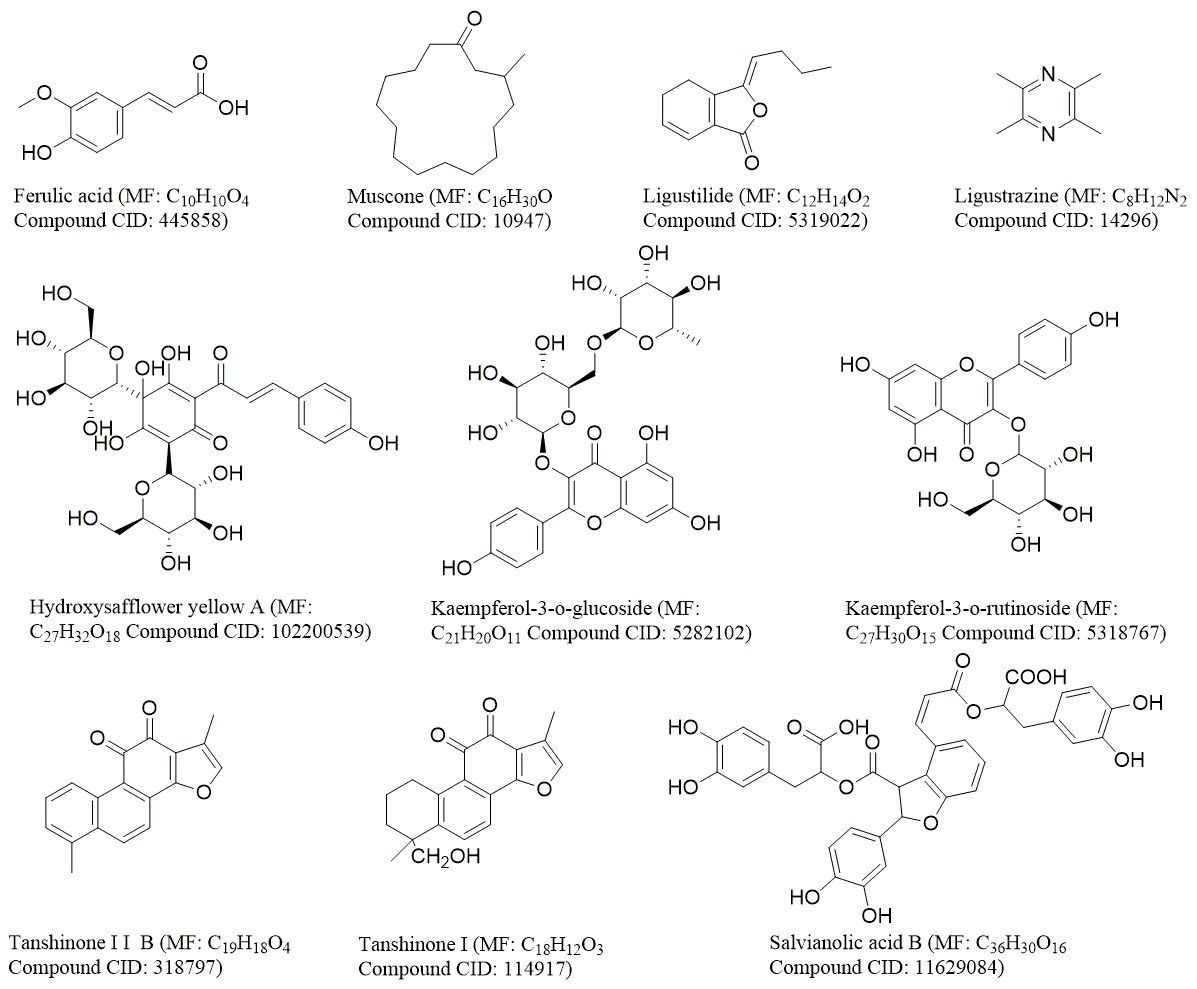 Fig. 7.
Fig. 7.The structure of the main compounds of common medicinal materials. The chemical structures of compounds were derived from PubChem (https://pubchem.ncbi.nlm.nih.gov/).
Chuanxiong Rhizoma (known as Chuanxiong in Chinese) is the dried rhizome of Umbelliferae L. chuanxiong Hort. This rhizome is produced in Dujiangyan, Pengzhou, Chongzhou, and other places in Sichuan Province [97]. In TCM theory, Chuanxiong Rhizoma is warm in property and pungent in flavor and activates qi, promotes blood circulation, expels wind, and alleviates pain [101]. Chuanxiong Rhizoma is clinically used in prescriptions for the treatment of migraine, myocardial ischemia, and cerebral ischemia. Its anti-inflammatory, antiapoptotic, antioxidative stress activities, activate autophagy, and its neuroprotection effects are related to the treatment of cerebral ischemia [102]. At present, more than 170 compounds in Chuanxiong Rhizoma have been isolated and identified. These compounds include phthalic acid, terpenoids and enols, polysaccharides, alkaloids, organic acids, and esters [97]. Ferulic acid and ligustrazine are the main bioactive and pharmacodynamic components and are also used as indicators of the quality of Chuanxiong Rhizoma in drug standards.
The extract of Chuanxiong Rhizoma has been proven to have some pharmacological
activities related to cerebral ischemia. For example, ferulic acid has a
neuroprotective effect on rats with transient middle cerebral artery occlusion
(MCAO) [103]. The mechanism is related to the expression of superoxide radicals,
intercellular adhesion molecule-1, and NF-
Safflower is the dried flower of Carthamus tinctorius L. In China, safflower is widely cultivated in various locations in Henan, Sichuan, Xinjiang, and Zhejiang provinces. The safflower is warm in property and pungent in flavor; promotes blood circulation, relieves stasis, and relieves pain; and is used to treat coronary heart disease, stroke, gynecological diseases, angina pectoris, and hypertension [106].
More than 200 compounds have been isolated from safflower, including flavonoids, phenylethanoid glycosides, coumarins, fatty acids, steroids, and polysaccharides [98]. Among these compounds, hydroxysafflor yellow A, kaempferol-3-o-rutinoside (KRS) and kaempferol-3-o-glucoside (KGS) are related to cerebral ischemia. Hydroxysafflor yellow A and kaempferide are often used as quality control markers in the pharmaceutical industry and drug standards. The level of hydroxysafflor yellow A is required to be greater than 1.0%, and the kaempferide content should be greater than 0.05% [107].
Some active components and extracts of safflower have pharmacological effects on cerebral ischemia. The water extract of safflower can improve brain injury in rats with CI/RI by antioxidation and anti-inflammatory mechanisms. Sun and Yang [108] reported that hydroxysafflor yellow a can reduce the formation of nitrotyrosine, inhibit iNOS mediated no production, clear peroxynitrite, and down regulate NMDA receptor containing NR2B to protect neurons from death. Yu [109] reported that KRS or KGS can improve the shape and structure of cortical neurons, reduce the number of apoptotic cells, down regulate the expression of p-JAK2, p-STAT3, caspase-3, and Bax, reduce the immune activity of Bax, and increase the protein expression and immune response of Bcl-2 [110].
Safflower is also used in combination with other drugs to clinically treat cerebral ischemic diseases. For example, the combination of A. propinquus Schischkin and safflower can ameliorate cerebral infarction with qi deficiency and blood stasis syndrome. Xu [111] reported that the combination of salvianolic acid A in Salvia miltiorrhiza Bge. and hydroxysafflor yellow A in safflower has a synergistic effect on CI/RI in rats.
Angelica sinensis (Oliv.) Diels is an important Chinese material medicine. Its use in China can be traced back to “Shennong Bencao Jing” nearly 2000 years ago [112]. Angelica sinensis is one of the most commonly used Chinese material medicines for tonification, hematopoiesis, tumor inhibition, and inflammation inhibition [113]. This medicine is used to treat menstrual disorders, amenorrhea and dysmenorrhea. Currently, more than 70 formulas containing Angelica sinensis are recorded in the Chinese Pharmacopoeia [114]. Angelica sinensis naturally grows in cool, high-altitude mountainous regions between 2500 m and 3000 m in Gansu, Hubei, Shaanxi, Sichuan, and Yunnan provinces [115]. Among them, Gansu is the main production area of Angelica sinensis and accounts for 90% of the total output. Min County has the largest yield among the cultivation areas in Gansu and produces more than 6000 tons, which makes up 70% of the nationwide production and 80% of the exported materials. Angelica sinensis that is cultivated in Gansu is thought to be the best quality and is regarded as an authentic and superior medicinal material (Daodi yaocai) [116]. Angelica sinensis can be divided into three distinct parts, namely, the head, body, and tail. The three parts have different therapeutic effects. The head is used to stop bleeding, the body is used to nourish the blood, and the tail is used to quicken the blood. The effect of Angelica sinensis on the nervous system is related to pain relief [117]. Angelica sinensis has many chemical components, and ferulic acid and volatile oils are its main bioactive and pharmacodynamic components and quality control index.
Angelica sinensis essential oil (AO), a major pharmacologically active component of Angelica sinensis (Oliv.) Diels, possesses hemogenesis activities, analgesic activities, and sedative effects and is used to treat a range of conditions, including menstrual disturbances and anemia. Over 40 compounds have been identified in AO, with the major constituent being ligustilide (LIG) [118].
It has been reported that AO can promote the production of IL-10 by inhibiting
the secretion of pro-inflammatory cytokines (TNF-
Musk is the dried secretion of the musk pod of the adult male deer. The medical use of musk was first recorded in “Shennong Bencao Jing” (approximately 200 AD), an ancient TCM book. Musk has been listed in the Chinese Pharmacopoeia for the treatment of stroke, tumors, traumatic injury, and cardiopathy with an oral dose of 0.03–0.1 g/day. In addition to being used as medicine, musk is also used as a spice base in East Asia [120].
Musk is rare and expensive. Thus, artificial musk has been studied based on the chemical composition and pharmacological effects of natural musk to protect wild musk sources. The results showed that artificial musk and natural musk have the same pharmacological activity [121]. In 1993, the Ministry of Health approved the trial production of artificial musk as a new Chinese medicine alternative to natural musk. Wang used an animal model of focal CI/RI and subarachnoid hemorrhage and found that the oral administration of low-dose artificial musk (10 mg/kg) could considerably improve the behavioral score and cerebral infarction volume of rats with transient CI/RI [122].
The chemical constituents of musk include steroids, lipids, peptides, and macrocyclic musk compounds with pharmaceutical activities, such as antitumor, antiulcer, anti-inflammatory, adrenergic stimulation, and androgen-like effects [123].
Muscone is the most important monomer in musk. Muscone has a neuroprotective effect against stroke injury by inhibiting apoptosis in the brain. Muscone has neuroprotective effect on stroke injury by inhibiting apoptosis of brain cells. The protective effect of Muscone on cerebral ischemia depends on the activation of PI3K/Akt signaling pathway. PI3K/Akt signaling pathway, as the central mediator of neural stem cell signal transduction, plays an important role in the proliferation and differentiation of neural stem cells [124]. Muscone can prevent PC12 cells and cortical neurons from damage after various injuries, and inhibit glutamate induced apoptosis of PC12 cells and cortical neurons. Wei reported that Muscone may be a small active molecule with neuroprotective properties, and inhibition of apoptosis and Fas is an important mechanism of Muscone induced neuroprotection [99].
The MCAO rat model was established by a transient filament method and treated
with musk ketone (0.9 or 1.8
Salvia miltiorrhiza is the dried root and rhizome of Salvia miltiorrhiza Bge. Salvia miltiorrhiza is one of the most popular Chinese materia medicines and is grown in the hilly areas of West, Southwest, and Southeast China. Numerous pharmaceutical forms of Salvia miltiorrhiza, including tablets, capsules, granules, injectables, oral liquids, sprays, and dripping pills, are commercially available in China [100]. Among all of the available forms, the Fufang Salvia miltiorrhiza tablet and Fufang Salvia miltiorrhiza dripping pill are the two most widely used products in China and have been officially listed in the Chinese Pharmacopoeia. The Fufang Salvia miltiorrhiza dripping pill has also been registered as a drug in several countries, including Vietnam, Russia, Cuba, the Korean Republic, and Saudi Arabia. This medicine has been used to treat stroke (since 1970), angina, and heart attack and is used as an antihypertensive and sedative. The SCED can alleviate cerebral ischemic injury, and its mechanism of action is related to the inhibition of thrombosis and platelet aggregation and activation of the PLC/PKC pathway [125].
The chemical components of Salvia miltiorrhiza are lipophilic and hydrophilic [100]. The liposoluble components are tanshinone, and the water-soluble components are salvianolic acids. In addition, Salvia miltiorrhiza also contains baicalin, ergosterol, ursolic acid, and other components. Tanshinone and salvianolic acid are the main bioactive and pharmacodynamic components of Salvia miltiorrhiza [126]. Salvianolic acid B is the most abundant and bioactive compound of the various Salvia miltiorrhiza phenolic acids extracted from Salvia miltiorrhiza mixtures. Salvianolic acid B can prevent brain ischemia-reperfusion injury in rats by reducing free radicals, improving energy metabolism, improving regional cerebral blood flow in the ischemic hemisphere, inhibiting platelet aggregation, and promoting the recovery of motor function after CI/RI.
Tanshinone can effectively resist ischemic injury to the central nervous system and protect neurons in the ischemic area. Park [127] used NeuN immunohistochemical analysis and F-J B histofluorescence staining to observe the protective effect of tanshinone I on ischemia-reperfusion injury in CA1 pyramidal neurons in gerbils. The results showed that tanshinone I pretreatment could protect against ischemia-induced neuronal death in the CA1 area by increasing or maintaining endogenous inflammatory cytokines. Tanshinone IIB has a protective effect against brain injury in rats. An intraperitoneal injection of 5 or 25 mg/kg tanshinone IIB could substantially reduce the infarct volume, brain injury, and apoptosis in rats with MCAO [128]. In addition, tanshinone IIA can play an important neuroprotective role in an animal model of ischemic brain injury by inhibiting inflammation, oxidative stress, and apoptosis [129].
Tanshinone can effectively resist ischemic injury of central nervous system and protect neurons in ischemic area. Park [127] reported that tanshinone I preconditioning can protect CA1 area ischemia-induced neuronal death by increasing or maintaining endogenous inflammatory cytokines. Tanshinone IIB can significantly reduce the infarct volume, brain injury and apoptosis in MCAO rats [128]. In addition, tanshinone IIA can play an important neuroprotective role in the animal model of ischemic brain injury by inhibiting inflammation, oxidative stress and apoptosis [129]. There are two main metabolic pathways of tanshinone, (a) hydroxylation, dehydrogenation, oxidation and furan ring cleavage of tanshinone, (b) Tanshinone is first metabolized to dehydro tanshinone, then hydroxylation, methoxylation, oxidation and furan ring cleavage are carried out on its molecule [130].
Salvia miltiorrhiza and Carthamus tinctorius are valued Chinese material medicines for the treatment of cardiovascular and cerebrovascular diseases and have multiple pharmacological effects, such as anti-inflammatory and antioxidant effects. Danhong injection combined with Salvia miltiorrhiza and Honghua has been widely used in clinical practice to treat patients with cerebrovascular disease [111].
Some chemical compounds in Chinese material medicine are also effective in the treatment of ischemic stroke. In this section, we introduce some chemical components in detail. The chemical structures of these compounds are shown in Fig. 8.
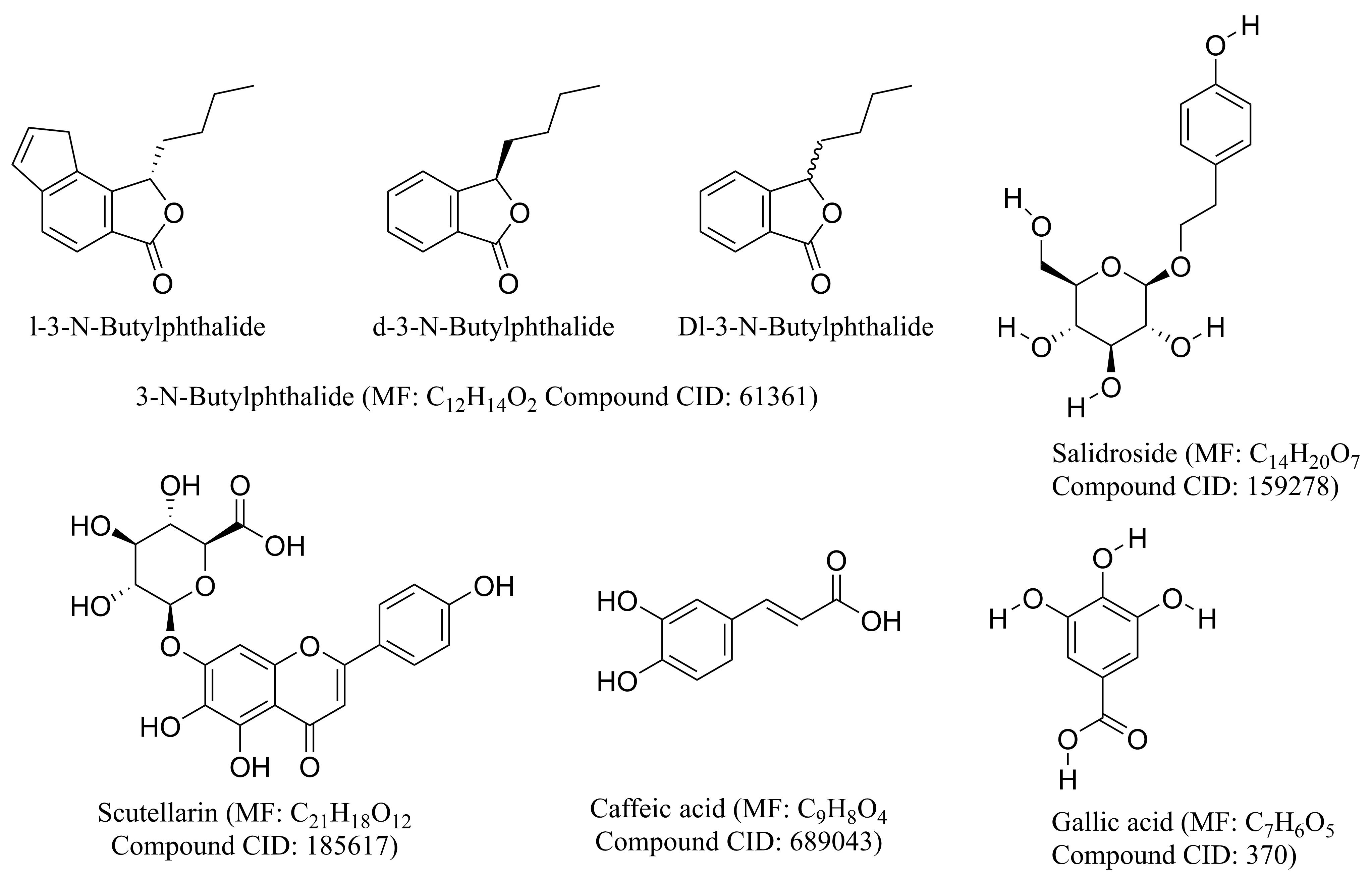 Fig. 8.
Fig. 8.The structure of the main chemical compounds in common use. The chemical structures of compounds were derived from PubChem (https://pubchem.ncbi.nlm.nih.gov/).
NBP, a family of compounds initially isolated from the seeds of Apium graveolens Linn., has shown considerable neuroprotective effects. NBP is composed of the optical isomers l-3-N-butylphthalide (l-NBP) and d-3-N-butylphthalide (d-NBP). In addition, dl-3-N-butylphthalide (Dl-NBP) is synthesized from l-NBP. In 2002, l-NBP was approved by the Food and Drug Administration of China for the treatment of ischemic stroke [131].
Xu [132] reported that l-nbp can reduce the number of glial fibrillary acidic protein positive astrocytes induced by chronic cerebral ischemia, and has a protective effect on hippocampal injury induced by chronic cerebral ischemia. Li [133] reported that l-nbp treatment can significantly reduce ischemic brain injury and promote the early recovery of neurological function in patients with ischemic stroke. This effect may be related to the transformation of M1 microglia/macrophage to M2 phenotype. Sun [134] reported that Dl-NBP could significantly increase the number and length of CST fibers in denervated cervical spinal cord, significantly increase the expression levels of PSD95 and vglut-1, increase the number of BrdU+/DCX+ cells in the SVZ; and markedly reduce Rho-A+, rock+, Nogo-A+, and nogo-r+ cells in the peripheral cortex.
Studies focused on human metabolism and the pharmacokinetics of NBP showed that NBP is safe for human usage and undergoes oxidation by cytochrome P450 after oral administration to form 23 identified metabolites, among which 10-keto-NBP, 3-hydroxy-NBP, 10-hydroxy-NBP, and NBP-11-oic acid are considered the major metabolites [135].
Scutellarin is an herbal flavonoid glucuronide with multiple pharmacological
activities and is the main active component of Erigeron breviscapus
(Vant.) Hand-Mazz. Scutellarin has been used clinically to treat stroke,
myocardial infarction, and diabetic complications owing to its multiple
beneficial effects, such as antioxidant, anti-inflammation, vascular relaxation,
antiplatelet, anticoagulation, and myocardial protection effects [136].
Scutellarin inhibits oxidative damage by directly scavenging free radicals and NO
produced during oxidative damage [137]. Apoptosis was inhibited by inhibiting
PARP-dependent mitochondrial dysfunction and the translocation of
apoptosis-inducing factors [138]. Scutellarin (50
Scutellarin is minimally toxic or nontoxic in rodents. The 50% lethal dose of scutellarin could not be determined. Similar to many plant-derived flavonoid glucuronides, scutellarin has low solubility in body fluids, unfavorable bioavailability, and a short half-life in mammalian systems. In addition, the hydrolyzed form, scutellarein, is relatively easily absorbed into the blood and can be metabolized into methylated, sulfated, or glucuronidated forms [143].
CA is a phenolic compound that is widely distributed in medicinal plants, such as Perillafrutescens, Dendranthema morifolium (Ramat.) Tzvelev, Patrinia villosa, and Taraxacum mongolicum Hand.-Mazz. CA is an inhibitor of 5-LO and an intriguing compound because it possesses various pharmacological activities, including antibacterial, antiviral, antioxidant, anti-inflammatory, antiatherosclerotic, immunostimulatory, antidiabetic, cardioprotective, antiproliferative, hepatoprotective, anticancer, and anti-hepatocellular carcinoma activities [144].
Liang [145] studied the effect of CA on global CI/RI by biochemical and
histological analyses. The results showed that CA has a remarkable protective
effect against global CI/RI in rats. This neuroprotective effect may be mediated
by the inhibition of 5-LO. Yang [146] injected aluminum (5.0
The pharmacokinetic effect of CA begins with its ingestion as a bound form (esterified) and its absorption upon arriving in the stomach. CA is subjected to three main types of enzymatic conjugation (known as detoxification), namely, methylation, sulphation, and glucuronidation, immediately after absorption through the action of sulfotransferase enzymes, UDP-glucotransferases, and catechol-o-methyltransferases, respectively [147].
Salidroside (Sal) is a bioactive extract principally from traditional herbal medicine such as Rhodiola crenulata (Hook. f. et Thoms.) H. Ohba., which has been commonly used for hundreds of years in Asia countries. Sal is mainly isolated from Crassulaceae and others plants such Oleaceae, Labiatae and Loganiaceae [148]. Previous studies revealed that Sal possessed a wide range of biological activities, such as anti-inflammatory, anti-apoptotic, anti-hypoxic, anti-depressive and anti-oxidative effects [149, 150, 151], for treatment of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, epilepsy, cancer, diabetes, liver damage and addiction [152, 153, 154].
Recently, accumulating researches has shown that Sal could be used as an
effective neuroprotection agent due to its significant effect on preventing
neuronal injury after cerebral ischemia. Sal can inhibit excitotoxicity,
oxidative stress, inflammation, apoptosis and BBB injury. It was found that Sal
induced activity of SOD, GSH-Px, and GST in MCAO models, significantly
attenuating cerebral I/R injury [155]. By inhibiting the activation of MMP-9 (a
member of MMPs) and reversing the decrease of tight junction proteins such as
claudin-5 and occludin, it shows the improvement of BBB injury in experimental
stroke rat model [156]. Xia Chen [157] reported Sal efficiently protected
hippocampal neurons against glutamate-induced excitotoxicity by inhibiting
excessive Ca
Sal, a principal physiologically active constituent from Rhodiola genus herb drug which has been widely verified to be no toxicity or less side effects in a multitude of animal experiments and clinical trials [161], is recognized as a safe natural ingredient agent similarly. The results of pharmacokinetic studies showed that Sal was widely distributed into multiple tissues and organs after entering circulation system, and can still converge in brain spite of keeping a low-rise concentration [162].
Gallic acid (GA), a class of phenolic compounds, also known as 3,4,5-
trihydroxybenzoic acid, is a naturally occurring secondary metabolite found in
various plants, vegetables, nuts and fruits [163]. The pure GA are white or light
brown needle-like crystals or powders with melting points of 235–240
As a histone acetyltransferase inhibitor, GA could counteract amyloid-induced
neurotoxicity by selectively suppressing NF-
Promisingly, toxicity studies have shown that GA scarcely has obvious toxicity or side effects in a variety of animal experiments and clinical trials [172]. According to pharmacokinetic studies, GA is firstly absorbed by the gastrointestinal tract, and then mainly distributed in the kidneys. And it is metabolized by the liver and excreted by the kidney, respectively. Noteworthily, methylation, glucoside acidification and sulfated products are the main forms of GA in vivo. In brief, the absorption and elimination of GA after oral administration are fast, while the structural optimization or dosage form adjustment of GA is beneficial to increase its bioavailability [173, 174, 175].
Modern medicine [176] holds that the main pathogenesis of cerebral infarction is atherosclerosis and plaque deposition on the blood vessel wall, which leads to lumen infarction, decreased vascular elasticity, increased blood viscosity, slowed blood flow, plaque shedding, and arterial and vascular blockage and results in a series of clinical changes, such as ischemia, hypoxia, and the degradation and necrosis of blood and brain tissues. Therefore, clinical treatments focus on neuroprotection, anticoagulation, thrombolysis, increasing blood flow, and lowering blood pressure. However, many patients lose the opportunity for thrombolytic therapy because of the limitation of the treatment window. Anticoagulant and antiplatelet drugs, such as aspirin, clopidogrel and prasugrel, are commonly used clinical drugs. Among them, aspirin inhibits the formation of TXA2, and clopidogrel and prasugrel inhibit the P2Y12 (ADP) receptor to prevent thrombus formation [177]. Although these drugs have low prices and notable curative effects, they are prone to hemorrhagic injuries during treatment, such as secondary hemorrhage from infarct focus and visceral hemorrhage [178]. The MATCH trial included 7599 subjects from 28 countries, and the result was that clopidogrel + aspirin were used for some subjects with ischemic stroke, transient ischemic attack, and bleeding adverse events. The frequent increase occurred, endangering patient lives, and no good results were obtained [179]. Chinese medicines have a great advantage in the treatment of stroke compared with modern medicine [180] because of the advantage of prescriptions. The complementary therapeutic effects of different prescriptions and specific effects of TCM are conducive to the smooth recovery after stroke under the guidance of the overall concept and theory of syndrome differentiation and treatment. Moreover, traditional medicines have stable and long-lasting effects on cerebral ischemia. Furthermore, traditional medicines can greatly reduce the side effects and adverse reactions of drugs and ensure the safety of clinical medication. However, traditional medicine decoctions are not easy to extract completely because of the large number of medicinal components, and the fat-soluble and insoluble components are decocted in water. Therefore, the absorption and the effect are slow.
Traditional Chinese patent medicines have been widely used clinically with the development of the modern pharmaceutical industry [181]. Compared with traditional Chinese medicine decoctions, Chinese patent medicines are easy to take and carry. Furthermore, different traditional medicines use different processing methods, and the effective substances are completely extracted. Traditional Chinese patent medicines have certain effects on treating ischemic stroke by improving daily life, the symptoms of neurological deficits and TCM syndromes. Some biological indicators, such as plasma NO levels, serum tumor factors, and circulating endothelial cell levels, can also explain their mechanism of action [182]. At present, traditional Chinese patent medicines can assist Western medicine in preventing vascular spasms, improving the cerebral vascular supply, and generally improving the therapeutic effect in patients with stroke [183].
This article summarizes and identifies the name, original species, family, medicinal parts, traditional use, pharmacological effects and other aspects of traditional medicines for the treatment of ischemic stroke. The results showed that most of these traditional medicines are botanical medicinal materials and are distributed among 132 families. Leguminosae was the most common. We found several traditional medicines that are commonly used to treat ischemic stroke, such as L. chuanxiong, safflower, Angelica sinensis, and musk, through literature searching and data mining. Pharmacology, phytochemistry, metabolomics and clinical trials were performed to evaluate the biological activity and clarify the mechanism of action. In addition, special attention should be paid to several active compounds isolated from the most commonly used medicines. We believe that ligustrazine, ferulic acid, hydroxysafflor yellow A, and LIG may be good and promising therapeutic candidates for the treatment of ischemic stroke because they have the neuroprotective effects and are present in high concentrations in corresponding species. In the future, we can find new perspectives for the treatment of ischemic stroke by studying the effective components and mechanisms of relevant preparations and traditional medicines. For example, Li explored the use of traditional medicines to treat ischemic stroke in the context of the mitochondrial permeability transition pore and found that traditional medicines inhibit neuronal apoptosis induced by excessive opening of the mitochondrial permeability transition pore [184]. Furthermore, the effective components in the preparations and traditional medicines were extracted and further studied, and these components may become potential drugs for the treatment of ischemic stroke. In addition, in ethnic medicine, there are many traditional medicines that are not common traditional Chinese medicines, such as Haematitum,Hydragyrum and Nardostachys jatamansi (D. Don) DC. These traditional medicines increase the probability of discovering novel effective components and are also conducive to the development of more stable and safe new drugs.
TCM, traditional Chinese medicine; WHO, World Health Organization; RNS, reactive
nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase;
CAT, catalase; GSH-PX, glutathione peroxidase; GSH, glutathione; MDA,
Malondialdehyde; NO, nitric oxide; 3-NT, 3-nitrotyrosine; NMDA,
N-methyl-D-aspartic acid; MLB, Magnesium lithospermate B; TNF-
MX, RXW and ZW designed the review. XLL, YSZ, JYL, KF, YL collects information. RXW arranges data. MX wrote the manuscript. ZW helped prepare the figures and revised the manuscript. All authors discussed, edited and approved the final version.
Not applicable.
We thank Chengdu University of Traditional Chinese Medicine for providing us with a research platform.
This work was supported by National Natural Science Foundation of China (81102895), China Postdoctoral Science Foundation (2012M511916), Sichuan application basic research plan (2016JY0017).
The authors declare no conflict of interest.
