The hallmark of Multiple Sclerosis (MS) pathophysiology is the damage to the myelin sheath around axons. The cerebellum is a predilection site for demyelination with a well-recognized role in motor and a rather understudied contribution to cognitive functions. The aim of this study is to investigate patterns of cerebellar grey and white matter pathology, expressed as reduced volume, as well as cortical thickness and their potential contribution to cognitive performance and disability status of patients with MS. 24 patients with MS underwent extensive neuropsychological assessment using paper and pencil tests and the Brain Health Assessment (BHA) tablet-based battery. Cerebellar lobular volumes and thickness were calculated using a volumetric analysis with automated segmentation of the cerebellum and its lobules. The main findings are a reduction of cerebellar grey matter (CGMV) and white matter volumes (CWMV) in lobule X and a widespread cerebellar cortical thinning in patients. Overall disease severity and neurological disability, assessed with the Expanded Disability Status Severity Scale, was correlated with fatigue and information processing speed tasks, but not with CGMV and CWMV. CWMV and CGMV of lobule I–II was negatively correlated with information processing speed, as well as visuospatial memory tests and, finally, inverse cortical thinning associations were noted between the whole cerebellum, lobule I–II, lobule III, lobule VI, Crus I, lobule VIIIA and information processing speed and verbal fluency tasks. The inverse associations observed may represent a compensatory mechanism activated in MS engaging additional high-level cortical areas functionally interconnected with the damaged cerebellum, in order to cope with the cognitive demands of a task.
Multiple Sclerosis (MS) is a heterogeneous, neurodegenerative disease, characterized by the presence of inflammation, demyelination and loss of axons and neurons in the Central Nervous System (CNS) that leads to progressive physical disability and cognitive impairment. MS can cause a wide spectrum of signs and symptoms, depending on the location and the extent of the lesions in the CNS affecting the physical, emotional and cognitive functioning of persons with Multiple Sclerosis (PwMS). Cognitive impairment is estimated to affect 40%–65% of PwMS [1] and may be present even in the absence of physical disability. The cognitive domains affected in MS can range across patients, but typically include attention and concentration, executive functions, information processing speed, learning and memory and visuospatial abilities. Cognitive impairment increases individuals’ disease burden and is directly related to social functioning, employability and independence [2].
Progressive and widespread brain volume loss is a common finding in MS. The cerebellum, in particular, is a site of atrophy in this population of patients [3], even at the early stages of the disease [4]. The mechanisms contributing to cerebellar atrophy in PwMS include demyelination, axonal/neuronal loss [5] and synaptic reduction [4]. Although demyelination affects more often the cerebellar white matter and the deep nuclei, histopathological studies have shown demyelination in the cortical cerebellar grey matter, as well, which could be even more extended, on average covering from 14% up to 90% of the total cerebellar GM atrophy for a review see Parmar et al. [6]. The few available MRI studies have demonstrated a significant reduction in cerebellar GM and WM volume in PwMS compared to healthy controls, which is related not only to impaired motor function, but also impaired performance on cognitive tests. Anatomical evidence suggests a dichotomy of a motor-cognitive functional organization of the cerebellum. Posterior cerebellar volume loss is mainly associated with cognitive impairment, whereas anterior cerebellar volume loss is associated with motor dysfunction [6, 7].
The role of cerebellum in cognition and more specifically, the relationship between cerebellar abnormalities and cognitive deficits in MS remains elusive. Functional imaging and clinical studies have provided evidence for the cerebellar contribution to attention (mainly divided and alternating attention), learning and memory [8], various aspects of executive functions, such as working memory, multitasking, inhibition [9, 10], task-switching [11], verbal fluency and concept formation [8]. Language impairments (writing and speech) can also be related to cerebellum pathology [12]. In individuals with dyslexia cerebellar dysfunction has been suggested as a potential etiological factor [13, 14, 15]. Furthermore, cerebellar lesions have also been related to visuospatial deficits and impaired performance in judgment of line orientation, mental rotation and cube drawing tasks [8, 16]. Finally, a constellation of cognitive deficits involving the cerebellum has been described by Schmahmann and Sherman [17], who introduced the cerebellar cognitive- affective syndrome (CCAS), a condition observed in adults and children, after acquired cerebellar lesions, tumors, cerebellar degeneration, Chiari malformation, cerebellar hypoplasia and agenesis [17, 18, 19]. The symptoms involve deficits in executive functions (planning, organization, set-shifting, working memory, verbal fluency, abstract thinking, mental flexibility), difficulties with spatial cognition (organization of visual short-term memory), personality changes (disinhibition, blunted affect) and language impairments (dysprosody and agrammatism).
The contribution of cerebellar pathology to cognitive difficulties of PwMS in particular, remains largely unknown. Of note, PwMS with cerebellar lesions have a different neurocognitive profile compared to those without cerebellar lesions, but its exact characteristics warrant further investigation [7, 20]. In several studies, GM loss in the posterior cerebellar lobules and, more specifically, in vermis (lobule VI) was associated with impairment in information processing speed (Symbol Digit Modalities Test; SDMT) [7, 21, 22, 23, 24]. In fact, lobule Crus I emerged among the other posterior cerebellar lobules, as an independent predictor of SDMT and California verbal learning test performance, a verbal learning and memory task [25]. The authors hypothesized that the cerebellum in PwMS is involved in the cognitive automation of a task, leaving the more demanding functions to higher-order associative cerebral areas, such as the prefrontal cortex [22].
Correlations of cerebellar volumes with disease severity and neurological disability, such as that assessed with the Expanded Disability Status Scale (EDSS) [26] were less consistent across studies, even though the trend was towards a negative relationship. For example, D’ Ambrosio and his colleagues [7], found a correlation between higher cerebellar EDSS subsystem score and lower total cerebellar volume. Similar findings were reported by other studies as well [27, 28]. On the other hand, Weier et al. [29] found no correlation between normalized total cerebellar volume and EDSS.
Studies investigating regional brain atrophy in PwMS, especially in relation to cognition have important theoretical and clinical implications. The potentially observed associations cannot infer causality. However, they constitute a starting point in our efforts to understand how different aspects of cognitive impairment can be explained by neuropathology-in the present investigation, atrophy of specific cerebellar lobules. On a clinical level, delineating the neuropathological basis in MS could potentially contribute to the discovery of new biomarkers of cognitive impairment and guide evidence-based neuropsychological rehabilitation interventions for treatment of MS cognitive deficits. Therefore, studying cerebellar focal atrophy could be of both theoretical and clinical importance.
Given the limited information on the cerebellar pathology expressed as cerebellar atrophy in MS and the much-disputed role of the cerebellum in cognitive functioning our aim was twofold: first, we wanted to determine the extent of cerebellar atrophy in patients with MS using a novel automatic magnetic resonance imaging (MRI) segmentation analysis, and second to investigate if cerebellar atrophy is related to cognitive deficits-measured with widely used neuropsychological procedures and experimental computerized tasks – and to the disability status of PwMS, assessed with the EDSS. We expected that PwMS will exhibit low WM and GM cerebellar volumes and cerebellar cortical atrophy and that both indexes (CGMV and CWMV), as well as cerebellar cortical thickness, will be correlated with performances on cognitive tasks. Finally, an association of EDSS with CGMV and CWMV indexes was expected.
A total of 24 (14 female) patients with MS and mean age of 38.83 years (SD = 9.9), were recruited from the outpatient clinic of the Multiple Sclerosis Center of the 2nd Department of Neurology of the AHEPA University Hospital of Thessaloniki, in Greece, between November 2019 and June 2020. All patients underwent a neurological examination, including the EDSS assessment, by a neurologist specializing in MS. Mean disease duration was 11 years (SD = 5.5) and median EDSS was 5 (range 1.5–7). Written informed consent was obtained from all participants prior to the assessment. The present study was approved by the local Ethical Committee (4.291/4) and was performed in accordance with the Declaration of Helsinki. A complete list of the demographic and clinical information of all patients with MS included in the study is summarized in Table 1.
| Mean (SD) or frequency (%) | Range | ||
| Age (years) | 38.83 (9.9) | 21–57 | |
| Education level in years | 13.25 (2.3) | 9–18 | |
| Gender (male/female) | 10/14 (41.7%/58.3%) | ||
| Hand (right/left) | 21/3 (87.5%/12.5%) | ||
| MS type (RRMS/SPMS/PPMS) | 13/2/9 (54.2%/8.3%/37.5%) | ||
| EDSS | 4.8 (1.7) | 1.5–7 | |
| Disease duration (years) | 11 (5.5) | 2–23 | |
| MFIS total | 37.8 (19.4) | 36–100 | |
| CRIq | 95.3 (7) | 84–107 | |
| DASS-21 | 17.8 (2.8) | 0–50 | |
| BDI | 4.6 (4.1) | 0–14 | |
| DMT (yes/no) | 19/5 (79.2%/20.8%) | ||
| Natalizumab | 8 (39.3%) | ||
| Ocrelizumab | 7 (29.2%) | ||
| Fingolimod | 1 (4.2%) | ||
| Alemtuzumab | 1 (4.2%) | ||
| Interferon Beta-1a | 1 (4.2%) | ||
| Glatiramer acetate | 1 (4.2%) | ||
| Note: Data are shown as M, mean; SD, Standard Deviation or as frequency in %; EDSS, Expanded disability status scale; MFIS, Modified Fatigue Impact Scale total; CRIq, Cognitive Reserve Index questionnaire; DASS-21, Depression, Anxiety, Stress Scale; BDI, Beck Depression Inventory; DMT, disease modifying therapy. | |||
Patients were diagnosed with MS based on the revised McDonald criteria [30]. Inclusion criteria were a recent MRI (within 3 months), and no clinical relapses and steroid treatment within the preceding 1 month. Patients with a history of psychiatric disorder, epilepsy, substance abuse and alcoholism and visual impairment that may interfere with cognitive testing were excluded from the study.
The Expanded Disability Status Scale (EDSS) was used to assess disease progression and disability via seven functional domains—visual (optic), brainstem (cranial nerve function including speech and swallowing), pyramidal (motor function), cerebellar (coordination), sensory (touch and pain), bowel and bladder, and cerebral (cognition and mood). Each domain is scored from 0 (no disability) to 5 or 6 (severe disability). The total EDSS score ranges from 0 (normal examination) to 10 (death) [26].
A list of the neuropsychological tasks administered in each cognitive domain is presented in Table 2. The neuropsychological assessment included the Brief International Cognitive Assessment for MS (BICAMS) and Brain Health Assessment (BHA-TabCAT).
| Domain | Paper and pencil | Computerized |
| BICAMS | BHA | |
| Learning and memory | ||
| Visuospatial abilities | ||
| Language | ||
| Processing speed/executive functions | ||
The BICAMS [31, 32] is a paper and pencil battery, validated in the Greek population [33] that includes three tests: SDMT, the California Verbal Learning Test-2 (CVLT-2) validated in the Greek language [34]—hereinafter referred to as GVLT (total score across the five learning trials, immediate recall)- and the Brief Visuospatial Memory Test-Revised (BVMT-R) (total score across the three trials) [35].
The BHA [36] is a 10-minute tablet-administered cognitive assessment developed at UCSF and programmed in the TabCAT software platform. It consists of five subtests: Favorites (associative memory), Match (executive function and speed), Line Orientation (visuospatial), and Animal Fluency (language). For the purposes of the present study, we added an additional executive function/sustained attention test, the Set-Shifting task, also on TabCAT. We used the Greek translation of the platform (see the Supplementary material for the translation procedure). In more detail, from the Favorites task, the two variables included in the analyses were (a) the total number of correct responses across both learning trials and the delay trial and (b) recognition score which is a d-prime score/variable; from the Match test, the variable used in the analyses was the total correct score in two minutes; from the Line Orientation test, the variable used was the threshold score, which represents for the line orientation the average angle difference between the non-match orange line and the white line at which probability of the examinee’s correct response is 75%. Finally, for the Set-Shifting task, the variable used was the set shifting score that combines accuracy and reaction time metrics.
We added to the neuropsychological assessment the Letter Fluency task, to complement the semantic condition of the verbal fluency test. The version administered was developed by Kosmidis et al. [34], where the patient is requested to orally produce words that begin with the Greek letter “X” in one minute.
Participants also completed the Cognitive Reserve Index Questionnaire (CRI-q), Greek adaptation, which is administered through a semi-structured interview [37]. CRI-q assesses three domains as indexes of cognitive reserve: education, working activity and leisure time activities.
Finally, we included in the study three self-assessment questionnaires (Greek adaptations). The Modified Fatigue Impact Scale (MFIS) was administered for the assessment of fatigue [38] and the Depression, Anxiety, Stress Scale (DASS-21) [39] and the Beck Depression Inventory (BDI) for the assessment of the mood [40].
All MRI scans were performed in a 1.5 Tesla Philips MRI scanner, as part of the
routine follow up of each patient. No scan presented new or enlarging T2 lesions
or gadolinium enhancing T1 lesions. All scans were performed with the same
imaging protocol. After checking for movement artifacts, we used 3 dimensional
T1-weighted sagittal brain images (160 slices, with a slice thickness of 1.2 mm
and 1.2 mm spacing between slices). Volumetric analysis of the cerebellum was
performed using the CERES [41] pipeline of the VolBrain automated brain volumetry
system [42] (see also Fig. 1). In brief, pseudo- anonymized images were
automatically segmented according to high resolution cerebellum atlases [43]. The total cerebellar volume, cortical thickness and lobule volumes were
calculated as absolute (cm
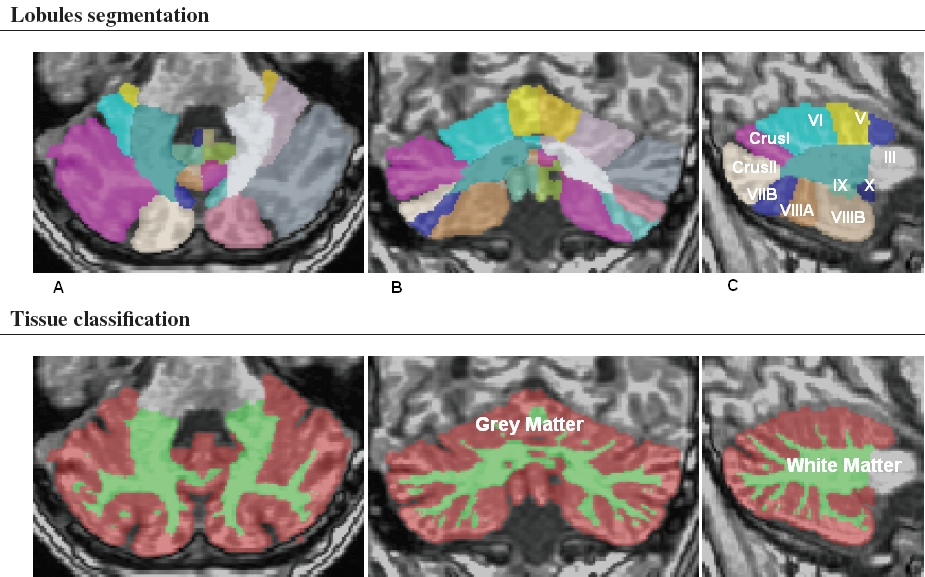 Fig. 1.
Fig. 1.Cerebellar magnetic resonance imaging (MRI) segmentation map of a representative MS patient provided by the brain volumetric program CERES. The different colors show the lobular parcellation. (A) axial, (B) coronal and (C) sagittal.
Statistical analyses were performed with the Statistical Package for Social
Sciences (SPSS), version 25.0 for Windows (IBM Corp., Armonk, NY, USA). Normal
distribution was tested for all variables with the Shapiro-Wilk test. Demographic
and clinical characteristics of the patients were presented as means (SD) or
frequencies (%). The proportion of patients whose cerebellar volumes and
cortical thickness were below the expected bounds based on their age and sex,
were automatically provided by the CERES tool (VolBrain). The association
of CWMV, CGMV and cerebellar cortical thickness with clinical and
neuropsychological measures was estimated with Pearson’s correlation coefficient
(r). When CGMV, CWMV and cerebellar cortical thickness were associated with
clinical and neuropsychological measures, a linear regression model including
age, sex and years of education was run to control for the potential effects of
demographic variables on the observed associations. Statistical significance was
set at p
Mean performances and standard deviations on neuropsychological tests are presented in Table 3.
| Neuropsychological tests | Mean (SD) | Range |
| SDMT | 42.13 (13.8) | 19–70 |
| BVMT-R | 19.8 (9.7) | 0–34 |
| GVLT | 51.6 (14.3) | 23–77 |
| Animal fluency | 18.3 (7.3) | 6–38 |
| Phonemic fluency | 8.4 (5.4) | 0–22 |
| Favorites (total) | 11.5 (5.9) | 2–22 |
| Favorites (recognition) | 5.8 (1.9) | 1–8 |
| Line orientation | 8282.61 (4305) | 2500–18750 |
| Match test | 39.8 (13) | 8–60 |
| Set-shifting | 6973.8 (808.3) | 5435–8342 |
| Note: Data are shown as M, mean; SD, Standard Deviation; SDMT, Symbol digit modalities test; BVMT-R, Brief Visuospatial Memory Test-Revised; GVLT, Greek Verbal Learning Test. | ||
Cerebellar volumes for each lobule obtained are presented (Fig. 1) in absolute
value (measured in cm
| Cerebellar structure | Mean (SD) | |||||
| Grey matter | White matter | |||||
| Total | Left | Right | Total | Left | Right | |
| Whole cerebellum | 90.7 (17.1) | 45.7 (9.1) | 45 (8.3) | 125.4 (20.6) | 62.9 (10.7) | 62.4 (10.1) |
| Lobule I–II | 0.03 (0.03) | 0.03 (0.07) | 0.04 (0.01) | 0.04 (0.04) | 0.02 (0.02) | 0.02 (0.02) |
| Lobule III | 1 (0.2) | 0.5 (0.1) | 0.5 (0.1) | 1.3 (0.3) | 0.6 (0.2) | 0.8 (0.3) |
| Lobule IV | 3.36 (0.56) | 1.65 (0.32) | 1.71 (0.28) | 3.9 (0.59) | 1.9 (0.33) | 2 (0.31) |
| Lobule V | 6.23 (1.1) | 3.13 (0.54) | 3.11 (0.57) | 7.52 (1.23) | 3.73 (0.61) | 3.79 (0.67) |
| Lobule VI | 14.5 (3.7) | 7.3 (2) | 7.1 (1.9) | 16.6 (3.9) | 8.3 (2.1) | 8.2 (2.1) |
| Crus I | 19.4 (5.5) | 10.1 (3.1) | 9.4 (2.7) | 24 (5.8) | 12.6 (3.2) | 12 (2.9) |
| Crus II | 13.04 (3.16) | 6.68 (1.7) | 6.38 (1.62) | 15.45 (3.45) | 7.91 (1.8) | 7.54 (1.86) |
| Lobule VIIB | 8.02 (1.58) | 3.91 (0.76) | 4.1 (0.91) | 9 (1.71) | 4.42 (0.84) | 4.58 (0.97) |
| Lobule VIIIA | 10.1 (2.3) | 5.1 (1.2) | 5.1 (1.2) | 11.4 (2.6) | 5.7 (1.4) | 5.7 (1.3) |
| Lobule VIIIB | 7.6 (1.58) | 3.89 (0.9) | 3.71 (0.76) | 7.6 (1.58) | 3.89 (0.9) | 3.71 (0.76) |
| Lobule IX | 7.49 (1.98) | 3.66 (0.96) | 3.84 (1.04) | 7.49 (1.98) | 3.66 (0.96) | 3.84 (1.04) |
| Lobule X | 1.6 (0.4) | 0.8 (0.3) | 0.8 (0.2) | 1.6 (0.5) | 0.8 (0.3) | 0.8 (0.2) |
In lobule X, all patients showed reduced GM volumes and 96% of them showed reduced WM volumes (total, left, right) (see Table 5).
Cerebellar subregions with cortical thickness below the bounds expected for age and sex are summarized in Table 6. Diffuse regional cortical thinning has been observed in all lobules apart from lobule I–II for over 75% of the patients with most of them showing cortical thinning in lobule X.
| Cerebellar structure | WMV | GMV | ||||
| Total | Left | Right | Total | Left | Right | |
| Cerebellum | 9% | 17% | 9% | 26% | 22% | 22% |
| Lobule I–II | 30% | 13% | 35% | 22% | 13% | 26% |
| Lobule III | 22% | 22% | 13% | 0% | 0% | 0% |
| Lobule IV | 0% | 9% | 0% | 4% | 4% | 0% |
| Lobule V | 0% | 0% | 0% | 0% | 0% | 0% |
| Lobule VI | 17% | 26% | 22% | 22% | 22% | 17% |
| Crus I | 9% | 9% | 17% | 30% | 17% | 22% |
| Crus II | 4% | 4% | 4% | 4% | 4% | 9% |
| Lobule VIIB | 4% | 0% | 4% | 4% | 4% | 0% |
| Lobule VIIIA | 17% | 13% | 13% | 13% | 9% | 13% |
| Lobule VIIIB | 9% | 4% | 9% | 4% | 4% | 9% |
| Lobule IX | 0% | 4% | 4% | 0% | 0% | 0% |
| Lobule X | 96% | 96% | 96% | 100% | 100% | 100% |
| Cerebellar structure | Mean | Left | Right |
| Cerebellum | 78% | 83% | 78% |
| Lobule I–II | 74% | 74% | 74% |
| Lobule III | 74% | 78% | 74% |
| Lobule IV | 74% | 74% | 74% |
| Lobule V | 74% | 74% | 74% |
| Lobule VI | 74% | 74% | 74% |
| Crus I | 74% | 74% | 74% |
| Crus II | 87% | 87% | 87% |
| Lobule VIIB | 87% | 87% | 83% |
| Lobule VIIIA | 83% | 83% | 78% |
| Lobule VIIIB | 83% | 83% | 78% |
| Lobule IX | 56% | 61% | 61% |
| Lobule X | 93% | 93% | 87% |
| Note: Cortical thickness is normalized in relation to the cube root of the intracranial volume (adimensional). N = 24. | |||
Since most of PwMS showed reduced WMV/GMV and lower cortical thickness in lobules I–II, III, VI, VIII A and X, we focused our analysis on these subregions to further examine potential correlations with neuropsychological performances. In order to study the relationship between WMV/GMV and cortical thickness with the clinical and neuropsychological measures, a series of Pearson’s r correlation coefficients were calculated followed by linear regression analysis to control for demographic factors such as age, sex, education level. For all correlations only relative values were considered (measured in relation to the total intracranial volume).
A negative correlation was found between WMV of lobule I–II and SDMT, BVMT-R,
GVLT and Match tests, such that larger volumes were associated with worse
performance. After controlling for age, sex and education level, this strong
negative relationship remained for SDMT (R
| Neuropsychological measures | Cerebellar structures | ||||||
| Whole cerebellum | Lobule I–II | Lobule III | Lobule VI | Crus I | Lobule VIIIA | Lobule X | |
| SDMT | 0.037 | –0.583**† | –0.082 | –0.034 | –0.144 | 0.021 | 0.080 |
| BVMT-R | 0.080 | –0.592**† | 0.048 | –0.185 | –0.230 | 0.036 | 0.179 |
| GVLT | –0.140 | –0.446* | –0.166 | –0.214 | –0.399 | –0.040 | 0.049 |
| Animal fluency | 0.070 | –0.334 | –0.170 | 0.062 | –0.200 | 0.100 | –0.016 |
| Phonemic fluency | 0.083 | –0.375 | –0.145 | –0.050 | –0.201 | –0.057 | –0.014 |
| Favorites (total) | 0.204 | –0.272 | –0.066 | 0.201 | –0.105 | 0.250 | 0.033 |
| Favorites (rec) | –0.012 | –0.125 | 0.447 |
–0.289 | –0.240 | 0.103 | 0.352 |
| Line orientation | –0.147 | –0.020 | –0.153 | 0.289 | 0.000 | –0.060 | –0.253 |
| Match test | 0.237 | –0.461 |
0.037 | 0.024 | –0.011 | 0.121 | 0.260 |
| Set-shifting | 0.139 | –0.053 | –0.169 | 0.065 | 0.013 | 0.203 | –0.157 |
| Note: *, Correlation is significant at the 0.05 level (2-tailed); **, Correlation is significant at the 0.01 level (2-tailed). †, Correlation is significant after correction for multiple comparisons. SDMT, Symbol digit modalities test; BVMT-R, Brief Visuospatial Memory Test-Revised; GVLT, Greek Verbal Learning Test. | |||||||
Negative correlations were found between lobules I–II, III, VI and Crus I and
neuropsychological scores. Larger volumes were again associated with worse
performances. More specifically, GMV of lobule I–II was correlated with scores on
SDMT, BVMT-R, GVLT and Match test; GMV of lobule VI with Line Orientation scores
and Crus I with GVLT scores. After controlling for age, sex and education level,
the correlations that remained significant were the ones between lobule I–II with
SDMT (R
| Neuropsychological measures | Cerebellar structures | ||||||
| Whole cerebellum | Lobule I–II | Lobule III | Lobule VI | Crus I | Lobule VIIIA | Lobule X | |
| SDMT | –0.065 | –0.593**† | 0.073 | –0.091 | –0.214 | 0.214 | 0.056 |
| BVMT-R | –0.098 | –0.419* | 0.084 | –0.208 | –0.300 | 0.230 | 0.148 |
| GVLT | –0.245 | –0.604**† | –0.059 | –0.245 | –0.459* | 0.081 | 0.032 |
| Animal fluency | –0.100 | –0.303 | –0.297 | –0.009 | –0.261 | –0.082 | –0.046 |
| Phonemic fluency | –0.180 | –0.373 | –0.119 | –0.126 | –0.269 | –0.138 | –0.046 |
| Favorites (total) | –0.013 | –0.305 | –0.166 | 0.103 | –0.218 | 0.167 | –0.014 |
| Favorites (rec) | –0.110 | –0.059 | 0.370 | –0.330 | –0.344 | 0.159 | 0.374 |
| Line orientation | 0.114 | –0.021 | –0.212 | 0.344 | 0.118 | 0.011 | –0.233 |
| Match test | 0.017 | –0.500* | 0.056 | –0.051 | –0.151 | 0.155 | 0.215 |
| Set-shifting | 0.002 | –0.109 | –0.134 | 0.009 | –0.049 | 0.118 | –0.192 |
| Note: *, Correlation is significant at the 0.05 level (2-tailed); **, Correlation is significant at the 0.01 level (2-tailed). †, Correlation is significant after correction for multiple comparisons. SDMT, Symbol digit modalities test; BVMT-R, Brief Visuospatial Memory Test-Revised; GVLT, Greek Verbal Learning Test. | |||||||
In conclusion, the pattern of correlations was the same for grey and white matter volumes. White matter and grey matter atrophy of lobules I–II can predict higher performances on tests across different cognitive domains, such as information processing speed, whereas grey matter atrophy of lobule VI can predict higher performances on visuospatial tasks.
Cortical thickness was correlated mainly with performance on the Match test and Line Orientation. More specifically, after controlling for demographic factors, cortical thickness of the whole cerebellum, lobule I–II, lobule III, Crus I and lobule VIIIA, was inversely associated with scores on the Match test and positively with scores on Line Orientation (higher scores indicate worse performance); cortical thickness of lobule VI was negatively correlated with the Match as well as SDMT scores. Finally, lobule X was negatively associated with the Match, SDMT and Phonemic fluency test scores. The observed correlations appear in Table 9, Fig. 2 and results of the linear regression analyses appear in Table 10. On further analysis, when we additionally controlled in the linear regression for disease severity and neurological disability, assessed with EDSS, the results remained unchanged with very small variations in the significance value. For example, the p-value between SDMT scores and cortical thickness of lobule VI and lobule X was 0.022 and 0.027 respectively, instead of 0.023 and 0.020 (see Table 10). Furthermore, although the present investigation is exploratory in nature, when we applied correction for multiple comparisons, most of the observed correlations remained significant. We applied partial Bonferroni correction that accounts for both the number of the comparisons conducted (10) and the mean correlation among the outcome variables (mean Pearson r = 0.39), and this yielded a partial Bonferroni adjusted p-value of 0.01 (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm) (see Tables 7,8,9).
 Fig. 2.
Fig. 2.Scatterplots showing the relationship (Pearson’s correlation r) between Match test and the whole cerebellum and lobules III, VI and X.
| Neuropsychological measures | Cerebellar structures | ||||||
| Whole Cerebellum | Lobule I–II | Lobule III | Lobule VI | Crus I | Lobule VIIIA | Lobule X | |
| SDMT | –0.435* | –0.271 | –0.429* | –0.471* | –0.441* | –0.372 | –0.538**† |
| BVMT-R | –0.343 | –0.364 | –0.360 | –0.406 | –0.322 | –0.301 | –0.444* |
| GVLT | –0.426* | –0.196 | –0.374 | –0.452* | –0.429* | –0.356 | –0.415* |
| Animal fluency | –0.338 | –0.323 | –0.319 | –0.348 | –0.372 | –0.282 | –0.434* |
| Phonemic fluency | –0.484* | –0.488 | –0.441* | –0.479* | –0.487* | –0.446* | –0.565**† |
| Favorites (total) | –0.394 | –0.599* | –0.409 | –0.388 | –0.419* | –0.324 | –0.473* |
| Favorites (rec) | –0.234 | –0.396 | –0.265 | –0.296 | –0.206 | –0.195 | –0.247 |
| Line orientation | 0.504 |
0.546 |
0.507 |
0.483 |
0.491 |
0.492 |
0.372 |
| Match test | –0.577**† | –0.741**† | –0.616**† | –0.579**† | –0.571** | –0.531* | –0.611**† |
| Set-shifting | –0.158 | –0.304 | –0.105 | –0.139 | –0.191 | –0.068 | –0.260 |
| Note: *, Correlation is significant at the 0.05 level (2-tailed); **, Correlation is significant at the 0.01 level (2-tailed). †, Correlation is significant after correction for multiple comparisons. SDMT, Symbol digit modalities test; BVMT-R, Brief Visuospatial Memory Test-Revised; GVLT, Greek Verbal Learning Test. | |||||||
| Model | Independent predictors | ||||||
| R |
F | p-value | t-value | p-value | |||
| Match test | |||||||
| Whole cerebellum | 0.659 | 8.229 | 0.001 | –0.781 | –5.195 | ||
| Sex | 0.057 | 0.356 | 0.726 | ||||
| Age | –0.580 | –3.729 | 0.002 | ||||
| Education | 0.048 | 0.289 | 0.776 | ||||
| Lobule I–II | 0.623 | 4.125 | 0.031 | –0.772 | –3.937 | 0.003 | |
| Sex | 0.177 | 0.716 | 0.490 | ||||
| Age | –0.237 | –1.199 | 0.258 | ||||
| Education | –0.083 | –0.333 | 0.746 | ||||
| Lobule III | 0.708 | 10.319 | –0.811 | –5.861 | |||
| Sex | 0.050 | 0.342 | 0.737 | ||||
| Age | –0.568 | –3.977 | 0.001 | ||||
| Education | 0.079 | 0.574 | 0.614 | ||||
| Crus I | 0.637 | 7.457 | 0.001 | –0.762 | –4.926 | ||
| Sex | 0.052 | 0.318 | 0.755 | ||||
| Age | –0.581 | –3.606 | 0.002 | ||||
| Education | 0.000 | 0.002 | 0.998 | ||||
| Lobule VI | 0.683 | 9.158 | –0.804 | –5.501 | |||
| Sex | 0.047 | 0.307 | 0.763 | ||||
| Age | –0.600 | –3.973 | 0.001 | ||||
| Education | 0.066 | 0.413 | 0.685 | ||||
| Lobule VIIIA | 0.602 | 6.418 | 0.002 | –0.742 | –4.539 | ||
| Sex | 0.054 | 0.312 | 0.759 | ||||
| Age | –0.555 | –3.311 | 0.004 | ||||
| Education | 0.102 | 0.571 | 0.576 | ||||
| Lobule X | 0.701 | 9.963 | –0.818 | –5.753 | |||
| Sex | 0.910 | 0.612 | 0.549 | ||||
| Age | –0.625 | –4.229 | 0.001 | ||||
| Education | –0.134 | –0.853 | 0.405 | ||||
| SDMT | |||||||
| Lobule VI | 0.450 | 3.682 | 0.023 | –0.638 | –3.405 | 0.003 | |
| Sex | 0.010 | 0.055 | 0.957 | ||||
| Age | –0.340 | –1.792 | 0.090 | ||||
| Education | 0.303 | 1.606 | 0.126 | ||||
| Lobule X | 0.459 | 3.825 | 0.020 | –0.639 | –3.481 | 0.003 | |
| Sex | 0.018 | 0.097 | 0.924 | ||||
| Age | –0.346 | –1.833 | 0.083 | ||||
| Education | 0.173 | 0.926 | 0.367 | ||||
| Phonemic fluency | |||||||
| Lobule X | 0.460 | 3.832 | 0.020 | –0.683 | –3.719 | 0.002 | |
| Sex | –0.060 | –0.328 | 0.747 | ||||
| Age | –0.383 | –2.033 | 0.057 | ||||
| Education | 0.053 | 0.286 | 0.778 | ||||
| Note: Match Test (BHA); SDMT, Symbol digit modalities test. | |||||||
As expected, after controlling for demographic variables (age, sex, years of
education), EDSS was positively correlated with fatigue (R
The associations of EDSS with cerebellar volumes of all cerebellar lobules
(I–X) was examined with Pearson’s r correlational analyses. The lobule
that was mainly associated with EDSS was lobule IX. Significant negative
correlations were found between EDSS and right cerebellar WM and GM volume, as
well as, with the total volume of lobule IX. However, after controlling for
demographic variables, the observed associations failed to reach statistical
significance (p
In the present exploratory study, we performed a comprehensive volumetric assessment of the cerebellum and investigated its association with cognitive functioning and disability status of patients with MS. The use of advanced MRI techniques such as diffusion-weighted imaging and volumetric imaging, in the study of cerebellum, can reveal microstructural changes, assess regional and global volumes, as well as measure lesion load (for a review see Mormina et al. [44]). The semiautomatic computerized volumetric method used in this study to assess lobular GM and WM cerebellar volumes, is appropriate for the investigation of cerebellar morphometry per se, objectively and reliably quantifies cerebellar tissues and is also expected to provide important biomarkers for cerebellar disease [45].
A major finding of the present investigation is that almost all PwMS had reduced GMV and WMV in lobule X and over 75% presented with widespread cerebellar cortical thinning. This was expected, as cerebellum is among the brain structures most vulnerable to focal and diffuse (i.e., thinning) damage in MS compared to other brain regions [27]. Furthermore, cerebellar pathology involving neuroinflammatory processes, as well as white and grey matter demyelination is a finding well established in MS (for a review see Wilkins et al. [20]).
A second finding is that disease severity and disability, measured with the EDSS was positively associated with fatigue and inversely associated with measures of processing speed/executive functions/associative memory (SDMT and Match). More specifically, the higher the disability, the higher the fatigue and the lower the processing speed ability. Fatigue in MS refers to low motor and/or mental energy and is one of the most common symptoms of the disease. Similarly, the primary cognitive domain affected by MS is information processing speed. Recently, Eizaguirre and colleagues also found that patients with higher EDSS and greater degree of fatigue had slow processing speed [46]. Similar findings on the correlations between disability status and cognitive impairment in MS have also been reported by others [47]. It seems that motor disability, fatigue and slow processing speed in MS are intertwined, although their underlying pathophysiologic mechanisms remain elusive.
Disease severity and disability did not correlate with cerebellar CGMV and CWMV. The absence of EDSS correlations with cerebellar volumes is in line with most previous studies. Damasceno, Damasceno, and Centers [48] found that cerebellar lesion load and not grey and white matter volumes correlated with EDSS. Other studies employing similar or different volumetric analysis of the cerebellum have also failed to detect associations between CGMV/CWMV and EDSS [25, 29, 49]. The observed lack of correlation in these studies was interpreted potentially as a result of low specificity and sensitivity of the existing imaging markers for pathology. An alternative explanation was that clinical measures, such as the EDSS, may not be sensitive enough to assess the high clinical heterogeneity of the disease. On the other hand, Calabrese and colleagues [27] and D’ Ambrosio and colleagues [7], did report a correlation between cortical cerebellar volume and EDSS, although a modest one. A potential explanation for the discrepant findings of the above-mentioned studies, may be the inclusion of patients with different disease severity. At the early stages of the disease, disability could be mainly determined by subcortical white matter lesions whereas at the later stages, it could be determined by the extent of widespread cortical pathology [50].
Regarding the associations between cerebellar volumetric indexes and cognition, we found that WMV and GMV of lobule I–II were both negatively correlated with SDMT and BVMT-R. This negative relationship observed between WMV and GMV of lobule I–II in the anterior cerebellum and information processing speed test (SDMT), as well as visuospatial memory (BVMT-R), means that PwMS with more white and grey matter atrophy in this lobule had higher scores on processing speed and visuospatial tasks and vice-versa; that is, larger volume correlated with worse performance on cognitive tests. Even if these are unexpected findings, similar results in healthy adults have been previously reported, either for the cerebellum or other brain structures. Of note, Bernard et al. [51] found that larger volume in the anterior cerebellum was associated with worse performance on a processing speed test and larger volume in the left posterior cerebellum was associated with shorter spatial span. Foster et al. [52] found that larger hippocampal volume was associated with worse delayed recall performance. The authors of these studies provided a very interesting neurodevelopmental interpretation, namely that larger volume resulted from insufficient neural pruning process during development or even from altered neuronal migration, which might have affected cognition [51, 52]. Although these studies involved healthy adults, this could be the case with PwMS as well. That is, by analogy to the hippocampus, “an inadequately pruned cerebellum mediates cognitive functions less efficiently than a well pruned cerebellum” [52]. Therefore, despite the small sample size, our findings cannot be easily dismissed as being random. An alternative interpretation for this counterintuitive finding relies on the functional connectivity between cerebellum and higher-order cortical areas. Functional and not structural alterations in the cerebellum may be primarily related to cognitive impairment, and cerebro-cerebellar connectivity and not cerebellar atrophy may be a better predictor for cognitive impairment in MS, than atrophy in the cerebellum per se. Of note, functional neuroimaging findings appear to corroborate this interpretation. Cerebellar dysfunction or damage correlates with enhanced activity either within the cerebellum, or with other brain areas anatomical connected to the cerebellum. Interestingly, Rocca et al. [53] reported that structural alterations (i.e., atrophy) and accumulation of focal lesions in the cerebellum correlated with increased activation of prefrontal areas (inferior and superior frontal gyrus) in RRMS patients with better cognitive performances. In fact, despite the presence of neural damage, measured by T2 lesion load, an extensive over-activation in different brain areas (frontal, parietal and temporal) was observed in patients with MS whose performance was similar to healthy controls. On the contrary, PwMS with worse performance showed less activation [54]. In summary, the above findings suggest that a compensatory mechanism may be activated in MS, employing additional high-level cortical areas interconnected with the cerebellum, in order to cope with the cognitive demands of a task. Other fMRI studies have shown decreased functional connectivity and lower intensity of spontaneous fluctuations among different brain areas in PwMS, which in conjunction with the absence of differences between PwMS and healthy controls on cognitive tests, could indicate subclinical disability undetected by neuropsychological assessments [50, 55].
In the present study, inverse associations between cortical thinning of the whole cerebellum, lobule I–II, lobule III, lobule VI, Crus I and lobule VIIIA and information processing speed and verbal fluency (phonemic fluency) tasks (mainly timed tests, as well as those assessing visuospatial abilities, such as judgement of line orientation) were found. In fact, we found more and stronger correlations between cerebellar cortical thickness and cognitive performance than between white and grey matter volumes and cognitive performance. Longitudinal and cross-sectional studies have consistently reported widespread cortical thickness reduction in PwMS [56, 57]. Cerebellar together with thalamic cortical atrophy has been detected in MS and patients with the clinically isolated syndrome compared to healthy controls revealing that regional cortical thinning is an early feature in MS and progresses along with the disease progression [58]. Specifically, cerebellar lobules VI, Crus I and VIIIa atrophy were independent predictors of 9-HPT, SDMT, BVMT and CVLT performances in PPMS patients confirming the contribution of these lobules to cognitive functions [25]. On the other hand, Calabreze et al. [27], found a different pattern of cortical thinning in cognitively unimpaired and impaired PwMS. The former presented with significant cortical thinning at frontotemporal areas, whereas the latter a more widespread cortical thinning, involving the whole brain. Perhaps, cognitive impairment in MS requires a diffuse generalized cortical thinning.
Several limitations of this study are noted. First, due to the small and uneven (as it pertains to MS subtypes), sample size, we did not investigate cerebellar volumetric differences among MS subtypes. It is, therefore, possible that distinct subtypes may present different patterns of pathology and associations with cognition. For example, we would expect greater cerebellar volume reduction in SPMS and PPMS patients in accordance with the theory that posits that atrophy is related to progressive disability [3, 27, 59]. Second, the majority of the patients included were males, contrary with the well-known female dominance in MS epidemiology. However, we did not observe any sex differences on either cerebellar volumes or test performances. Third, the present study is cross-sectional. Longitudinal investigations may shed light into how the accumulation of cerebellar pathology relates to clinical manifestations in MS. A further potential limitation may be resulting from the automated brain segmentation methods per se, as these methods require optimal imaging planes for accurate segmentation. Nevertheless, when compared to other cerebellar volumetric tools, CERES was ranked in the first two out of eight segmentation methods in accuracy and fidelity [41]. In addition, tiny cerebellar volumes, considered in the present analysis (but also previous studies), such as lobule I–II, may not produce as reliable measures as bigger volumes, i.e., lobule VI. A final methodological limitation is the lack of a control comparison group. However, we did rely on the normative data included in the CERES volbrain dataset library to estimate abnormal cerebellar volumes in our clinical sample.
This is a preliminary and exploratory in nature study. Future work with larger clinical samples should examine the replicability of the current findings and possibly investigate the rate of cerebellar regional volume loss and its association with the rate of clinical and cognitive progression in PwMS using a longitudinal research design.
In conclusion, we observed cerebellar cortical, grey and white matter atrophy in PwMS and inverse associations with cognitive functions, mainly information processing speed. In our study, the “bigger is better” hypothesis, as it pertains to the associations of cerebellum with information processing speed in MS, was not confirmed. It should be noted, however, that the cause of cognitive impairment in MS is multifactorial and cannot be interpreted solely by the pathological findings captured by conventional MRI in the cerebellum, in particular. The complex relationship between a structure’s volume and its functional efficiency warrants further investigation.
MS, Multiple Sclerosis; BHA, Brain Health Assessment; CGMV, cerebellar grey matter volume; CWMV, cerebellar white matter volume; CNS, central nervous system; PwMS, persons with Multiple Sclerosis; CCAS, cerebellar cognitive-affective syndrome; SDMT, symbol digit modalities test; MRI, magnetic resonance imaging; EDSS, Expanded Disability Status Scale; BICAMS, Brief International Cognitive Assessment for MS; CVLT-2, California Verbal Learning Test-2; GVLT, Greek Verbal Learning Test; BVMT-R, Brief Visuospatial Memory Test-Revised; CRI-q, Cognitive Reserve Index Questionnaire; MFIS, Modified Fatigue Impact Scale; DASS-21, Depression, Anxiety, Stress Scale; BDI, Beck Depression Inventory; SPSS, Statistical Package for Social Sciences; RRMS, relapsing-remitting Multiple Sclerosis; SPMS, secondary progressive Multiple Sclerosis; PPMS, primary progressive Multiple Sclerosis.
PIliad conceived and designed the experiments; PIliad and CB performed the experiments and collected the data; PIliad and EA designed the methodology; EA reviewed and validated the methodology; SS analyzed the MRI data; SZ and KP contributed materials; PIliad wrote the original draft; EA, KP, PIoan, NG, CB, and SZ reviewed and edited the draft. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All the reported experiments on humans were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from participants prior to the commencement of study procedures.
We would like to thank all the MS patients who accepted willingly to participate in this study.
This research received no external funding.
The authors declare no conflict of interest.
