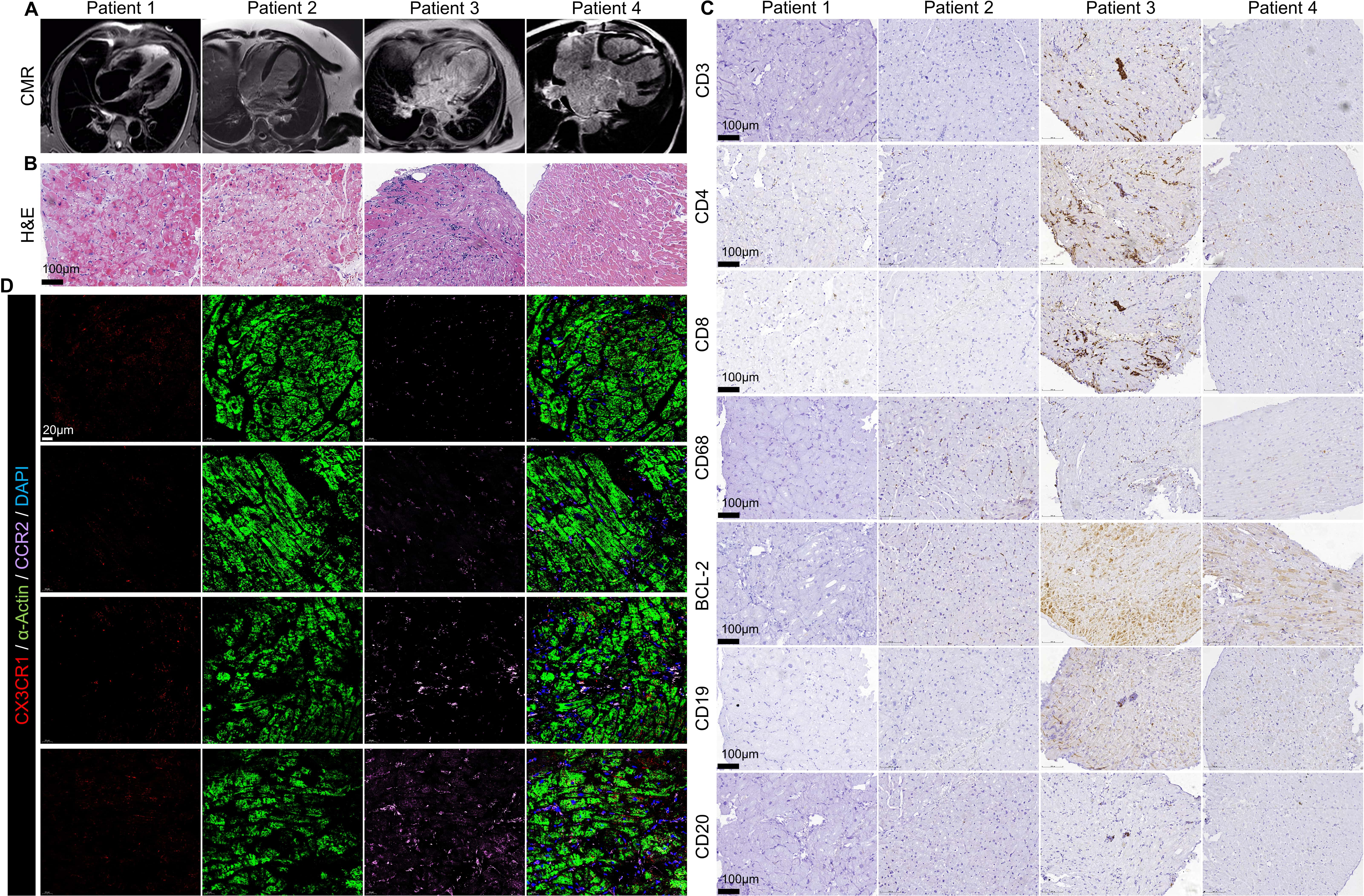
Background: Macrophages expressing CC chemokine receptor 2 (CCR2)
possess characteristics and performance akin to M1 polarized macrophages, which
promote inflammation. Advanced heart failure (HF) patients with higher abundance
of CCR2 macrophages are more likely to experience adverse remodeling. The
precise mechanism of CCR2 macrophages in how they affect the progression of
dilated cardiomyopathy remains unknown. Methods: Cardiac biopsy samples
from dilated cardiomyopathy patients (DCM) were used for immunohistochemistry and
immunofluorescence staining. PCR is employed to identify the
IL-1, IL-6, TNF-, TGF-, MMP2, MMP9, PKM1, PKM1,
GLUT1, GLUT2, GLUT3, GLUT4, PDK1, PFKFB3, PFK1 and HK2 mRNA expression of
CCR2 monocytes/macrophages from the peripheral blood of DCM patients.
Seahorse was used to evaluate the oxygen consumption rate (OCR) and extracellular
acidification rate (ECAR) of CCR2 monocytes/macrophages. 2-DG was used to
simulate a lack of glucose. Lentivirus containing GLUT1 inhibitory sequence was
used to knockdown GLUT1 gene expression of CCR2 monocytes/macrophages.
Western Blot and immunofluorescence staining was used to evaluate the expression
of NLRP3. Results: Immunostaining results of cardiac biopsy tissue from
dilated cardiomyopathy (DCM) patients demonstrated that the progression to HF was
associated with an increase in the number of CCR2 macrophages. PCR results
demonstrated that CCR2 monocytes and macrophages derived from the blood of DCM
patients expressed elevated levels of inflammatory factors and up regulation of
glycolysis related genes. In addition, OCR and glucose uptake experiments
confirmed that increased glucose uptake of these cells was associated with
greater inflammation and correlated with a worsening of cardiac function.
limiting the glucose supply to CCR2 monocytes and macrophages, or
suppressing the activity of glucose transporter 1 (GLUT1) could reduce
inflammation levels. Conclusions: These results suggest that CCR2
monocytes and macrophages rely on metabolic reprogramming to trigger inflammatory
response and contribute to myocardial injury and the progression of DCM.