Aim: To analyze second trimester risk factors to predict small for gestational age and intrauterine growth restriction (IUGR) fetuses. Materials and Methods: The authors retrospectively analyzed clinical files of 5,390 women, who delivered between 2007 and 2009, 4,071 of which were included in the study. Adequate for gestational age (AGA), small for gestational age (SGA), and IUGR fetuses were included. Results: The authors found IUGR to be delivered significantly earlier than SGA and AGA. Moreover, they found a higher prevalence of nulliparity in SGA and IUGR fetuses than in AGA, and a significant higher prevalence of bilateral notch in SGA than AGA. SGA fetuses at 20 gestational weeks present a significantly higher value of umbilical artery pulsatility index (PI) and mean uterine arteries resistance index (RI) than AGA. In multivariate logistic regression analysis, the second trimester factors to predict SGA at delivery were: mother age, nulliparity, academic title, umbilical artery PI at 20 gestational weeks, mean RI of uterine arteries, and bilateral notch. In case of IUGR the most predictive factors were: mother age, nulliparity, and bilateral notch at 20 gestational weeks. Conclusions: Clinical interview and sonographic examination at 20 gestational weeks were capable to predict fetal growth potential.
Suboptimal intrauterine growth occurs in approximately 5-10% of pregnancies and it is often referred to intrauterine growth restriction (IUGR) or small for gestational age (SGA) in an inconsistent and confusing manner. This inconsistency in terminology leads to uncertainties regarding management, surveillance of these pregnancies, and time of the delivery [1, 2]. IUGR is a pathological condition in which fetuses cannot achieve full in utero growth potential. A possible cause of IUGR, when not attributable to structural or genetic defects of the fetus, is placental insufficiency and placental senescence [3, 4]. Low birth weight is one of the most important determinants of neonatal morbidity and mortality, and many studies suggest that is associated with metabolic syndrome development in adult life [5, 6]. Also among the SGA with a constant fetal growth trajectory during gestation, there could be a suboptimal intrauterine growth with an increased incidence of negative outcomes [2, 7]. However, SGA generally are associated with only a slight increase in perinatal mortality and morbidity in comparison to normally grown fetuses, while IUGR fetuses have an elevated risk of perinatal mortality and morbidity. In both cases negative outcome could be reduced if identified prenatally, through close monitoring, timely delivery, and prompt neonatal care [2, 8].
The traditional approach to identify pregnancies at high risk of delivering SGA neonates is maternal abdominal palpation or measurement of the symphysis-fundus height, but the performance of such screening is poor, with a detection rate of less than 30%. Ultrasound exams have increased the capability to identify SGA or IUGR fetuses through biometry and maternal Doppler [9], however still a large proportion of SGA are unknown before birth [2, 10].
It would be useful to improve the SGA or IUGR development prediction beginning from second trimester to increase pregnancies’ surveillance in a limited population at high risk of impaired fetal growth. The aim of the present study was to evaluate the second trimester risk factors, including fetal Doppler, to predict SGA and IUGR fetuses.
In this observational retrospective clinical chart review study the period between January 1st, 2007 and December 31st, 2009 was considered. The authors included in the study all pregnancies that presented a second trimester anomaly scan and that after gave birth in the present center. Indeed, they included all adequate for gestational age (AGA), SGA, and IUGR fetuses. They excluded all pregnancy-related hypertensive disorders, chronic hypertension, fetal malformations known at birth, twin pregnancies, large for gestational age (LGA) fetuses, and all SGA without a known sonography before delivery to confirm IUGR. Furthermore, clinical information (up to maternal discharge from the hospital) were gathered from the clinical database of Obstetrics and Gynecology Clinic. This study was approved by the internal review board and it was in accordance with Helsinki Declaration, and followed the dictates of the general authorization to process personal data for scientific research by the Italian Data Protection Authority. Pregnancy and maternal information considered were the following: maternal age, parity, macro-region of origin, mode of conception, gestational age at delivery, pregnant admissions in hospital over childbirth, mode of labor, and mode of delivery. Neonatal data taken into account were the following: neonatal weight, sex, placental weight, Apgar score at the first and fifth minutes, IUGR, SGA, neonatal cardio-pulmonary resuscitation (CPR), admission to neonatal intensive care unit (NICU), and intrauterine death. The authors defined the macro-regions of origin as previously stated (first Italy and Western Europe that encompasses European Union before 2004, Switzerland, Norway, and Iceland, second Eastern Europe, third Asia that included Nepal, Bangladesh, Bhutan, North, Central, East and Southeast Asia, fourth Arabian countries that included North Africa, Southwest and South Asia (excluding Nepal, Bangladesh, and Bhutan), fifth Sub-Saharan Africa, and sixth other countries) [11].
 Figure 1.
Figure 1.— Receiver operating characteristic (ROC) curve of the multivariate prediction model for SGA; AUC is 64% (CI.95 60-68%).
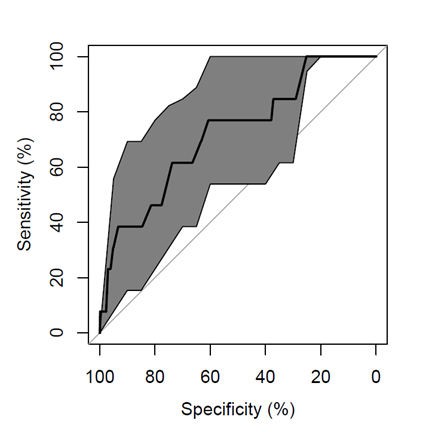 Figure 2.
Figure 2.— Receiver operating characteristic (ROC) curve of the multivariate prediction model for IUGR; AUC is 72% (CI 95 58-87%).
The gestational age at delivery was taken in consideration as a continuous value. The authors considered pregnancy-related hypertensive disorders: pre-eclampsia (a systemic diastolic blood pressure greater than or equal to 90 mmHg or a systolic blood pressure greater than or equal to 140 mmHg, in association with a proteinuria (loss of proteins in the urine greater than or equal to 0.3 grams of protein in the collection of 24 hours), gestational hypertension (hypertension in the absence of proteinuria), and pre-ecplampsia arising in a patient with preexistent chronic hypertension [12, 13]. Mode of labor was considered as follows: medically induced, spontaneous, or augmented by oxytocin. Placental index was considered as previously stated (the ratio between placental and the fetal weight) [11]. The SGA neonates were considered as previously stated all infants with a weight below the 10th centile [11]. LGA were defined as a weight above the 90th centile, and AGA were considered between the 10th and the 90th centiles [11].
IUGR was defined as a sonographically estimated fetal weight below the 10th centile in relation to gestational age and a umbilical artery pulsatility index (PI) greater than two standard deviations from the mean [14]. In addition neonatal weight multiple of the median (MoM) was calculated as previously stated: neonatal weight/50th centile of neonatal weight at the same gestational age adjusted per neonatal sex [15]. Intrauterine fetal demise was defined as the intrauterine cessation of heartbeat after the 22nd gestational week [16].
Statistical analysis was performed using the program R (version 3.2.3) and was considered as significant a p-value < 0.05. The distribution normality was assessed by Kolmogorov-Smirnoff test. Data were presented by mean (± standard deviation) or median and interquartile range (IQR). A specified reference value and 95% confidence interval (CI) were also used [e.g., odds ratio and 95% CI or area under the curve (AUC) and 95% CI].
The missing values were considered as NA. During the analysis the authors used the following statistical tests: in case of categorical variables chi-square or Fisher’s exact test; in the case of continuous variables t-test, one way ANOVA, Wilcoxon test or Kruskal-Wallis test. The authors performed also multivariate logistic regression analyses. In multivariate logistic regression model, the development of IUGR or SGA were considered as the dependent variables, whereas clinical information and feto-maternal Doppler data at 20 weeks of gestation were considered as as independent variables. Moreover, in the initial multivariate model, all factors (that had a p-value < 0.300 in univariate analysis) and their interactions were considered in a single analysis, except when the interaction term was non-significant (in which case they analyzed the no-interaction model). The final multivariate models were selected by a stepwise analysis. Finally, the model performance was assessed by the area under the receiver operating characteristic (ROC) curve with the relative 95% CI.
| AGA (3913) | IUGR (13) | SGA (145) | p | |
|---|---|---|---|---|
| Maternal age at delivery (years) | 31.92 (±5.48) | 33.77 (±6.04) | 31.32 (±6.22) | NS |
| Nulliparity | 53.00% (2074/3913) | 84.62% (11/13) | 68.28% (99/145) | 1, 2 |
| Sub-Saharan-African origin | 4.02% (157/3902) | 0.00% (0/13) | 10.34% (15/145) | 2 |
| Neonatal weight (grams) | 3259.30 (±514.02) | 1284.69 (±670.07) | 2546.76 (±427.32) | 1, 2, 3 |
| Neonatal weight MoMs | 1.01 (±0.08) | 0.73 (±0.14) | 0.78 (±0.08) | 1, 2 |
| Placental weight (grams) | 584.30 (±111.68) | 276.92 (±118.11) | 479.70 (±96.69) | 1, 2, 3 |
| Placental index | 0.18 (±0.04) | 0.22 (±0.04) | 0.19 (±0.05) | 1, 2, 3 |
| Apgar score 1st minute | 8.10 (±1.27) | 5.85 (±1.68) | 7.87 (±1.41) | 1, 3 |
| Apgar score 5th minute | 8.87 (±0.81) | 7.46 (±0.97) | 8.79 (±1.05) | 1, 3 |
| Gestational age at delivery (weeks) | 38.67 (±2.38) | 31.54 (±3.78) | 38.57 (±1.73) | 1, 3 |
| PI umbilical artery (20 weeks) | 1.19 (±0.19) | 1.38 (±0.42) | 1.26 (±0.21) | 2 |
| Mean RI uterine arteries (20 weeks) | 0.55 (±0.09) | 0.69 (±0.28) | 0.59 (±0.11) | 2 |
| Bilateral notch (20 weeks) | 2.25% (88/3913) | 7.69% (1/13) | 7.59% (11/145) | 2 |
The values reported in this Table refers to mean ±standard deviation or percentage and absolute values and the p-values refer to t-test, Chi-square test, or Fisher exact test. Significant differences between: (1) AGA vs. IUGR, (2) AGA vs. SGA, and (3) IUGR vs. SGA. NS = non-significant.
The study reviewed clinical charts of 5,390 pregnancies, 4,071 pregnancies met the inclusion and did not meet the exclusion criteria. The authors included in the study 3,913 AGA, 145 SGA, and 13 IUGR. They excluded 1, 319 women because of pregnancy-related hypertensive disorders, LGA, SGA with unknown Doppler data before delivery to confirm IUGR, twin pregnancies, fetal malformations, or chronic hypertension. They found IUGR to be delivered significantly earlier than SGA and AGA (Table 1). Moreover, they found a higher prevalence of nulliparity in SGA and IUGR fetuses than AGA ones (84.62% in IUGR and 68.28% in SGA), and a significant higher prevalence of bilateral notch in SGA than AGA. IUGR presented a significantly higher placental index than SGA and AGA. SGA fetuses at 20 gestational weeks presented a significant higher value of umbilical artery PI (1.26 vs. 1.19) and mean uterine arteries resistance index (RI) than AGA (0.59 vs. 0.55). Finally, IUGR fetuses presented a significantly higher placental index than SGA and AGA (0.22 versus 0.18-0.19) (Table 1). Multivariate regression analysis demonstrated that maternal age, nulliparity, academic title, PI of umbilical artery at 20 gestational weeks, mean RI of uterine arteries at 20 gestational weeks, and bilateral notch were the second trimester factors to predict SGA at delivery (Table 2 and Figure 1). In particular nulliparity, PI umbilical artery at 20th gestational week and mean RI uterine arteries (20 weeks) were significantly associated with the risk of delivering a SGA neonate. There was a correlation between bilateral notch (20 weeks) of uterine arteries and the risk of delivering a SGA neonate even if this was not statistically significant (p = 0.054). Nulliparity was a risk factor also for delivering a IUGR neonate (p < 0.05) (Table 3 and Figure 2). Othersmost predictive factors to forecast at 20 gestational weeks the development of IUGR neonates were maternal age and bilateral notch of uterine arteries that had a 0.072 and 0.219 p-value, respectively (Table 3 and Figure 2).
| OR (IC95%) | p | |
|---|---|---|
| Maternal age at delivery | 1.01 (0.99-1.04) | 0.328 |
| Nulliparity | 1.97 (1.44-2.69) | <0.05 |
| University degree | 0.74 (0.54-1.01) | 0.062 |
| PI umbilical artery (20 weeks) | 2.81 (1.33-5.94) | <0.05 |
| Mean RI uterine arteries (20 weeks) | 10.58 (2.29-48.91) | <0.05 |
| Bilateral notch (20 weeks) | 1.72 (0.99-2.99) | 0.054 |
| OR (IC95%) | p | |
|---|---|---|
| Maternal age at delivery | 1.1 (0.99-1.22) | 0.072 |
| Nulliparity | 5.88 (1.28-26.93) | <0.05 |
| Bilateral notch (20 weeks) | 3.66 (0.46-28.91) | 0.219 |
To discover elements in pregnancy that can help to predict a pathological fetal outcome is an hot topic. Ultrasound monitoring is an important source that may reveal signals of suspicion in pregnancy, especially when it is enriched with medical history and information about pregnancy.
This study was conducted with the aim to correlate the medical history of patients with ultrasound measurements detectable in the second trimester of pregnancy, alone or in combination, in order to discover elements of suspicion that could help to predict SGA and IUGR fetuses, that may have severe problems in pregnancy and during labour.
The present results confirm that actually the second-trimester assessment did not detect term SGA with high accuracy according to the current available literature. However the present findings suggest that integrating maternal demographics, fetal biometry, and maternal and fetal Doppler assessment could be useful to discover early a group at risk in developing SGA fetuses. Previous studies investigated the role of the second-trimester parameters to estimate the risk for SGA neonates. Recently, Lesmes et al. [17], first described a combined screening by maternal characteristics and history with fetal biometry at 19-24 weeks that could predict 76%, 55% , and 38% of SGA < 5th delivering at < 32, at 32-36, and > 37 weeks of gestation, respectively, at a 10% false-positive rate. However, Doppler parameters were not included in this study. Later, Lesmes et al. [9] integrated maternal factors, fetal biometry with maternal Doppler at 19-24 weeks of gestation, and this increased the accuracy of SGA detection rate than their previous study; indeed they predicted 90%, 68%, and 44% of SGA < 5th neonates delivering < 32, 32-36, and ≥ 37 weeks’ gestation, at a 10% false-positive rate. However the performance of combined screening for SGA in the second trimester was poorer than in the third.
In the present study despite the mean gestational age of SGA population, delivery was 38.57 weeks (±1.73); the authors’ model including fetal Doppler presented an AUC of 64% (CI 95 60-68%). Even Familiari et al. identified almost 90% of SGA fetuses by the use of fetal biometry, mid-gestational UtA Doppler, maternal demographic characteristics and scheduling further ultrasound assessment [18]. Interestingly, also the present results showed that integrating maternal demographic parameters with ultrasound findings in the second-trimester is useful to predict pregnancies at risk to develop SGA fetuses. In particular, with the present study it was found that the second trimester factors to predict SGA at delivery were: maternal age, nulliparity, academic title, PI of umbilical artery at 20 gestational weeks, mean RI of uterine arteries at 20 gestational weeks, and bilateral notch. Especially, the authors found a significant role of fetal Doppler, and not only maternal Doppler, to predict the risk of developing SGA at term already at 20 weeks of gestation. In case of IUGR, the most predictive factors were: maternal age, nulliparity, and bilateral notch at 20 gestational weeks.
The major limitation of this study is the retrospective design and the limited number of IUGR fetuses in the considered population. Instead the strengths of this study are an uniform management of all pregnancies by a team of obstetricians following the same obstetrical policies.
The present study provides evidence that clinical interview and sonographic examination at 20 gestational weeks were capable to predict fetal growth potential. In particular the authors found fetal Doppler in addition to maternal Doppler at 20 weeks gestation to be useful to predict SGA development at term of pregnancy. Therefore, the present data suggest to improve actual forecasting algorithms to add fetal Doppler in future studies with the aim to predict SGA development.
