Objective: To investigate the age specific reference intervals of serum anti-Müllerian hormone (AMH) concentration of healthy women in infertility center. Materials and Methods: After exclusion of 642 participator who did not meet the inclusion criteria, a total of 1,253 healthy women aged 17 to 49 years were enrolled in the study between January and August 2016. This study population was divided to five age groups, The samples of this study collected from each participants and aliquoted and stored at -80℃ refrigeratory until assayed, The authors used AMH enzyme-linked immunosorbent assay (ELISA) kit for the assessment of AMH levels. Results: The serum AMH levels varied inversely correlation with patient age. The AMH concentration can be roughly calculated by formula (AMH =-0.327*age+15.98). This downward trend was significant among these women whom 40 years age. The coefficient of variation was larger even in the same age groups, The 2.5-97.5th percentile of AMH level of each age group was 2.30-16.7 ng/mL, 0.96-16.4ng/mL, 0.44-15.1ng/ mL, 0.14-12.5ng/mL, and 0.08-6.56ng/mL, respectively. Conclusions: This study determined reference values of serum AMH in China women with regular menstruation. These values can be applied to clinical evaluation and treatment of infertile women. It suggested that AMH is an individual medical index that women should establish their own baseline date for longitudinal axis comparison.
Ovarian reserve tests include ultrasonographic markers and serologic markers, such as the quantity and quality of antral follicle count (AFC) and ovarian volume. Basal FSH, estrogen, and inhibin B levels have been commonly measured by serologic tests, but have several limitations that AFC and basel serum FSH were relatively low in predicting poor ovarian response and pregnancy failure, and that exhibit intense variability within the menstrual cycle or between cycles, which has limited their reliability as a marker of ovarian reserve [1].
In recent years, the serum level of anti-Müllerian hormone (AMH) has been considered a new marker of ovarian reserve. AMH is a member of the transforming growth factor-beta superfamily that previously thought to causes regression of the Müllerian duct of the male embryo during male fetal sex differentiation. Serum AMH levels significantly correlate with the ovarian primordial follicle number and reflect the ovarian follicular pool even after adjustment for chronological age [2, 3]. Moreover AMH levels have very low variability throughout different menstrual cycle as well as within one menstrual cycle, as compared to other biomarkers of ovarian activity, such as FSH, which has a number of obvious clinical advantages. AMH might therefore also be used a marker of ovarian reserve and ovarian failure for individualized fertility counseling [4, 5]. Meanwhile AMH is independently associated with natural menopause in women with either regular or irregular menstrual cycles. AMH appears most useful in identifying women at risk of menopause within three years of AMH [6]. In addition, AMH assessment might also be used to diagnosis criteria of polycystic ovarian syndrome (PCOS) and reflect the severity of the phenotype of patients with PCOS [7, 8]. AMH also represents a good predictor of ovarian response to ovarian hyperstimulation syndrome [9, 10]. However, it is now recognized that serum AMH results can have dramatic variability due to common, biologic fluctuations within some individuals [11], use of hormonal contraception [12] or chemotherapy for breast cancer [13], certain surgical procedures [14], specimen treatment [15], and assay changes [16], smoking status [17], socioeconomic status [18], impact of ethnicity [19], and no international calibration obtained for AMH. All aforementioned factors lead to significantly different reference intervals and diagnostic cut-offs for AMH, therefore each laboratory should eastablished their own reference interval and diagnostic cut-off, and to avoid clinicians to use the same criterion causing problems with results’ interpretation. However, there are no age-related reference values for AMH levels based upon a large China population. The aim of this present study was to established the reference intervals of serum AMH of women of reproductive age who presented to infertility center in china, so that clinicians correctly interpret AMH fluctuations in various physiological and pathological conditions.
A total of 1,895 apparent healthy women were recruited to this study. All individual patients’ serum AMH levels were measured between January 2016 and August 2016 in Department of Clinical Labartory of Hospital 105 of People′s Liberation Army. The study received ethics committee approval, and all included patients signed a written informed consent form. Inclusion criteria of this studies included: 1) aged between 17-49 years, 2) participator who had at least bear one child, 3) regular menstrual cycles of 20-45 days duration, 4) no evidence of endocrine disorders, metabolic disorders, autoimmune disease, and cancer, 5) a BMI ranging from 18-28 kg/m2, 6) not received hormone therapy for previous three months, and 7) no history of gynecological operation. After exclusion of 642 participator who not meet the inclusion criteria, 1,253 subjects were included in this retrospective study as the basis for reference survey. The mean age of the women was 32.9 (range17.0-49.0) years. This study population was divided to five age groups: 17-25 years, 26-30 years, 31-35 years, 36-40 years, and 41-49 years.
The samples of this study were obtained from infertility center of Hospital 105 of People′s Liberation Army. Three millilitres of whole blood was drawn from each participants and allowed to clot at room temperature for at least 30 minutes, then centrifuged at 1000 g for 10 minutes at room temperature within two hours of collection. The serum samples were aliquoted and stored at -80°C refrigeratory until assayed. No samples were repetitive free-zethawing.
The AMH concentration was measured using the commercially available AMH enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s specified protocol. The AMH was captured by a monoclonal antibody bound to the microtiter plate, then another monoclonal antibody with streptavidin-peroxidase bound to the solid phase form antibody-antigen complex. After incubation, the antibody-antigen complex was detected by addition of a chromogenic substrate. The serum AMH concentration was presented by intensity of the coloration compare with standard curves. The limit of detection of the assay was 0.43 pmol/L. The intra-assay and inter-assay coefficients of variation was < 5% and < 15%, respectively. There is no detectable cross-reactivity with closely related compounds in samples.
All statistical analysis was performed using statistical software version 18.0. Normality analysis of the date was performed using Skewness-Kurtosis test. The value of Skewness and Kurtosis less than 1.96 SD of the date distribution and was recognized as normal distribution. Used “stem-and leaf & box plots” methods were used to remove outline data. Categorical variables were expressed as percentages and continuous variables were expressed as means ± SD or as medians and ranges. The correlation between continuous variables age and AMH was assessed by Pearson analysis. If there was a correlation, the reference interval was established according to age. The non-parametric sorting method was used and two sides of 95% ranges as reference intervals.
As Figure 1 shows, the serum AMH levels varied inversely correlation with patient age (r = 0.437, p < 0.01). The AMH concentration can be roughly calculated by formula (AMH = -0.327*age+15.98). This downward trend was significant among these women whom 40 years age.
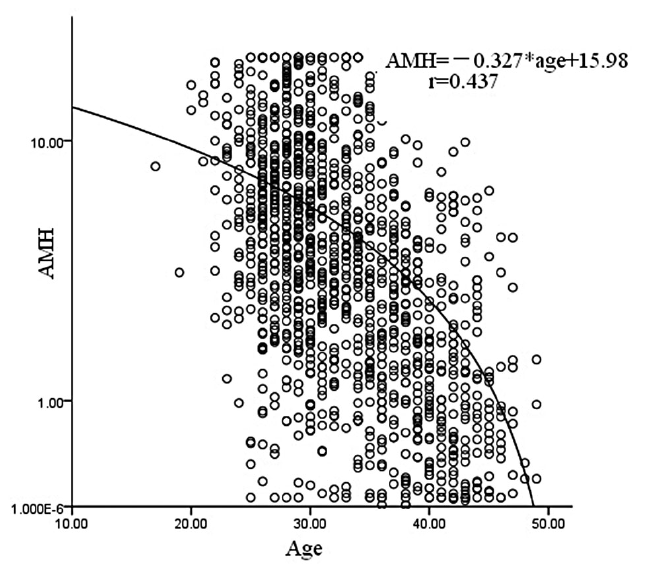 Figure 1.
Figure 1.— Age-specific AMH concentrations.
To present the age-specific AMH levels associated ovarian reserves, all about 1,253 participants were classified into 32 categories within representative single-year intervals. All age groups showed skewed distributions of AMH values not following the standard bell shaped curve found in the Gaussian distribution. The median AMH levels of each age group decreased steadily with increasing age, in contrast SD and CV of values becoming larger accompanied with an increasing age. As can be seen in Figure 2, some AMH levels outside 95% confidence interval that indicated the coefficient of variation was larger even in the same age groups. The same conclusion can be drawn from Table 1. From the aforementioned limited data, it can concluded than AMH is an individual medical index that women should establish their own baseline date for longitudinal axis comparison.
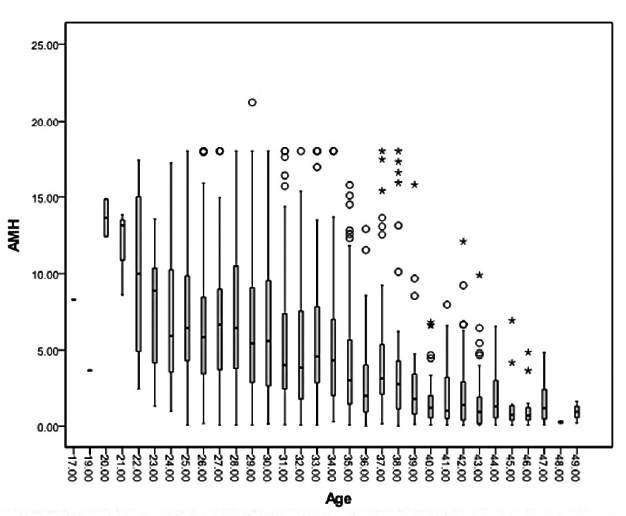 Figure 2.
Figure 2.— Serum AMH concentrations of the age group.

|
Values are median (lines), 2.5-97.5 the percentiles (upper lines and bottom lines) and variety marker indicate AMH levels outside 95% confidence interval.
In order to facilitate the AMH reference range application to clinical practice divided fertility women into five groups such as 17-25 groups, 26-60 groups, 31-35 groups, 36-40 groups, over 40 years groups. As can be seen in Table 2, there are statistically significant differences between each groups, the AMH levels steep down especially for 35-year-old women.

|
In women, AMH is exclusively produced by granulosa cells of ovarian follicles through reproductive life. The highest serum AMH concentrations are observed during puberty and decreases continuously until menopause. A large number of studies have described the correlation of serum AMH levels with age. Recently Seifer et al. study show that both median and mean of AMH levels decreased steadily in a manner highly correlated with advancing age beginning from 25 years. The median serum AMH value of average yearly decrease was 0.2 ng/ml/year through age 35 and then diminished to 0.1 ng/ml/year after age 35. The mean AMH values of decline rate was 0.2 ng/ml/year through age 40 and thereafter diminished to 0.1 ng/ml/year [20]. Yoo et al. have examined age-specific serum AMH values for 1,298 women who have regular menstrual cycles of reproductive age from 20- to 50-years-old within Korea. The study showed that serum AMH concentration was negative correlated with age, and continuous regression between AMH value and age (r2=0.183, p < 0.001). The calculation formula between AMH and age is AMH=12.6+-0.26*age. Approximately 75% of women showed a serum AMH level below 5.0 ng/mL [21]. The present study showed that the serum AMH levels varied inversely correlation with patient age (r = 0.437, p < 0.01). The AMH concentration can be roughly calculated by formula (AMH=-0.327*age+15.98). This downward trend is particularly obviously among these women older than 40 years of age. Mother’s age at natural menopause (ANM) usually provides an important information for daughters time to menopause (TTM) in the clinical setting. One study shows that AMH and mother’s age ANM both have added value in forecasting TTM for the daughter based on her age, suggests that a 47% improvement in predictive accuracy is offered by adding AMH to the model of age and mother’s ANM. In comparison, AMH is a more accurate added predictor of TTM than mother’s age of individual for the onset of menopause [22]. Given the increasing utility of AMH in clinical evaluations of ovarian reserve and fertility, trends in age-specific reference values for AMH may provide added perspective for clinicians and couples who are considering fertility treatment options.
The total variance includes intraindividual variability and interindividual variability. The present study supports that the high interindividual variability in AMH values, and the mean intraindividual coefficient of variation (CV) for AMH was revealed in 51.9%-136.6% for each age group. The results shows that can measured low AMH concentrations and exhibited variation greatly even in younger women groups. Aim of quantify intraindividual variability of AMH as analytical and biological coefficients of variation by a retrospective cohort study in Australia. The average total intraindividual AMH variability was 20% (range: 2.1% to 73%). Biological variation was 19% (range: 0 to 71%) and analytical variation was 6.9% (range: 4.5% to 16%). The majority roles of this result contributed by biological variation [11]. Intraclass correlation coefficient (ICC) which directly indicated the amount of variation between individuals. In a Netherlands large longitudinal study based on a total of 3,326 female participants from 1987 to 2007, the ICC of the mixed model for AMH decline with age was 0.87, indicating that 13% of the total variance could be accounted for by variability intraindividual [23]. Taken together, these results indicate that AMH is an individualized indicator, and each reproduction woman should realized baseline AMH value own oneself for future diagnostic, prognostic, and other clinical purposes.
Several studies have been performed to promulgate many factors that could affect the reproducibility of the test result included between laboratory differences, intra/interassay differences, samples’ stability in storage, and may be sometimes unknown factors. Moreover various several different reagents/kits and methods have been used by laboratories to measure AMH, which has led to inconsistencies in the literature and misuse of value ranges. There are reports on comparison of AMH values between the newer and older ELISA kits suggest that AMH concentrations in the newer kits are slightly higher than in the older ones [24]. Now the first commercial fully automated AMH assay systems have been development to measure AMH. The Access AMH assay showed good performance across the measuring range for both intra-assay (CV1.41-3.30 %) and interassay (CV 3.04-5.76 %) precision and acceptable sample stability. Fertile AMH reference range of the preceding ELISA kit is interchangeable with the new automated Access AMH assay [25]. Recently, the first fully automated AMH ELISA was developed and the median AMH values measured in healthy women was 4.0 ng/ml, 3.31 ng/ml, 2.81 ng/ml, 2.0 ng/ml, 0.882 ng/ml, 0.194 ng/ml, respectably. The fully automated AMH assay showed excellent precision, linearity, and functional sensitivity. The coefficient of variation was 1.8% for repeatability and 4.4% for intermediate precision [26]. Most recently, there has been another introduction of different reagent AMH ELISA kit which has begun replacing the prior systems in China and has different performance characteristics. The median AMH concentration measured by AMH assay are higher compared to the present research reagents. Caution is therefore advised when extrapolating ranges from one laboratory to another. The present research shows age-specific serum AMH reference values in Chinese women with regular menstruation (serum AMH levels correlated negatively with age). The median AMH level of each age group was 7.54 ng/mL, 5.93 ng/mL, 3.16 ng/mL, 2.10 ng/mL, and 0.94 ng/mL, respectively. This between-laboratory variability suggests clinicians should use the same laboratory to avoid problems with results’ interpretation.
In the present study, the authors established age-specific reference values for circulating AMH levels in the female population. AMH measurement continues to play an increasingly important role in the evaluation of a woman’s follicular supply and fertility treatment options. This study provides an analysis of the trends in AMH reference values determined uniformly at one laboratory based on a large number of unselected women being evaluated in China. It is important to note that in this study, without clinical outcome analysis, future validation with longitudinal data is still needed. The provided reference values for AMH cannot be used in isolation to provide counseling about a woman’s chance for a successful ovulation induction or ability to have a child. However, this information may serve as one component among others that improves the evaluation of a woman’s reproductive potential and treatment options that she may consider to conceive.
