- Academic Editor
Transient receptor potential (TRP) channels, particularly those involved in nociception (nociceptive TRP channels), are implicated in both pain and cancer development. Activation of these channels by diverse stimuli triggers calcium influx, leading to mitochondrial oxidative stress and reactive oxygen species (ROS) accumulation. This ROS production contributes to both nociceptive signaling (causing pain) and aging processes, including genomic instability, a key driver of carcinogenesis. Although a direct causal link between pain and cancer onset remains elusive, the shared involvement of nociceptive TRP channels strongly suggests a correlation. This opinion article proposes targeting the crosstalk between nociceptive TRP channels and ROS as a promising therapeutic strategy to mitigate cancer and cancer-associated pain simultaneously. While further research is needed to definitively establish a causal relationship between pain and cancer risk, the available evidence suggests that inhibiting this pathway may offer significant benefits for both cancer prevention and treatment.
Transient receptor potential (TRP) channels are central to both carcinogenesis and advanced tumor progression [1]. Targeting TRP channel-mediated Ca2+ signaling offers a novel therapeutic approach for cancer [2]. Nociceptive signaling involves several TRP channels, including TRPA1, TRPC1/C3/C5/C6/C7, TRPM2/M3/M8, and TRPV1/V2/V3/V4, which contribute to pain perception. Interestingly, these TRP channels are implicated in carcinogenesis, where genomic instability—a crucial driver of cancer development—is linked to Ca2+ signaling-regulated aging process [3]. While nociception may play a role in cancer development, the correlation between pain and cancer progression remains largely unexplored.
PubMed searches on “pain” and “cancer” show that “cancer pain” is the most significant problem to address in cancer patients. This pain arises from the complex interplay of cellular, tissue, and systemic changes associated with tumor growth, invasion, and metastasis. Cancer cells and infiltrating immune cells release numerous mediators that modulate primary afferent nociceptive signaling, contributing to the multifaceted nature of pain perception [4]. As shown in Fig. 1, upregulation and activation of nociceptive TRP channels in cancer promote tumor development and result in pain by inducing the release of excitatory amino acids (EAAs) and substance P (SP) [5]. Excessive Ca2+ signals cause mitochondrial Ca2+ overload, leading to reactive oxygen species (ROS) accumulation and activation of the Ca2+/calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII) signaling pathway [6]. This cascade promotes nociceptive signaling and contributes to aging by activating the DNA damage response (DDR), senescence-associated inflammatory response (SIR), and senescence-associated secretory phenotype (SASP) [7, 8]. These processes can drive cancer development through mechanisms like genomic instability, telomere shortening, epigenetic alterations, and metabolic dysregulation [9]. The DDR is triggered by various DNA structural changes, including single-strand breaks (SSBs) and double-strand breaks (DSBs). These changes compromise genome stability and contribute to epigenetic alterations [10, 11]. Telomere shortening contributes to persistent DDR during replicative senescence [12]. SASP, a characteristic of senescent cells, drives aging through the secretion of proinflammatory cytokines, chemokines, growth factors, and proteases, leading to chronic inflammation and tissue damage [10]. Epigenetic mechanisms also promote the expression of nociceptive TRP channels in cancer cells, thereby accelerating cancer malignancy [3].
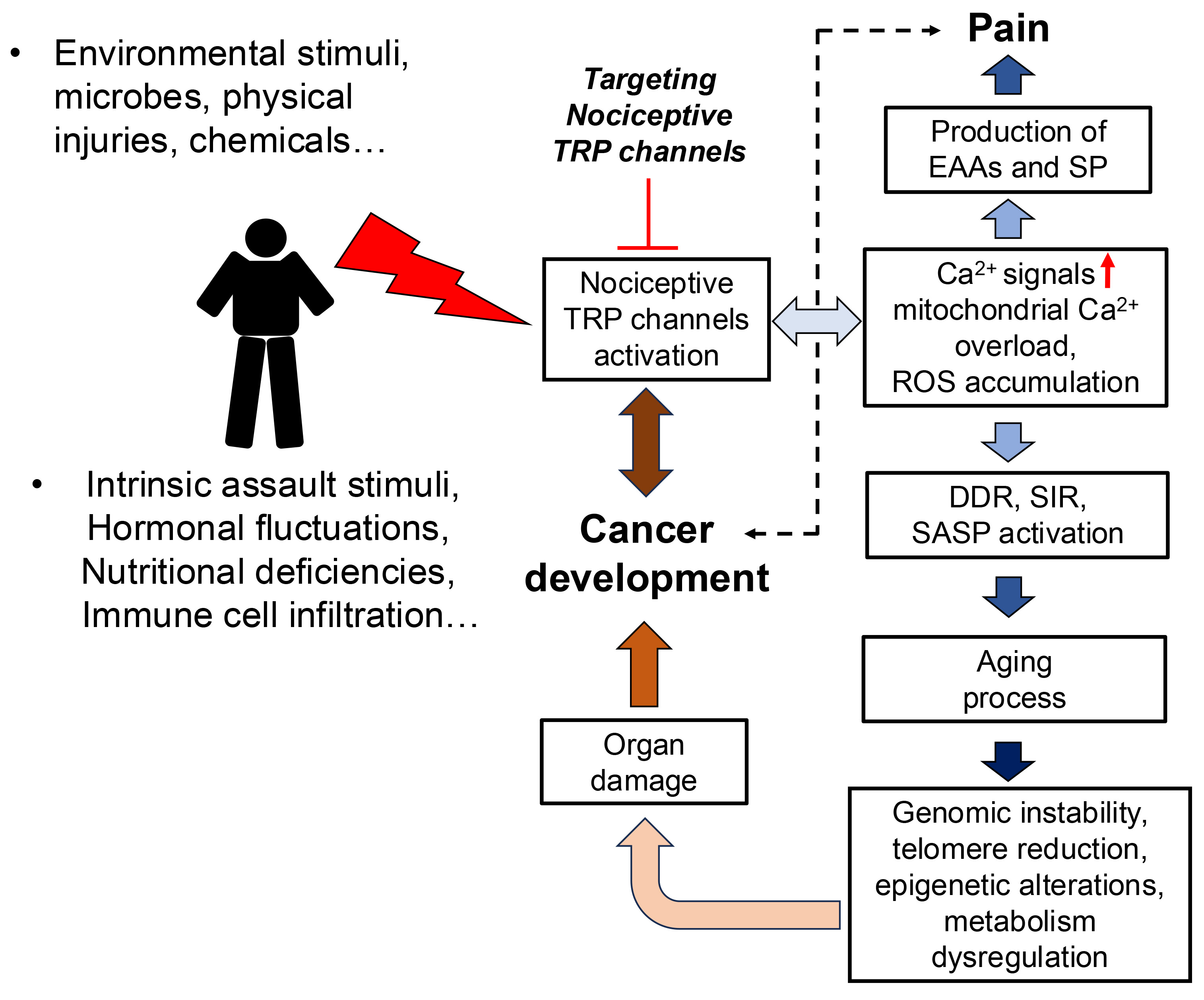 Fig. 1.
Fig. 1.
The complex interplay between nociceptive transient receptor potential (TRP) channels and reactive oxygen species (ROS) significantly contributes to both pain and cancer development. Targeting this crosstalk may present a promising therapeutic strategy for the simultaneous mitigation of cancer and cancer pain. EAAs, excitatory amino acids; SP, substance P; DDR, DNA damage response; SIR, senescence-associated inflammatory response; SASP, senescence-associated secretory phenotype.
Nociceptive TRP channels respond to various stimuli, including environmental factors such as microbes, chemicals, and physical injury, as well as intrinsic factors like hormonal fluctuations, nutritional deficiencies, and immune cell infiltration [3] (Fig. 1). Activation of these channels leads to increased Ca2+ influx, causing mitochondrial oxidative stress and activating Ca2+/CaMKII signaling pathway. This initiates both aging processes and pain signaling, which is transmitted from nerve terminals to the spinal cord’s dorsal horn via the dorsal root ganglia (DRG) [13, 14]. Thus, chronic pain prevalence increases significantly with age, becoming much higher in older adults [15]. While no clear evidence establishes a correlation between pain and cancer onset, aging increases the risk of both.
While evidence is still inconclusive, targeting the crosstalk between nociceptive TRP channels and ROS may offer a novel approach to mitigating aging and its associated pain and cancer. Previous study suggests a link: TRPV1 blockade extends lifespan in animal models, potentially through neuropeptide signaling [16], and TRPC7 deficiency inhibits age-related tumor formation [7]. These findings support a correlation between nociceptive pain, aging, and cancer development. Therefore, manipulating nociceptive TRP channels-ROS crosstalk could potentially increase lifespan by inhibiting age-related cancer development.
Nociceptive TRP channels are implicated in regulating cancer malignancy [3]. Activated nociceptive TRP channels generate Ca2+ signals and moderate levels of ROS, which can enhance cancer metabolism and growth signaling while inhibiting antioxidants, ultimately activating mitogenic signaling molecules [17, 18, 19]. Targeting the crosstalk between nociceptive TRP channels and ROS may therefore alleviate both cancer malignancy and cancer pain (Fig. 1). Several inhibitors of nociceptive TRP channels demonstrate both anticancer and analgesic effects in animal models. For example, the TRPV1 antagonists capsazepine and N-(4-tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl) tetrahydropyrazine-1(2H)-carbox-amide (BCTC) exhibit analgesic and anti-inflammatory effects in neuropathic pain models [20] and also suppress cancer malignancy [21, 22]. Capsazepine has demonstrated the ability to inhibit Janus kinase/Signal transducer and activator of transcription 3 (JAK/STAT3) signaling, leading to reduced tumor growth and cell survival in prostate cancer in preclinical models [23]. BCTC, also an inhibitor of the nociceptive TRPM8 channel, shows potent anti-tumor activity in prostate cancer through inhibited cell proliferation, migration and invasion. This effect is achieved through the modulation of Mitogen-activated protein kinase (MAPK) signaling pathways [24].
Despite advancements in targeting the interplay between nociceptive TRP channels and ROS, several challenges persist. Although multiple TRP channels are implicated in nociceptive signaling, the specific links between individual channels and particular cancer types remain unclear. Effective treatment of both pain and cancer necessitates identifying cancers that overexpress specific nociceptive TRP channels. Our previous studies have used clinical data to illustrate the expression patterns of these channels in various cancers; for example, TRPV1 and TRPM8 are overexpressed in breast cancer [3], suggesting that targeting these channels, such as with BCTC, might be beneficial in breast cancer and related pain management. However, cancer cells may exhibit mutant forms of nociceptive TRP channels due to epigenetic mechanisms. For instance, pancreatic ductal adenocarcinoma (PDAC) with a mutant TRPM2 subtype might present unique challenges in both malignancy and pain management. To efficiently manage both pain and cancer, a comprehensive approach is needed. This approach should move beyond a one-size-fits-all perspective and involve characterizing cancer features, particularly nociceptive TRP channels, using techniques like DNA sequencing, transcriptomics, proteomics, and metabolomics. This detailed characterization will be crucial for effectively targeting nociceptive TRP channels for both cancer and pain treatment.
The intricate crosstalk between nociceptive TRP channels and ROS plays a significant role in both pain and cancer development. Although the direct causal relationship between pain and cancer onset remains elusive, the shared involvement of nociceptive TRP channels in pain signaling, the aging process, and carcinogenesis suggests a potential link. Targeting the nociceptive TRP channels-ROS axis represents a potential therapeutic strategy for cancer pain management and may correlate with a reduced risk of cancer development and malignancy.
Conceptualization: TYC, TY, WLH; Funding: WLH; Original Draft: TYC, WLH; Review & Editing: WLH. All authors contributed to editorial changes, read and approved the final manuscript, participated sufficiently in the work, and agreed to be accountable for all aspects of the work.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This work was supported by the National Center for Geriatrics and Welfare Research at National Health Research Institutes (CG-112-GP-11 and CG-113-GP-11).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
