- Academic Editor
†These authors contributed equally.
The CD47 molecule (CD47) performs a novel role in regulating immunoreactions by binding to signal-regulatory protein alpha (SIRPα), resulting in the tumorigenesis of multiple malignant neoplasms. However, its effects and mechanisms in breast cancer (BC) remain unknown.
To explore the molecular mechanisms and explicit impacts of CD47, we screened two databases for CD47-associated signaling pathways and cellular functions. BC samples and patients’ basic information were collected to identify the statistical significance of CD47 expression. We also constructed experiments to validate the regulatory role of CD47 in BC cell proliferation.
Analysis of the TCGA-BRCA, GSE42568, and GSE15852 datasets demonstrated an elevated level of CD47 in BC tissues. A Venn diagram revealed 11,194 co-expressed genes, and pathway analysis linked elevated CD47 levels to critical signaling pathways, such as cytokine-receptor interactions and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling, which are integral to cell proliferation and invasiveness. Clinical data from 108 BC specimens showed that CD47 localization was primarily membranous, with higher levels correlating with proliferation marker Ki-67 (Ki-67) expression (p < 0.0001) and advanced tumor/node/metastasis (TNM) stage (p < 0.0001). Additionally, functional assays demonstrated that CD47 depletion reduced cell viability (p < 0.01), migration (p < 0.001), and invasion (p < 0.05 in 4T1 cells; p < 0.001 in MDA-MB-231 cells) in vitro and led to smaller tumor volumes (p < 0.0001) in vivo.
CD47 is a key regulator of BC cell proliferation and invasiveness and serves as a potential marker for assessing tumor aggressiveness and guiding therapeutic strategies.
Breast cancer (BC) continues to be one of the most common cancers impacting women around the world, with projections indicating approximately 2.3 million new diagnoses in 2024 [1]; therefore, it continues to represent a significant public health challenge. Recent data have highlighted demographic shifts in BC incidence, particularly an alarming increase among younger women and diverse populations, and disparities in access to healthcare exacerbate outcomes in underrepresented groups. Moreover, the mechanisms of metastasis and invasion in BC involve complex interactions between cancer cells and their microenvironment, contributing to the metastatic cascade [2, 3]. Notably, key genes such as BRCA1 DNA repair associated (BRCA1) and BRCA2 DNA repair associated (BRCA2) have been identified as crucial players in BC development, and mutations in these genes are associated with a significantly increased risk of developing the disease and with aggressive tumor behavior [4]. In response to these challenges, the treatment landscape has evolved to include targeted therapies, such as trastuzumab for Human epithelial receptor-2 (HER2)-positive tumors and immunotherapies that show promise in triple-negative BC [5]. Overall, the fight against BC continues to evolve rapidly, and future studies should concentrate on understanding the fundamental mechanisms of metastasis and enhance interventions that are customized for the various populations impacted.
CD47 molecule (CD47), a transmembrane protein primarily found on the surface of diverse cells, functions significantly in modulating immune responses. It does this by interacting with signal-regulatory protein alpha (SIRPα) present on macrophages, which consequently inhibits phagocytosis and enables cells to avoid immune clearance [6, 7, 8]. CD47 is often up-expressed in carcinomas and contributes to immune evasion and poor prognosis in various cancers, including leukemia, lymphoma, and solid tumors [9]. This overexpression facilitates tumor progression, metastasis, and resistance to therapies, prompting research into CD47 as a therapeutic target through strategies such as monoclonal antibodies that block its interaction with SIRPα [8, 10, 11]. Although some studies have indicated CD47 overexpression in specific BC subtypes, its precise involvement and clinical significance in this malignancy remain unclear, necessitating further investigation to elucidate its therapeutic potential and role in BC progression. Overall, the implications of CD47 in cancer biology are expanding; however, more research is required to determine its impact, specifically in the context of BC.
Given the unclear applications of CD47 gene expression in BC, this study conducted an in-depth investigation using several methodologies, including bioinformatics analysis through database retrieval, statistical analysis of clinical sample data, and immunohistochemical staining of tissue samples. By integrating these approaches, our goal was to clarify how CD47 is expressed in breast cancer and to evaluate its possible significance in relation to disease progression and patient outcomes. These comprehensive analyses support valuable insights into the role of CD47 in BC and contribute to inform future therapeutic strategies targeting this gene.
The Cancer Genome Atlas-Breast Cancer (TCGA-BRCA)-related data were downloaded from the Gene Expression Datasets Associated with BC using the Genomic Data Commons (GDC) data portal (https://portal.gdc.cancer.gov/) and the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/). The analysis of the differential expression of CD47 in breast cancer and adjacent non-cancerous tissues was conducted using Gene Expression Profiling Interactive Analysis (GEPHIA2; http://gepia2.cancer-pku.cn/).
In the TCGA-BRCA, GSE15852, and GSE42568 datasets, using the “DESeq2” software
package (dds
This research conducted a retrospective analysis of breast cancer cases stored in the Pathology Department at Shandong Third Hospital from 2020 to 2022. The analysis included 108 patients who had been diagnosed with invasive ductal carcinoma of the breast, all of whom did not receive preoperative chemotherapy, radiotherapy, or endocrine treatment. This study was conducted in accordance with the Declaration of Helsinki and was approved by the relevant ethics committee. The Ethics Committee and Institutional Review Board of the Third Hospital of Shandong Province approved the study, and the necessity for informed consent was signed (No. KYLL-2021066). An immunohistochemical staining assay was used to detect 108 paired BC tissue samples. Rabbit anti-human CD47 monoclonal antibody was purchased (ab300124, Cell Signaling Technology, Inc. Boston, USA; Dilution concentration: 1:200). Staining results were analyzed using ImageJ software (Version 1.8.0.345; National Institutes of Health; NY, USA) [15] and interpreted according to the semi-quantitative method utilized to assess the expression of CD47 in BC and paraneoplastic samples.
Human BC cells (MDA-MB-231 cell line; ZQ0118) and mouse BC 4T-1 cells (ZQ0201; Shanghai Zhongqiao Xinzhou Biological Company, Shanghai, China) were inoculated in Dulbecco’s Modified Eagle Medium (Gibcp 11885084, Suzhou, Jiangsu, China) with 10% fetal bovine serum (Suolaibao technology company, Beijing, China) and 100 IU/mL penicillin/streptomycin at 37 °C with 5% CO2. All cell lines were validated by STR profiling and tested negative for Mycoplasma.
Cells were placed in six-well plates at a density of 2
Total RNA was extracted from cultured cells by Easy Pure RNA Isolation Kit (R0018S; Beyotime Biotechnology, Shanghai, China) and a reverse-transcription (RT) Kit (D7153; BeyoRT M-MuLV, Beyotime Biotechnlogy Company, Shanghai, China). The cDNA was subjected to qRT-PCR with gene-specific primers in the presence of qPCR Master Mix (BeyoRT II cDNA, Beyotime Biotechnlogy Company, Shanghai, China). Relative mRNA expression levels were calculated using the 2-ΔΔCt method, where ΔCt represents the difference in threshold cycle (Ct) values between the target gene and a reference gene (e.g., GAPDH (Supplementary Table 3). All samples were analyzed in triplicate.
The total protein was extracted from the cells using the Radio
Immunoprecipitation Assay (RIPA) extraction reagent (Beyotime Biotechnology
Company, Shanghai, China), and the protein concentration was determined using a
bicinchoninic acid protein assay (Beyotime Biotechnology Company, Shanghai,
China). Subsequently, the samples were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The primary antibody
(anti-CD47:1:2000, 20305–1-AP; anti-
Experimental cells were plated in a 96-well plate with a density of 5000 cells per well. The proliferation rates of 4T-1 and MB-231 cells from both the short hairpin (sh)-CD47 and control groups were assessed. Cell viability measurements occurred every 12 hours. Daily, 10 µL of CCK-8 reagent (MCE, Shanghai, China) was added to each well of the 96-well plates and incubated at 37 °C for one hour. Optical density values were then determined using a microplate reader (Infinite® M1000PRO, TECAN, Männedorf, Switzerland) at a 450-nm wavelength.
In summary, 400 cells were placed in a six-well plate and allowed to incubate in culture medium containing 10% fetal bovine serum at a temperature of 37 °C with 5% CO2 for a duration of 2 weeks. The culture medium was replaced every three days. Prior to staining with 0.1% crystal violet, the cells were fixed using 4% paraformaldehyde for 30 minutes. The clones were then quantified using ImageJ software.
Cells from each group were inoculated in six-well plates (2
The capability of cell migration was assessed using 24-well transwell chambers
(with an 8-µm pore size; Sigma-Aldrich, Corning, NY, USA). In the upper
compartment, 100 µL of serum-free medium was combined with 2
Culture the cells until they reach 70–80% confluence, then detach and re-suspend them in fresh medium. Use diluted Matrigel (3 mg/mL; M8370, Suolaibao company, Beijing, China) coated on the upper surface of the transwell inserts and add a predetermined number of cells to the upper chamber, while adding complete medium to the lower chamber. This process should be performed on ice, allowing the Matrigel to solidify at 37 °C for 45 minutes. Incubate the setup at 37 °C with 5% CO2 for 24–48 hours. After incubation, fix the migrated cells on the lower surface of the insert using a fixative solution, followed by staining with a crystal violet. Once stained, rinse to remove excess dye, allow the inserts to dry, and then examine them under a microscope to count the number of invaded cells.
Female BALB/C nude mice (5-weeks-old; 17.54
Statistical analyses were conducted utilizing IBM SPSS Statistics software (IBM Corp., Armonk, NY, USA) version 29.0, along with GraphPad Prism 10.0 (GraphPad Software, La Jolla, CA, USA), and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) [12]. The results of the IHC were evaluated employing the chi-square test. A p-value of less than 0.05 was deemed statistically significant. To compare the two sample groups, an independent samples t-test was used, whereas a one-way ANOVA was applied for comparisons involving multiple groups. Following one-way ANOVA, post-hoc multiple comparisons were performed using the Tukey’s HSD (Honestly Significant Difference) test to evaluate differences between group means. This method was chosen for its ability to control the Type I error rate while comparing all pairs of means. All statistical tests were two-sided, and each experiment was conducted three times for every group.
To elucidate the divergence in CD47 expression in BC, we analyzed two datasets derived from TCGA-BRCA databases: GSE42568 and GSE15852. Our findings indicated that CD47 expression was significantly high in BC samples comparing to normal breast samples (Fig. 1A,B). Subsequently, we used a Venn gram to identify approximately 11,194 genes co-expressed across the three datasets (Fig. 1C,D). To conduct a comprehensive analysis of the signaling pathways correlated to the regulation of activated CD47 from KEGG database [13], we established that elevated CD47 levels in BC were linked to several critical pathways, including cytokine-cytokine receptor interactions, the JAK/STAT signaling pathway, chemokine signaling pathway, and cell adhesion molecule pathways. These pathways are pivotal in driving processes such as cell proliferation, apoptosis, metabolism, and invasiveness (Fig. 1E; Supplementary Fig. 3). Additionally, the GO analysis indicated that differentially expressed genes (DEGs) associated with elevated CD47 expression were connected to the enhancement of cytokine production, activity of receptor ligands, and the activation of signaling receptors (Fig. 1F).
 Fig. 1.
Fig. 1.
Bioinformatic analysis of CD47 in two databases. (A) Column diagrams of CD47 expression in tumors and normal tissues based on The Cancer Genome Atlas and Gene Expression Omnibus databases. (B) Volcano plots of differentially expressed genes according to three databases. (C) Venn diagram of three databases showing co-expressed genes. (D) Gene numbers in the three databases. (E,F) The Gene Ontology/Kyoto Encyclopedia of Genes and Genomes clustering analysis of high CD4 expression-associated signaling pathways and functions. BP, Biological Process; CC, Cellular Component; MF, Molecular Function.
To investigate the relationship between CD47 level and
BC, we collected data from 108 clinical cases between January 2020 and January
2022. IHC staining demonstrated that CD47 was primarily localized on the
membrane of BC cells, and these levels were significantly higher in cancer
tissues than in para-cancerous tissues (Fig. 2A,B; Table 1; p
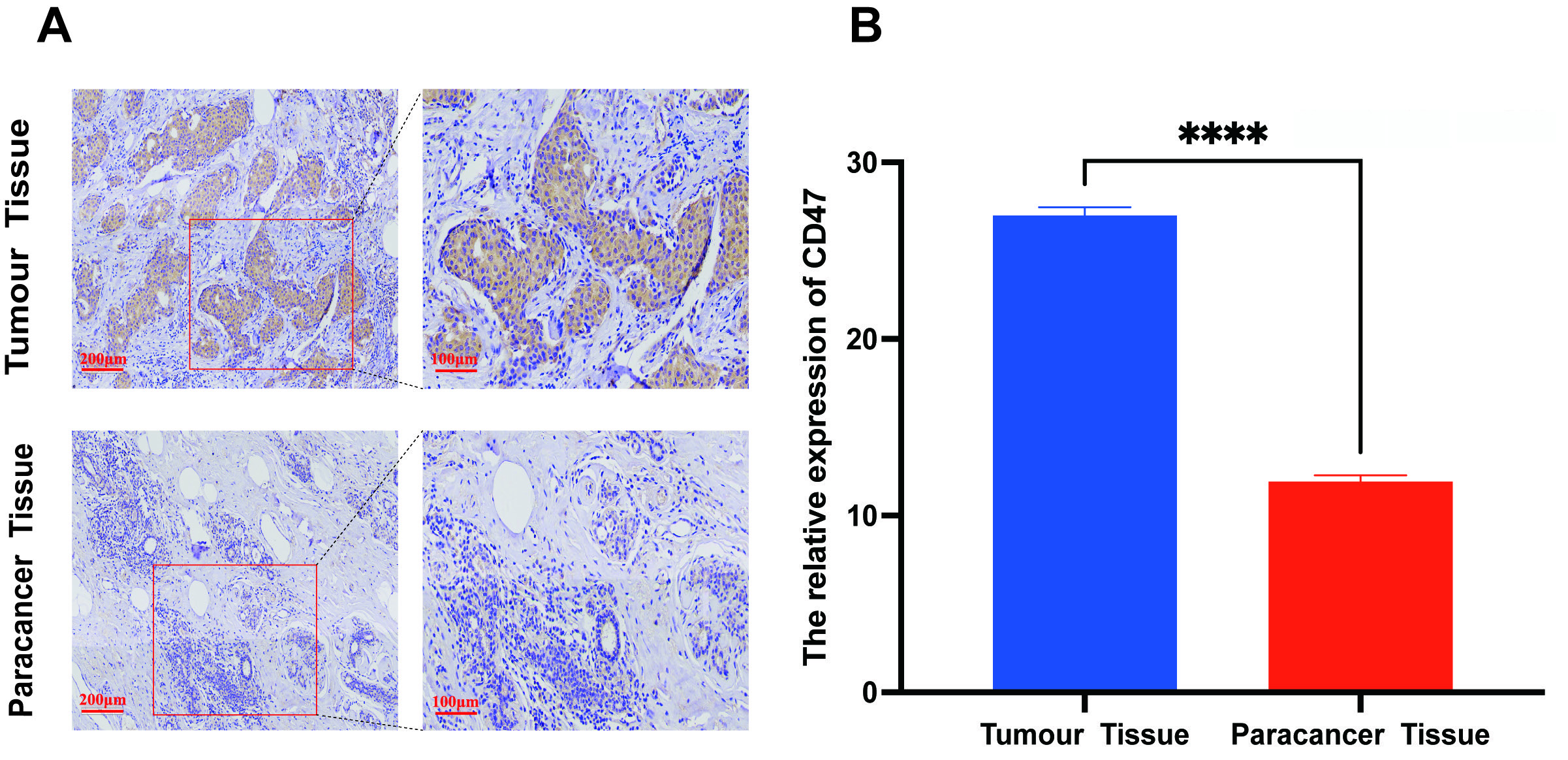 Fig. 2.
Fig. 2.
The clinical significance of CD47 expression in breast cancer.
(A,B) CD47 expression in cancer and paired para-cancerous tissues by
immunohistochemical staining. The scale bar = 100 or 200 µm. All
experiments had three biological replicates with similar results. Data are
presented as mean
| CD47 Expression (n = 109; %) | T test | p value | |
| Cancer | 26.996 |
271.200 | |
| Para-cancer | 11.932 |
n = 108. All experiments had three biological replicates with similar results. Data are
presented as mean
| N | CD47 Expression (%) | T/F test | p value | ||
| Age (years) | |||||
| 47 | 28.709 |
9.048 | |||
| 61 | 26.362 |
||||
| ER | |||||
| Positive | 79 | 26.997 |
0.130 | 0.896 | |
| Negative | 29 | 27.046 |
|||
| PR | |||||
| Positive | 67 | 28.708 |
1.760 | 0.081 | |
| Negative | 41 | 28.023 |
|||
| HER-2 | |||||
| Positive | 62 | 27.268 |
0.330 | 0.272 | |
| Negative | 46 | 27.226 |
|||
| Ki-76 | |||||
| 32 | 25.930 |
11.580 | |||
| 76 | 26.997 |
||||
| pTNM stage | |||||
| I | 32 | 25.930 |
9.557 | ||
| II | 45 | 26.591 |
|||
| III | 13 | 26.020 |
|||
| IV | 18 | 25.414 |
|||
ER, estrogen receptor; PR, progesterone receptor; HER-2, Human Epidermal Growth
Factor Receptor-2; pTNM stage, pathological tunor/node/metastasis (TNM) staging;
Ⅰ, T1N0M0; Ⅱ,
T2N0M0/T2N1M0/T3N0−3M0; Ⅲ,
T3N0−3M0/T4N0−3M0; Ⅳ, M1. All experiments had
three biological replicates with similar results. Data are presented as mean
We performed in vitro and in vivo experiments to verify the
function of CD47 in BC. Given the elevated levels of CD47, we
transfected three shRNA plasmids into 4T1 and MB-231 cells to downregulate
CD47 expression (Supplementary Fig. 1). Notably, cell viability
was overtly lower in the sh-CD47 group than that in the control group, which was
further corroborated by monoclonal proliferation results using CCK-8 staining
(Fig. 3). The wound healing assay results for 4T1 and MB-231 cells demonstrated
that the migratory ratios at 24 and 48 hours were reduced in the sh-CD47 group
compared with the control groups (p
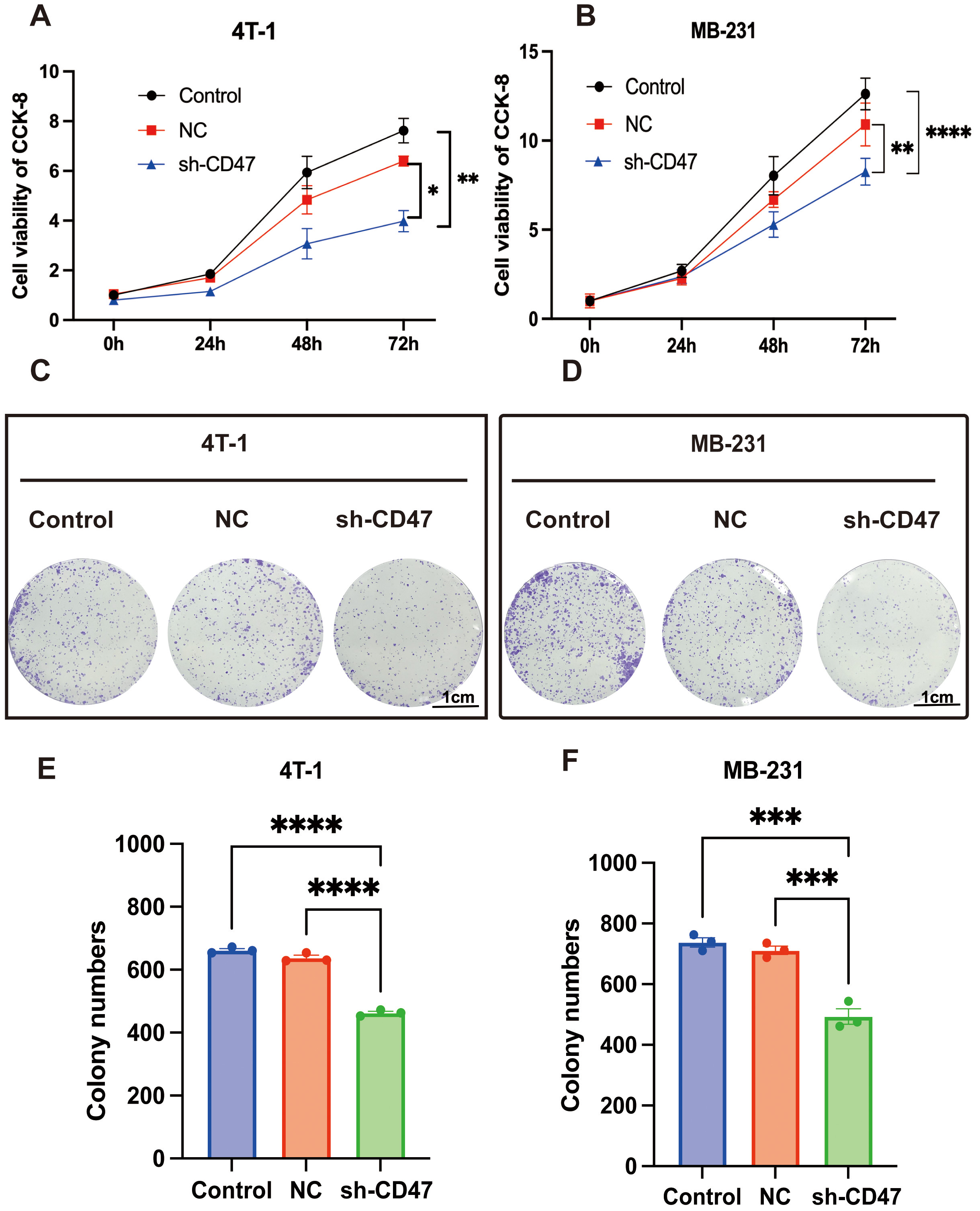 Fig. 3.
Fig. 3.
The functional experiment of CD47 in breast cancer
cells. (A,B) Cell viability of 4T1 and MB-231 cells transfected withshort hairpin-CD47, negative control (NC) plasmids were detected by Cell
Counting Kit-8 (CCK-8) staining assay. (C–F) Cell monoclonal proliferation of
4T1 and MB-231 cells. The scale bar = 1 cm. *p
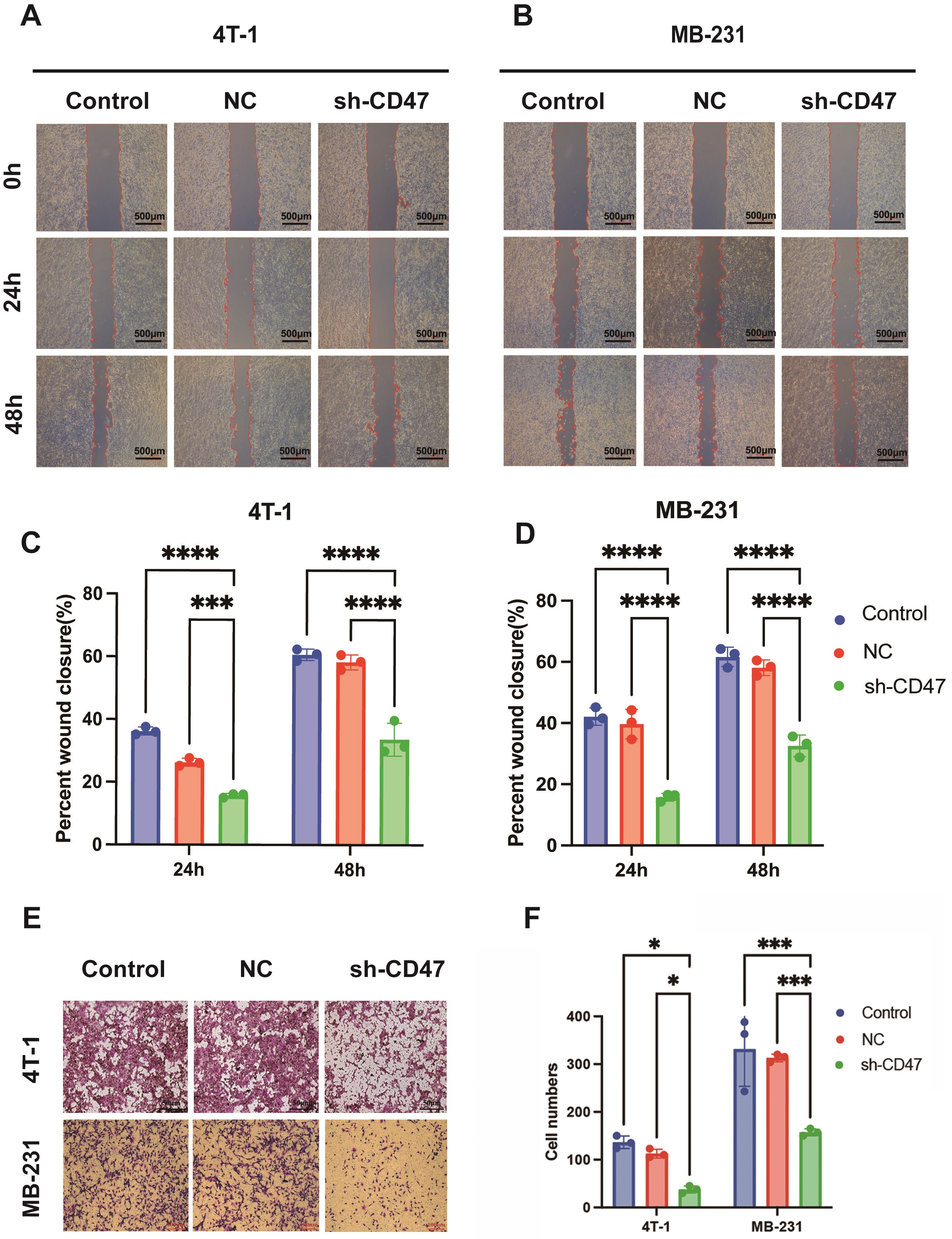 Fig. 4.
Fig. 4.
CD47 knockdown attenuated the invasion and migration
behavior of breast cancer cells. (A–D) Wound healing of 4T1 and MB-231 cells
transfected with sh-CD47 and sh-NC plasmids was measured at 24 h and 48 h. The scale bar = 500 µm. (E,F)
The transwell detection of 4T1 and MB-231 cells transfected with sh-CD47 and
sh-NC plasmids is shown. The scale bar = 50 or 100 µm. *p
 Fig. 5.
Fig. 5.
CD47 knockdown decreased xenograft tumor volume and
weight in nude mice. (A) Gross images of nude mice with BC tumors in the sh-CD47
and control groups. (B,C) Distinct tumor volumes and weights of nude mice are
shown. (D,E) The gross species of BC tumors in the sh-CD47 group and the control
group are shown; tumor weights were analyzed. (F,G) The cyclin dependent kinase
4/6 expression of BC tumors by immunohistochemistry staining assay. The scale bar = 100 or 500 µm. (H,I)
Relative expression values of cyclin dependent kinase 4/6 (n=5). (***p
The involvement of CD47 in cancer research has gained considerable attention since many tumors overexpress this protein to avoid being targeted by the immune system. High levels of CD47 expression have been correlated with poor prognoses in several malignancies, including breast, prostate, and lung cancers [9, 17, 18, 19]. Consequently, therapeutic strategies have emerged, such as the development of monoclonal antibodies against CD47, which have shown promise for enhancing the phagocytosis of cancer cells in preclinical and clinical trials.
The modulation of CD47 presents new avenues for therapies aimed at adjusting immune responses in autoimmune conditions. Finally, in infectious diseases, CD47 may influence the immune response to pathogens, with certain viruses potentially exploiting the CD47 pathway to evade phagocytosis, thereby prolonging survival in host cells [20]. In summary, CD47 serves as a critical regulator of the immune response and cellular homeostasis across a spectrum of human diseases. Ongoing research is focused on elucidating its complex role and exploring potential therapeutic interventions that target CD47 to improve treatment outcomes in various conditions. The CD47 gene plays a crucial role in the mechanism of malignancies primarily through its functions in immune evasion, tumor growth promotion, and modulation of the tumor microenvironment. As a “don’t eat me” signaling protein, CD47 interacts with SIRPα on macrophages, inhibiting phagocytosis and allowing tumor cells to escape immune surveillance [8, 10, 21]. CD47 contributes to tumor cell proliferation and survival through various signaling pathways, including integrin signaling, which promotes tumor cell migration and invasion. Moreover, CD47 affects the tumor microenvironment by regulating the activity and composition of immune cells. It can polarize tumor-associated macrophages towards a pro-tumor phenotype, further aiding tumor progression [22, 23, 24]. Therapeutically, CD47 has emerged as a promising target in cancer immunotherapy, with the development of monoclonal antibodies that block CD47-SIRPα interactions, thereby enhancing the phagocytic activity of macrophages against tumor cells. Overall, the multifaceted role of CD47 in malignancy represents a vital area for ongoing research and potential therapeutic interventions.
The role of CD47 in BC has emerged as a critical area of research, particularly due to its implications for tumor growth and immune evasion [18, 19, 25]. Our findings demonstrate that the knockdown of CD47 significantly decreases the proliferation of BC cells and reduces tumor size in animal models, which is consistent with the existing literature that highlights CD47’s essential role in maintaining BC. This conclusion was affirmed by the finding that high CD47 expression correlated with high Ki-67 levels. Ki-67 is a nuclear protein that is associated with cell proliferation [26]. It is widely used as a cellular marker to assess the growth fraction of a given tissue, particularly in tumor pathology. The presence of Ki-67 in a cell indicates that it is actively dividing or preparing to divide, making it a valuable tool for evaluating the proliferative activity of tumors [27, 28]. Additionally, CD47 is involved in promoting tumor survival through various signaling pathways, such as the Phosphoinositide 3-kinase/ Protein Kinase B (PI3K/Akt) pathway, which enhances cell proliferation and resistance to apoptosis [29].
This study centers on the phenotypic impacts of CD47 in enhancing the proliferation and invasion of BC cells. Nevertheless, we did not examine the fundamental mechanisms through which CD47 influences these processes, including the activation of cellular signaling pathways. Subsequent research should delve into these internal mechanisms to achieve a more thorough comprehension of CD47’s function in BC, potentially facilitating the identification of therapeutic targets.
Our study underscores the pivotal role of CD47 in BC pathogenesis and highlights its dual function in promoting tumor cell propagation. Elevated CD47 expression in BC tissues, as evidenced by bioinformatics analyses and clinical correlations, is associated with increased Ki-67 levels, suggesting a direct link to cellular proliferation. Furthermore, findings from in vitro and in vivo experiments reinforce the critical involvement of CD47 in maintaining the malignancy of BC cells. The potential of CD47 as a therapeutic target offers exciting avenues for the development of innovative treatments aimed at enhancing antitumor responses. Future research should focus on elucidating the intricate mechanisms governing CD47 signaling and exploring the clinical applicability of CD47-targeted interventions to improve outcomes in patients with BC.
During the preparation of this work the authors used ChatGPT in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
The datasets used and analyzed during the current study can be available from the corresponding author on reasonable request.
JW (co-first author): Data curation; Investigation; Formal analysis; Methodology. XW (co-first author): Data curation; Methodology; Conception. XL (second corresponding author): Conceptualization; Data curation; supervision. YX (First corresponding author): Conceptualization; Investigation; Resources; Writing—original draft, review and editing; Data curation; Supervision. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was approved by the research ethics committee Shandong provincial third hospital (KYLL-2021066 for clinical samples) and registered in Medical Research Registration Information system of China (https://www.medicalresearch.org.cn/). All patients signed informed consent. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All animal experimental processes were approved by the Laboratory Animal Center of Shandong Third Hospital (No. DWKYLL-2021023 for animal models). The study is reported in accordance with ARRIVE guidelines of experimental animals.
Not applicable.
(1) Science and Technology Development Program of Shandong Geriatrics Society (LKJGG2021Z005); (2) Scientific research cultivation fund of Shandong provincial third hospital (M2023004/sjzd004); (3) Medical and Health Science and Technology Project of Shandong Province (2023BJ000037).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/FBL28210.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
